To examine the effects of insulin treatment on the expression of phosphorylated protein kinase B (Akt or pAkt) and nitric oxide (NO), known targets of insulin action.
MethodsRetinas from Lewis rats were treated with insulin in vitro, and the levels of pAkt and NO expression assessed in lysed tissue homogenates at various time points. In addition, rats were treated in vivo with a single eye drop containing 1% insulin after which the levels of pAkt and NO were assessed in their retinas at various time points.
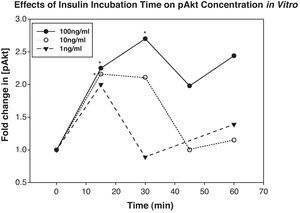
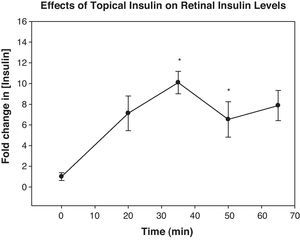
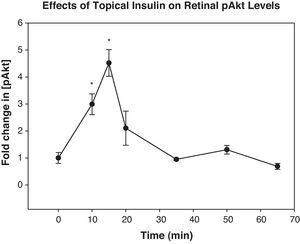
ResultsIncubation of retinas in media containing 10 or 100ng/mL of insulin resulted in a significant increase in their expression of pAkt 15–30min later. When insulin was topically applied in vivo, its levels peaked in the retina 35min. Topically applied insulin significantly increased retinal pAkt levels 10 and 15min post-treatment (p<0.02 and 0.01, respectively). NO levels were elevated 20min after insulin treatment both in vitro and in vivo (p<0.007 and 0.04, respectively).
ConclusionsInsulin upregulated retinal expression of pAkt and NO both in vivo and in vitro, suggesting that topically applied insulin that accumulates in the retina may be physiologically active.
Examinar los efectos del tratamiento con insulina sobre la expresión de las proteínas fosforiladas proteina kinasa B (Akt o pAkt) Akt y de óxido nítrico (NO), objetivos conocidos de la acción insulínica.
MétodosSe trataron las retinas de ratas Lewis con insulina in vitro, evaluándose los niveles de expresión de pAkt y NO en homogeneizados de tejidos lisados en diversos instantes temporales. Además, las ratas fueron tratadas in vivo con una única gota ocular que contenía un 1% de insulina, tras lo que se evaluaron los niveles de pAkt y NO en sus retinas en diversos instantes.
ResultadosLa incubación de retinas en medios con un contenido de 10 ó 100ng/ml de insulina originó un incremento significativo de la expresión de pAkt, pasados 15 ó 30minutos. Cuando la insulina se aplicó tópicamente in vivo, sus niveles se elevaron en la retina a los 35minutos. La insulina aplicada tópicamente incrementó los niveles de pAkt en la retina durante los 10 y 15minutos posteriores al tratamiento (p<0,02 y 0,01, respectivamente). Los niveles de NO se elevaron a los 20minutos del tratamiento de insulina, tanto in vitro como in vivo (p<0,007 y 0,04, respectivamente).
ConclusionesLa insulina incrementó la expresión en la retina de pAkt y NO, tanto in vivo como in vitro, lo que sugiere que la insulina tópicamente aplicada que se acumula en la retina puede ser fisiológicamente activa.
Retinopathy is a common complication of diabetes that poses a serious threat to vision by damaging the retinal vasculature and resulting in ischemia, retinal hemorrhages, and other complications.1–3 Currently, the most effective method of treating diabetic retinopathy and preventing further vision loss is retinal photocoagulation. This treatment is unable to restore losses in visual acuity, however, and may directly contribute to the further loss of peripheral vision, color vision abnormalities, and problems with night vision.4 Thus, it would be clinically beneficial to develop a non-invasive, topical therapy for preventing complications associated with diabetic retinopathy.
