To evaluate the possible use of soft contact lenses (CL) to improve the secretagogue role of diadenosine tetraphosphate (Ap4A) promoting tear secretion.
MethodsTwo conventional hydrogel CL (Omafilcon A and Ocufilcon D) and two silicone hydrogel (SiH) CL (Comfilcon A and Balafilcon A) were used. Ap4A was loaded into the lenses by soaking in a 1mM Ap4A solution during 12h. In vitro experiments were performed by placing the lenses in multi-wells during 2h containing 1ml of ultrapure water. 100μl aliquots were taken at time zero and every minute for the first 10min, and then every 15min. In vivo experiments were performed in New Zealand rabbits and both the dinucleotide release from SiH and tear secretion were measured by means of Schirmer strips and high-pressure liquid chromatography (HPLC) analysis.
ResultsAp4A in vitro release experiments in hydrogel CL presented a release time 50 (RT50) of 3.9±0.2min and 3.1±0.1min for the non-ionic and the ionic CL, respectively. SiH CL released also Ap4A with RT50 values of 5.1±0.1min for the non-ionic and 2.7±0.1min for the ionic CL. In vivo experiments with SiH CL showed RT50 values of 9.3±0.2min and 8.5±0.2min for the non-ionic and the ionic respectively. The non-ionic lens Ap4A release was able to induce tear secretion above baseline tear levels for almost 360min.
ConclusionThe delivery of Ap4A is slower and the effect lasts longer with non-ionic lenses than ionic lenses.
Evaluar el posible uso de las lentes de contacto blandas (LC) para mejorar la función secretagoga del diadenosín tetrafosfato (Ap4A), de cara a promover la secreción lagrimal.
MétodosSe utilizaron dos lentes de contacto convencionales de hidrogel (Omafilcon A y Ocufilcon D) y dos lentes de contacto de hidrogel de silicona (SiH) (Comfilcon A y Balafilcon A). Se cargó Ap4A en las lentes, mediante inmersión en una solución de 1mM de Ap4A durante 12h. Se realizaron experimentos in vitro, colocando las lentes durante 2h en pocillos con 1ml de agua ultra pura. Se tomaron 100μl de muestra en el momento inicial, y a cada minuto durante los primeros diez minutos, y posteriormente cada 15 minutos. Se realizaron experimentos in vivo con conejos de Nueva Zelanda, midiéndose tanto la liberación de nucleótido a partir de las lentes SiH como la secreción de lágrimas, mediante tiras de Schirmer y análisis cromatografía líquida de alta presión (HPLC).
ResultadosLos experimentos de liberación in vitro de Ap4A en las lentes de contacto de hidrogel presentaron un tiempo de liberación 50 (RT50), de 3,9±0,2min y 3,1±0,1min para las lentes no iónicas e iónicas, respectivamente. Las lentes de contacto de SiH liberaron también Ap4A con valores RT50 de 5,1±0,1min para las lentes no iónicas, y de 2,7±0,1min para las lentes iónicas. Los experimentos in vivo con las lentes de SiH mostraron valores RT50 de 9,3±0,2min y de 8,5±0,2min para las lentes no iónicas y las iónicas, respectivamente. La liberación de Ap4A de las lentes no iónicas fue capaz de inducir la secreción de lágrimas por encima de los niveles basales, durante casi 360min.
ConclusiónLa liberación de Ap4A es más lenta, y el efecto es más duradero con lentes no iónicas que con lentes iónicas.
The eye is characterized by its complex structure and high resistance to foreign substances including drugs that makes ocular drug delivery a major challenge. Currently, more than 90% of ophthalmic drugs are delivered in the form of solutions or suspensions.1 These conventional ophthalmic formulations generally show low ocular bioavailability due to various factors such as reflex tearing and blinking, non-productive absorption, nasolacrimal drainage, metabolic degradation, and the relative impermeability of the corneal epithelial membrane. As a consequence of these physiological and anatomical constraints, ophthalmic drugs have a short residence time of less than 5min and only 1–5% of applied drug is effectively absorbed.2 The drug loss to the systemic circulation leads to drug wastage and it could cause undesirable side effects.