Insulin plays a critical role in retinal health. Receptors for insulin are found extensively in the retina, and treatment with systemic insulin has been shown to reverse retinopathic changes.5–8 In addition to moderating blood glucose levels, insulin activates a biochemical cascade that indirectly helps to maintain the health of retinal blood vessels. In this pathway, the binding of insulin to its receptor results in the phosphorylation of Akt, a serine/threonine kinase involved in a variety of cellular responses such as proliferation, growth, metabolism, and survival.9,10 Endothelial nitric oxide synthase (eNOS) is one of many substrates recognized by phosphorylated Akt (pAkt). Once activated by pAkt, eNOS catalyzes the formation of NO, a vasodilating compound that plays a critical role in maintaining the patency of blood vessels and controlling blow flow and pressure.11 Hyperglycemia decreases NO synthesis and reduces its levels in the retina. It has therefore been suggested that diabetics might benefit from therapies that target the NO pathway.12
We previously reported that topically delivered insulin accumulates in the retina and optic nerve of normal and diabetic rats.13 Our preliminary data suggest that the topical application of low concentrations of insulin may be beneficial in preventing retinal pathology.14 One mechanism by which insulin may be exerting its effects is through the upregulation of its signaling intermediate as well as the levels of NO. Accordingly, we applied insulin eye drops to normal rats, confirmed that their retinas accumulated insulin following treatment, and then measured their retinal levels of pAkt and NO. We also examined the effects of insulin on the retinal expression of these mediators in vitro. Our demonstration that topically applied insulin upregulates physiological mediators in the retina that may be protective supports further assessment of the effects of such long-term treatment in diabetic animal models.
MethodsAnimalsTwo to three month old female Lewis rats, obtained from Harlan Laboratories (Indianapolis, IN) were housed in the animal facility at the New England College of Optometry. Animals were fasted overnight prior to experimentation in order to minimize the effects of circulating insulin levels. All experimental procedures were approved by the school's Institutional Animal Care and Use Committee and adhered to the ARVO Statement of the Use of Animals in Ophthalmic and Vision Research.
Retina samplesRetinas were harvested from untreated and insulin treated rats (n=4–15). Animals were sacrificed by decapitation after which their eyes were enucleated and their retinas carefully isolated free of choroid and sclera. Light microscopy was used to confirm that this technique yielded only retinal tissue (data not shown). The in vitro experiments were run in 96-well plates as described below. Following homogenization and centrifugation, all retinal pellets were assessed for their total protein content using the Pierce® BCA Protein Assay Kit (Thermo Scientific, Rockford, IL).
In vitro experimentspAktHarvested retinas were placed in 200μL of serum-free Waymouth's media (Invitrogen, Carlsbad, CA) supplemented with 0.1% BSA and incubated alone or with 100ng/mL, 10ng/mL, or 1ng/mL of insulin for different time periods (15, 30, 45, or 60min). The retinas were then removed from the media and placed in 100μL of assay lysis buffer in microfuge tubes on ice. After 15min, the retinas were homogenized on ice using a Kontes (Vineland, NJ) battery-powered, handheld homogenizer, and 500μL of assay sample diluent were added to each sample tube. Samples were spun for 1min in a Beckman microfuge, and the supernatants assayed for their pAkt content by ELISA (Surveyor™ IC Human/Mouse/Rat Phospho-Akt (Pan) (S473) Immunoassay, R&D Systems, Minneapolis, MN). Values were expressed as fold changes in pg of pAkt per mg protein.
Nitric oxideHarvested retinas were placed in placed in 100μL of serum-free Waymouth's media supplemented with 0.1% BSA and incubated for 20min alone or with 4ng/mL insulin, the approximate peak amount of insulin found in the retina following topical administration. Retinas were then removed from the media and placed in 150μL of lysis buffer in microfuge tubes on ice and immediately homogenized. Samples were left on ice for 15min, after which they were spun in a microfuge for 1min, and the supernatants assayed by ELISA (Parameter Total NO/Nitrite/Nitrate Immunoassay, R&D Systems) to determine their nitrite content. Values were expressed as μmol/L. The 20min time point was selected since our preliminary data suggested that it had the highest probability of showing an insulin-induced effect.