To overcome the low ocular bioavailability associated with topical ocular administration of drugs by eye drops, alternative drug delivery systems have been proposed.3,4 In this context, soft contact lenses (CL) are emerging as new vehicle for ophthalmic drug delivery. In the eye, CL are separated off the cornea by a thin fluid layer called post-lens tear film. The fluid in the post-lens tear film is not well-mixed with the remaining tear fluid. The mixing time of the fluid in the post lens tear film with the outer tear fluid is about 30min.5 Thus, ophthalmic drugs released from the soft CL would have a residence time in front of the cornea for at least 30min compared to less than 5min for eye drops.6 The enhanced residence time leads to improved drug bioavailability that would reduce the amount of drug that enters in the systemic circulation, thus potentially improving therapeutic efficacy and preventing side effects.
The potential of CL loading conventional drugs used to treat anterior eye disorders have been already explored.7–10 Most of the studies performed have been in vitro assays, whereas in vivo validation of therapeutic contact lenses has not been so widely examined.
Commercially available CL are manufactured using different materials presenting significant changes in their water content and electrostatic charge among other characteristic properties.11 These material variations can have a relevant effect on the CL–drug interaction, and thus affect rates of drug uptake and release.
Initial studies were developed soaking hydrophilic conventional soft contact lens based on poly (2-hydroxyethyl methacrylate) (pHEMA) hydrogels.11–15 However, in the last years, highly oxygen-permeable soft CL based on siloxane containing hydrogels, have also been tested.16–20 As silicone-hydrogel (SiH) contact lenses transmit more oxygen than conventional materials, thus, diminishing in most of the cases the clinical hypoxic signs associated with extended wear of hydrophilic contact lenses, they may represent a better option to develop extended drug delivery vehicles for therapeutic applications.
New substances are emerging as potential agents for the treatment of dry eye. Among these, dinucleotides are interesting candidates as their topical application induces tear secretion stimulating the secretion of tear fluid (water) as well as the secretion of mucins (key proteins contributing to maintenance and lubrication of the tear film). The dinucleotide diadenosine tetraphosphate (Ap4A) markedly increased tear production to around a 60% above normal tear values in New Zealand White rabbits.21 Likewise, the application of other dinucleotide, diuridine tetraphosphate (Up4U, INS365, diquafosol), induced a 1.5-fold transient increase in tear fluid secretion in a rat dry eye model.22 Interestingly, this dinucleotide has been under development as a new treatment for dry eye23 and, after completion of clinical trials, it has been recently launched in Japan by Santen Pharmaceutical.
The purpose of this study was to investigate the delivery of the dinucleotide Ap4A from commercial soft CL performing in vitro and in vivo assays. Furthermore, to obtain an indication of the clinical feasibility of this drug delivery system to treat dry eye pathology, we measured tear secretion induced by Ap4A soaked-soft contact lenses.
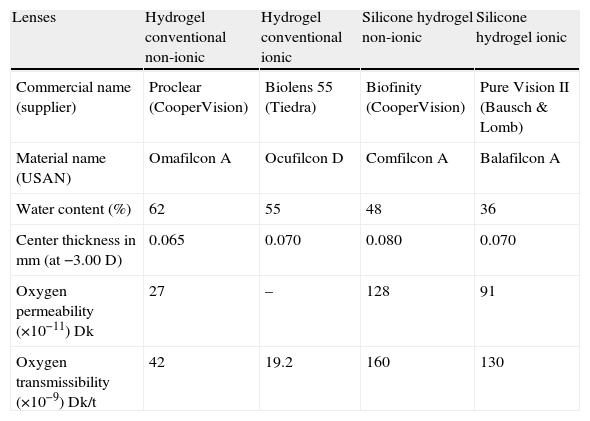
MethodsMaterialsFour commercially available CL, including two conventional hydrogel lenses (Omafilcon A and Ocufilcon D) and two SiH CL (Comfilcon A and Balafilcon A), which properties are outlined in Table 1, were evaluated in this study. All lenses had a power of −3.00D and base curve of 8.60mm. Diadenosine tetraphosphate (Ap4A) was purchased from Sigma–Aldrich Chemicals (St. Louis, USA). The Schirmer strips (Tear Flo test strips, HUB Pharmaceuticals Rancho Cucamonga, USA) were provided by Laboratorios Lenticon SA (Spain). All chemicals were of reagent grade purchased from Merck (Germany).
Characteristics of the contact lenses used along this study.
| Lenses | Hydrogel conventional non-ionic | Hydrogel conventional ionic | Silicone hydrogel non-ionic | Silicone hydrogel ionic |
| Commercial name (supplier) | Proclear (CooperVision) | Biolens 55 (Tiedra) | Biofinity (CooperVision) | Pure Vision II (Bausch & Lomb) |
| Material name (USAN) | Omafilcon A | Ocufilcon D | Comfilcon A | Balafilcon A |
| Water content (%) | 62 | 55 | 48 | 36 |
| Center thickness in mm (at −3.00D) | 0.065 | 0.070 | 0.080 | 0.070 |
| Oxygen permeability (×10−11)Dk | 27 | – | 128 | 91 |
| Oxygen transmissibility (×10−9)Dk/t | 42 | 19.2 | 160 | 130 |
Four male New Zealand white rabbits (2.5±0.5kg) were used in the in vivo experimentation. The animals were kept in individual cages with free access to food and water. Rabbits were submitted to 12h/12h light/dark cycles. All the protocols here described were approved by the Comité de Experimentación Animal of Complutense University which follows the tenets of the European Communities Council Directive (86/609/EEC).
Ap4A loading into contact lensesCommercial CL were removed from their original blister packs and were transferred to 24-well plate. Ap4A was loaded into the lenses by soaking in a 1mM Ap4A solution (diluted in saline solution sterile at 0.9% whose properties were the same than CL’ packaging solutions). The lenses were soaked overnight for 12h simulating a normal cleaning regime performed by wearers of contact lenses. The control CL was soaked in saline solution at 0.9%. At the end of Ap4A loading, the lenses were taken out and the excess Ap4A from the surface was removed before to beginning the Ap4A release experiments. The procedure was the same for “in vivo” and “in vitro” studies.
Ap4A release “in vitro” assaysFor each release experiment, three CL of each lens type were studied. After Ap4A loading, the lenses were placed in new well containing 1ml ultrapure water. Samples (aliquots of 100μl) were removed from the well at time zero and every minute for the first 10min, and then at t=15, 20, 30, 60, 90 and 120min, to assess the quantity of Ap4A released into the solution. The same volume, which was removed in each sample, was replaced with ultrapure water. The Ap4A release dynamics was determined by measuring the quantity of Ap4A in each sample, which was measured by means of high-pressure liquid chromatography (HPLC).
Ap4A release “in vivo” assaysThe in vivo experiments were done with SiH contact lenses, which were loaded with Ap4A as described above. In vivo assays study two topics, the dynamic of Ap4A release “in vivo” via CL and its effect on tear secretion. Both were measured by means of Schirmer strips. Firstly, baseline values were measured. Then, each animal received to right eye one drop of 10μl of Ap4A at 1mM, a Comfilcon A (non-ionic) contact lens loaded with Ap4A or a Balafilcon A (ionic) contact lens loaded with Ap4A. In each case, the contralateral eye receives the same lens but without Ap4A. After that, tear secretion was measured using the Schirmer test without anesthesia at t=0, 5, 10, 15, 30, 45, 60, 90, 120 and then every hour during 6h. Tear collection was always performed following a van Bijsterveld modified criterion.24 Briefly, the Schirmer strip was placed on the temporal tarsal conjunctiva of the lower lid for 2min25 with the eyes closed. The volume of tears was recorded as millimeters of moistened strip, and the wetted ends of the Schirmer strip were placed in Eppendorf tubes containing 500μl of ultrapure water, until the HPLC analysis was performed.
The reason for not using conventional hydrogel lenses was the problems we observed when these lenses were fitted to rabbit eyes. Due to the low frequency of blinking, the rate of dryness did not permit us to perform accurate measurements and therefore we decided to focus on SiH lenses.
Sample preparation for analysisSample from “in vivo” and “in vitro” assays were collected after the corresponding procedure (see above). Samples were stored in Eppendorf tubes at −20°C before processing. The treatment was based on the protocol described by Lazarowski and co-workers.26 Briefly, samples were heated at 98°C bath for 2min. Then, the samples were incubated in ice for 10min and finally the residues were removed by centrifugation at 13,000rpm for 10min. Finally, the supernatants were collected for HPLC analysis.