In vivo experimentsInsulin eye drop administrationPorcine insulin (Sigma Chemical Co., St. Louis, MO) was reconstituted in a 0.75% bicarbonate buffer containing 1% saponin, a permeation enhancer (Sigma). Rats in the pAkt experiments were anesthetized with ketamine and xylazine (2 parts ketamine (100mg/mL)+1 part xylazine (20mg/mL)−dosage: 0.3–0.8mL/rat) prior to eye drop administration. Rats were not anesthetized in the NO experiments, as studies suggest that anesthesia decreases the activity of eNOS.15,16 A 10μL drop of 1% insulin was applied to the left eye of treated rats, which was then held closed for one full minute to reduce lacrimal drainage; control animals were untreated.
InsulinRetina insulin levels following eye drop administration were assessed at various time points (20, 35, 50, 65min – time points selected based on our previous data) to determine the point at which insulin levels peak. A porcine insulin ELISA (Mercodia, Winston Salem, NC) was used to quantify insulin levels, which were expressed as fold changes in pg per mg protein.
pAkt and nitric oxideFor the determination of pAkt levels, rats were sacrificed by decapitation at various time points following insulin eye drop administration (10, 15, 20, 35, 50, or 65min), after which their retinas were harvested and prepared for pAkt ELISA analysis as described above. For the assessment of NO levels, animals were sacrificed 20min following insulin drop administration and the retinas harvested and prepared for ELISA analysis as described above.
StatisticsSignificant differences between groups were determined by ANOVA. The post hoc Dunnett's test was used to assess statistical significance at the various time points and a Bonferroni correction was applied to account for the multiple tests performed. Values are expressed as the mean±SEM.
ResultsIn vitro experimentsIncubation of rat retinas in media containing 100ng/mL or 10ng/mL of insulin resulted in an increase in their expression of pAkt. Specifically, tissues incubated in 100ng/mL of insulin showed statistically significant elevations in their pAkt levels above baseline at 15 and 30min (fold increases above baseline were 2.3±0.48 and 2.7±0.23, p<0.04 and 0.01, respectively), and tissues incubated in 10ng/mL of insulin showed statistically significantly increased levels at 15min only (fold increase was 2.2±0.1, p<0.01) (Fig. 1). pAkt levels in retinas incubated with 1ng/mL of insulin were not statistically significantly different from controls, though levels at 15min did approach significance.
Incubation of retinas with 100ng/mL of insulin significantly elevated their expression of retinal pAkt above controls 15 and 30min later (p<0.04 and 0.01, respectively). Similarly, 10ng/mL of insulin significantly elevated retinal pAkt expression after 15min (p<0.01). Asterisks indicate points that were significantly different than baseline.
NO levels were significantly elevated in insulin-treated retinas compared to controls. Treated retina nitrite levels were 60.2±3.2μmol/L while the nitrate levels of control retinas averaged 43±4.2μmol/L (p<0.007).
In vivo experimentsInsulin levels peaked in the rat retinas 35min after topical application and remained significantly elevated at 50min (p<0.001 and 0.02, respectively). Insulin levels peaked at approximately 10pg/mg protein (Fig. 2). Intravenous injection of an equal amount of insulin as is present in a 10μL insulin drop did not affect retinal insulin levels (data not shown).
Topically applied insulin led to a significant elevation in Akt levels relative to baseline at 10 and 15min (fold increases above baseline were 3.0±0.25 and 4.5±0.41, p<0.02 and 0.01, respectively) (Fig. 3).
NO levels were significantly elevated in the treated group relative to controls. Rats that received an insulin eye drop 20min earlier had average nitrite levels of 183±15μmol/L, while controls had average levels of 133±11.4μmol/L; this difference was statistically significant (p<0.04).
DiscussionThe above data not only confirm our previous findings that insulin accumulates in the rat retina following topical administration, but also support the conclusion that retina insulin increases the expression of its downstream mediators, pAkt and NO.