HPLC proceduresThe chromatographic system consisted of a Waters 1515 Isocratic HPLC pump, a 2487 dual absorbance detector, and a Reodyne injector, all managed by the software Breeze from Waters (Milford, MA). The column was a KromaPhase 100 C18 (250mm in length, 4.6mm in diameter) from Scharlab. The system was equilibrated overnight with 10mM KH2PO4, 2mM tetrabutylammonium (TBA), 21% acetonitrile, pH 7.5, and detection of nucleotides was performed under isocratic conditions with the mobile phase described above at a flow rate of 2ml/min.
After injection of 100μl of sample, detection was monitored at 254nm. Ap4A peak was identified comparing their retention time with the one of a commercial standard. The quantification was performed by comparing the areas under the curves with those of known amounts of commercial standard Ap4A.
Statistical analysisAll data are presented as the mean±SD. Due to the limited sample size, comparisons between lenses were done using non-parametric statistical analysis. Differences between time points were determined by Kruskal–Wallis test. Repeated measures analysis was conducted using Wilcoxon signed ranks test. All graphics were carried out by GraphPad Prism 5 software (GraphPad, La Jolla, CA). Ap4A release (decay constant) was calculated by fitting the corresponding curves to one phase exponential decay curve. From this analysis it was possible to calculate the Release Time 50 (RT50), which corresponds to the time in which the lens has released 50% of the maximum Ap4A measured. With this analysis it has been also possible to obtain the rate of release for each lens. A p value lower than 0.05 was considered statistically significant.
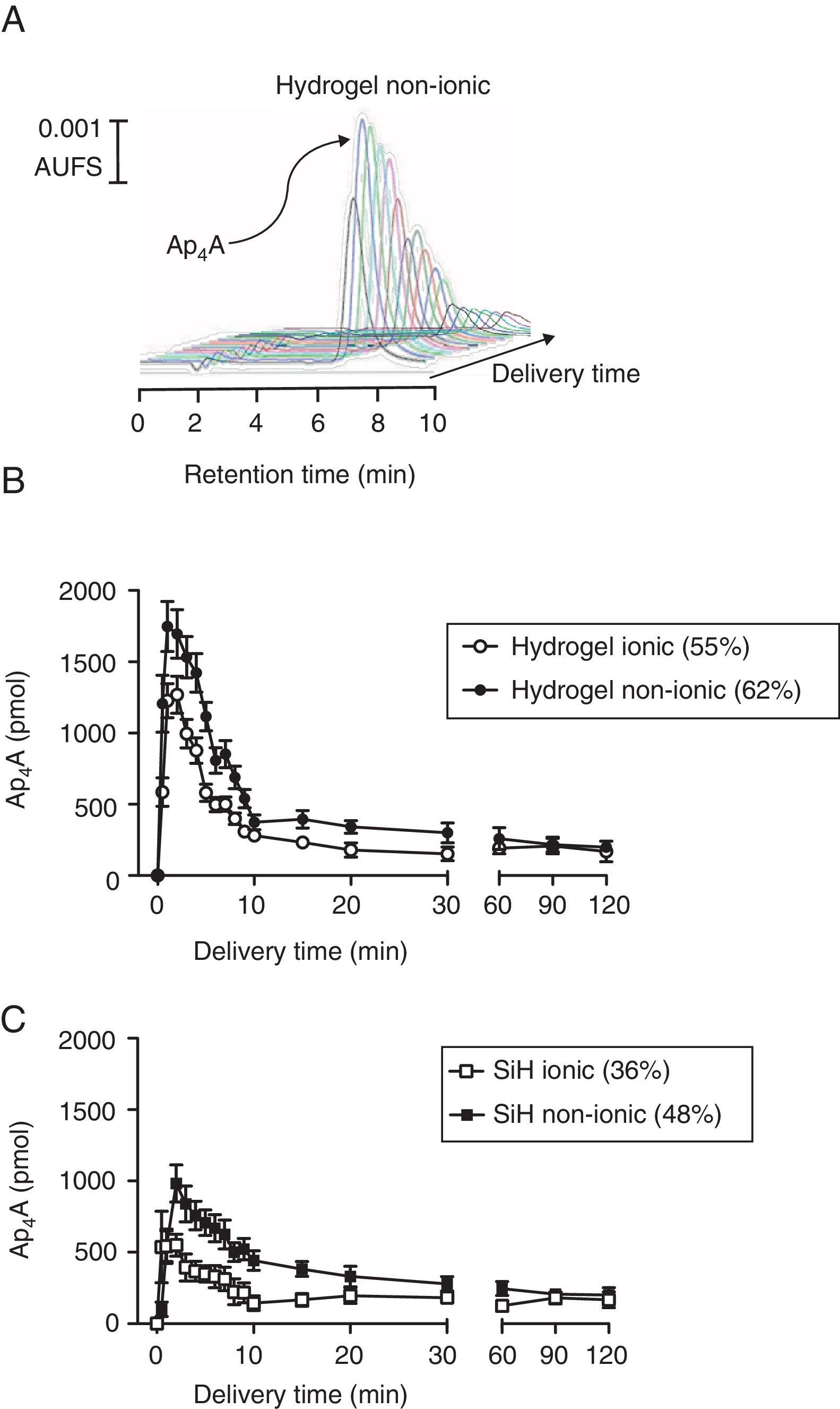
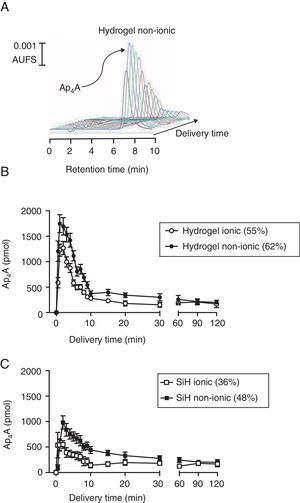
ResultsAp4A release “in vitro”The release of the dinucleotide Ap4A was followed by HPLC by measuring its absorbance at 254nm wavelength. As presented in Fig. 1A for a non-ionic hydrogel lens, where a series of HPLC injections are plotted, the release of the loaded dinucleotide presented a maximal release at 2min after the in vitro experiment started. After this maximal peak, the amount of released Ap4A was gradually diminishing until the end of the experiments (360min).
In vitro release of diadenosine tetraphosphate from soft contact lenses. (A) Representative chromatographic profile showing the release of Ap4A from a non-ionic hydrogel contact lens. (B) Comparative in vitro release of Ap4A from non-ionic and ionic hydrogel lenses following the protocol described in methods (n=3). (C) Comparative in vitro release of Ap4A from non-ionic and ionic silicon lenses following the protocol described in methods (n=3).
Four types of lenses were used in this study, two conventional hydrogel lenses, Omafilcon A (ionic; 55% hydration) and Balafilcon A (non-ionic; 62% hydration) and two SiH lenses, Confilcon A (ionic; 36% hydration) and Balafilcon A (non-ionic; 48% hydration). For a better understanding, the study of how Ap4A was released in vitro from the lenses was grouped by lens materials. As it can be seen in Fig. 1B, hydrogel lenses presented a peak of 1747±175pmol and 1227±120pmol, 2min after the experiment started, for the Omafilcon A (non-ionic) and the Oculfilcon A (ionic) lens respectively (p=0.017; Wilcoxon test). Both contact lenses presented release time 50 (RT50) of 3.9±0.2min and 3.1±0.1min for the Omafilcon A and Oculfilcon A lens respectively. The rate of the released of Ap4A permitted us to obtain decay constant (decay rate) values of 0.17±0.02min−1 for the Omafilcon A lens and a value of 0.22±0.02min−1 for Ocufilcon A lens (all the experiments n=3).
When the same protocol was performed with the SiH lenses, the Comfilcon A (non-ionic) lens presented a peak of 982±130pmol of Ap4A, and the Balafilcon A (ionic) provided a maximal release of 550±78pmol of Ap4A, both peaks 2min after the experiment started. (p=0.011; Wilcoxon test). The RT50 for both lenses were 5.1±0.1min for the Comfilcon A and 2.7±0.1min for the Balaficon A contact lens. The Ap4A decay constant values for these lenses were 0.13±0.01min−1 for the Comfilcon A and 0.25±0.04min−1 for the Balafilcon A lens (n=3 in all experiments).