In addition to modifying blood flow through other vessels such as the aorta, NO has been recognized as an important regulator of blood flow through the choroid, optic nerve, and retina.17 It is well established that the release of autoregulatory factors such as NO plays a critical role in maintaining the patency of retinal blood vessels. Insulin plays a key role as an NO inducer by binding to insulin receptors on the endothelial cells; inactivation of these receptors in knockout mice resulted in reduced expression of endothelial vasoactive mediators including NO and endothelin-1.18 A complete biochemical signaling pathway links the insulin receptor to the activation of eNOS in vascular endothelial cells, resulting in the enhanced production of NO.19 Insulin-mediated NO production involves the initial phosphorylation of IRS-1 and later downstream phosphorylation of the serine-threonine kinase Akt, the latter of which directly causes eNOS S1179 phosphorylation by a Ca2+ independent mechanism, leading to the enhanced production of NO within minutes.19–23 In addition to activating Akt, insulin increases eNOS mRNA and protein levels over a longer time course.24 Intraluminal insulin administration was shown to dilate potassium-contracted pig retinal arteries within minutes by a NO-mediated mechanism,25 and to increase fundus pulsations.26
In light of our above findings, the known effects of NO on retinal blood flow, and the fact that insulin receptors are expressed throughout the retina,5,27 it is fair to posit that the accumulation of insulin in the retina following topical application may help to protect it from the harmful effects of diabetes. The fact that NO was reported to be important in stabilizing platelets by blocking platelet activating factor and in preventing excessive activation and endothelial adhesion of circulating leukocytes could be an added benefit of induced NO release in the diabetic retina.28 Further evidence suggesting that insulin-induced NO release could be beneficial in diabetics includes the data showing reduced levels of constitutive eNOS expression in retinal vascular cells in diabetes29 as a result of increased PKC levels in endothelial cells,30 the reduction in NADPH induced by sorbitol pathway activation,31,32 and by the activity of advanced glycation endproducts (AGEs).33 Diabetic eNOS knockout mice were reported to experience more severe retinopathy than age-matched diabetic controls, exhibiting increased vascular leakage, acellular capillaries, basement membrane thickening, and gliosis.34 Retinal symptoms also occurred at an accelerated rate compared to diabetic controls. Likewise, others have related polymorphisms in the eNOS gene to an increased risk of developing retinopathic changes.35–38 High glucose was shown to inhibit insulin-stimulated NO production in endothelial cells.39 Finally, insulin itself was shown to rescue retinal neurons from apoptosis by an Akt-mediated mechanism that reduces the activation of caspase-340 and the retina was shown to express mRNA for all three anti-apoptotic Akt isoforms.41
We previously demonstrated that high dose insulin eye drop treatment increased the number of retinal acellular capillaries, with many of these capillaries exhibiting a highly degenerated, thread-like appearance.14 In other words, high doses of insulin drops administered for over a year led to a worsening of background retinopathy in a rat model. While our rationale for such treatment is described above, our dosage was apparently too high. The foregoing notwithstanding, our earlier results were not entirely unexpected in light of the data from the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), which showed that the 10-year incidence of retinopathy, progression of retinopathy, and progression to proliferative retinopathy were higher in individuals taking insulin as opposed to those who were not.27 Furthermore, insulin was shown to increase VEGF mRNA and protein levels in RPE cells, suggesting that insulin treatment could, at least transiently, lead to a worsening of retinopathy,42 and acute intensive insulin therapy was reported to exacerbate diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1 α and VEGF.43 These data suggest that there is a fine line between insulin's beneficial and detrimental effects in the retina. More recently, we treated streptozotocin-induced diabetic rats with a much lower dosage regimen of insulin eye drops and found significant reductions in retinal vascular cell apoptosis, acellular capillaries, and loss of pericytes (Okamoto T et al. Ophthalmol Vis Sci 2004;45:ARVO E-Abstract 3254). The data from the current study suggest that low dose retinal insulin may be mediating these effects through the upregulation of Akt and NO.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study was supported by the Massachusetts Lions Eye Research Fund and by a grant from the OneSight Research Foundation.