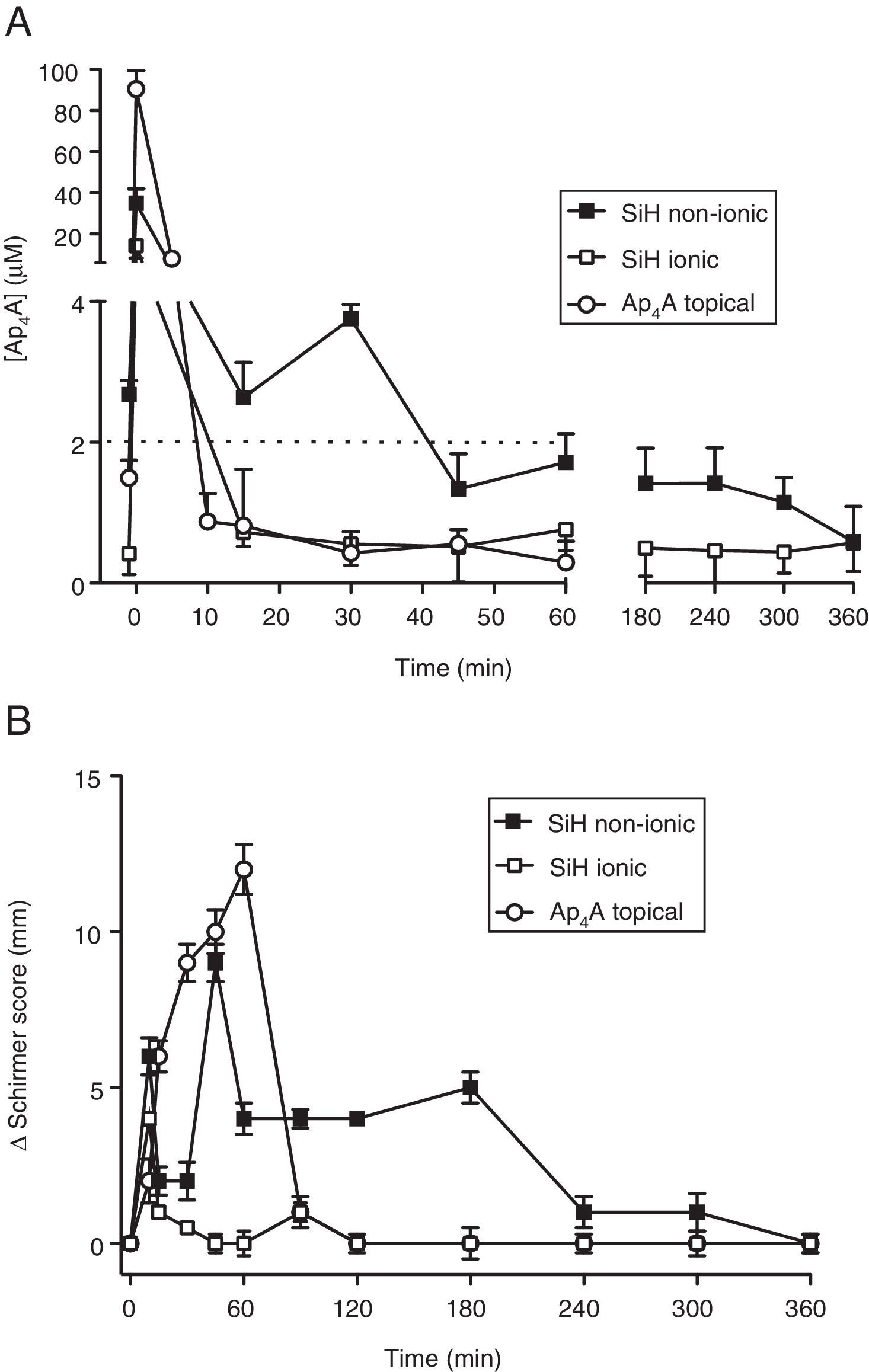
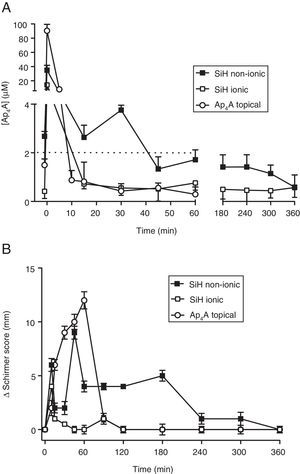
Ap4A release “in vivo”Since the lens that provided the longest RT50 value was the Comfilcon A (non-ionic) lens, we took this material for our “in vivo” studies in New Zealand rabbits. In this sense, we compared whether or not the ionic nature of the contact lens could be a relevant aspect considering the chemical characteristics of the dinucleotide Ap4A. In Fig. 2A is presented a comparison between the Comfilcon A (non-ionic) lens with the Balafilcon A (ionic), both loaded with Ap4A, and also the topical application of the dinucleotide, in New Zealand rabbits. The maximal Ap4A concentration released was provided by the Balafilcon A lens, with a peak of 34.9±7.0μM, while the Comfilcon A peak was 14.2±6.0μM (p=0.034; Wilcoxon test). The topical application of Ap4A presented a peak of 90.5±9.0μM.
In vivo release of diadenosine tetraphosphate from silicon contact lenses and effect on tear secretion. (A) In vivo release of Ap4A from non-ionic and ionic silicon contact lenses together with a topical application of 1mM Ap4A (10μl) (n=3). (B) Variation of tear secretion, induced by Ap4A released from non-ionic and ionic silicon contact lenses together with a topical application of 1mM Ap4A (10μl) (n=3). Zero represents the baseline tear secretion which corresponded to a Schirmer score of 12±1mm.
Interestingly, the way the two lenses and the topical application of Ap4A was released “in vivo”, demonstrated that the lens that delivered Ap4A more slowly was the Comfilcon A (non-ionic) lens. The RT50 for the Comfilcon A, Balafilcon A and topical application of Ap4A were, 9.3±0.2min, 8.5±0.2min and 4.9±0.1min respectively (n=3). Concerning the decay constant, the values obtained were 0.07±0.05min−1 for the Comfilcon A (non-ionic) lens and 0.08±0.06min−1 for Balafilcon A lens (n=3).
When the ability of the lens-released Ap4A to modify tear secretion was tested in New Zealand rabbits, it was possible to see that the longest effect on tear secretion was obtained by the Ap4A that was released from the Comfilcon A (non-ionic) lens, as presented in Fig. 2B. The effect was measurable up to 360min, compared to the Balafilcon A (ionic) which lasted less than 1h. Interestingly, the topically applied Ap4A although producing a strong effect, 12mm in Schirmer test, its effect only lasted 90min (n=3).
DiscussionTo date, there are no published data regarding the delivery of dinucleotides from CL for dry eye treatment or in another condition. The present work reports the first study that has assessed the in vitro and in vivo release of Ap4A from conventional soft CL.
We have chosen two conventional hydrogel lenses (Omafilcon A which is non-ionic and Ocufilcon D which is ionic) and two silicone-hydrogel contact lenses (Comfilcon A which is non-ionic and Balafilcon A which is ionic) with similar parameters (diameter, base curve and center thickness), because the aim of the assay has been to compare the different behavior in the Ap4A loading and release according to material and in relation to surface charge properties (ionic or non-ionic). Incorporation of a drug into the lens can be achieved by different techniques such as drug-loaded colloidal nanoparticles, molecular imprinting, or soaking.8 For this first stage, we have chosen soaking the lenses in the dinucleotide solution because is the simplest way to incorporate the drug into conventional soft CL. When CL are soaked in a solution containing the drug, the compound diffuses into the gel-like matrix of the lens, where it is trapped long enough to be released when transferred to the tear film.27
A number of research groups working on the development of therapeutic CL have focused on delivery of dry eye medications from the lenses. Various molecules acting as re-wetting agents such as hyaluronic acid or hydroxypropyl methylcellulose have been included in soft CL and their subsequent release has been analyzed.28–30 However, soaked CL with hyaluronic acid solution did not load sufficient amounts of hyaluronic acid and release was complete in less than 1h.29 Considering the inflammatory etiology of the disease, delivery of the anti-inflammatory drug cyclosporine A from soaked contact lenses has also been proposed for dry eye treatment, maintaining cyclosporine A delivery rates within the therapeutic window for 14 days.19 Our in vitro outcomes show that Ap4A was released at least 2h with all soaked contact lenses, being the SiH non-ionic lens with more slowdown release rate.
Chemical properties and water content have been suggested as important contact lens properties influencing drug delivery.11 Conventional hydrogel CL are made in polyHEMA which are hydrophilic,31 meanwhile SiH CL have various siloxane-based monomers and therefore the material is less hydrophilic.16 It is not clear if the release is correlated with the drug hydrophilicity.11 In our study it would be expected that Ap4A has a better interaction with conventional hydrogel lenses than silicone-hydrogel lenses, because of its hydrophilic properties. In fact, hydrophilic contact lenses release more dinucleotide than SiH lenses in the first minutes (see Fig. 1).
Regarding water content, drug release is higher in CL with more water content. Probably this is due to water solvates and carries the Ap4A through the CL material.32 Ap4A delivery correlated well with CL water content. But it seems that hydrophilicity and water content are not the main factors influencing the dinucleotide release. The ionic charge of the contact lens materials could be the major factor in Ap4A delivery. In this sense, it is important to note that Ap4A is a dinucleotide presenting four phosphates that in neutral pH present four negative charges.33 This could be the reason why those lenses with ionic charge, which is also negative, may repel Ap4A. This may produce two undesirable effects, either a poor load of the dinucleotide or/and a fast release form the lens due to the repulsion between the negative charges of Ap4A and the contact lens material. Moreover, the fact of ionic contact lenses to provide a slightly longer retention of the dinucleotide, strongly suggests that among some factors to be studied, charge and water content of the lens compete for both the loading as well dinucleotide release. This point deserves more attention and further analysis.
Another important difference between both SiH lenses is the presence of a plasma oxidation surface treatment in the ionic lens Balafilcon A, which is not present in the non-ionic lens Comfilcon A. Plasma treatment has been applied to the first generation of SiH CL in order to counterbalance the hydrophobic nature of the siloxane moieties. This distinctive characteristic could also play a role in the kinetics of Ap4A uptake and release that need to be further investigated in future studies.
There are several in vitro studies about drug delivery from CL but not so many in vivo evaluating the in vivo release performance. New Zealand white rabbit and beagle dog are the most commonly used models in drug delivery from CL.18,34–36 Initially, we wanted to evaluate all the contact lenses studied in vitro also in vivo, but the hydrogel contact lens were impossible to fit because the lenses were dried in the first hour of wear and the nictitating membrane expelled the lens out of the eye. The Comfilcon A, a non-ionic SiH, maintained the Ap4A concentration around 2μM (value established as a concentration necessary to increase tear secretion21) during 60min in tear film, compared with 5min achieved by topical administration. The effect of Ap4A delivered from Comfilcon A, a non-ionic SiH, on the tear secretion lasted up 300min, 5-fold compared with Ap4A presence time in the ocular surface. It appears that P2Y2 receptors present in the main lachrymal and accessory glands37 are stimulated by Ap4A and this effect remains when the levels of the dinucleotide has decreased on the ocular surface.
It is known the involvement of Ap4A in the ocular surface physiopathology stimulating tear secretion or improving the rate of corneal wound healing. Therefore the dinucleotide can act on the lachrymal gland to induce tear secretion and also on the corneal epithelium accelerating the rate of migration during wound healing.38,39 Both roles can be enhanced when Ap4A is delivered from CL. In particular, and talking about tear secretion, the dinucleotide it could provide great comfort in dry eyes and it could be particularly helpful for preventing CL induced dry eye and/or ocular injury. Nevertheless, it is necessary to be careful when extrapolating these in vivo results obtained in animal models to humans because there are some differences between species that could affect the dinucleotide release. The most important difference is the blinking rate, lower in rabbits,40 due to its role in the natural release of Ap4A41 and also its relevance in the CL wear. In this sense, it would be expected better results with humans, since they blink more often permitting higher tonic levels of Ap4A due to blink shear stress.41 Future studies should be conducted in order to evaluate the release rate with different dinucleotides concentrations. Also, it could be interesting to test its potential delivery slowdown with others drug-loaded techniques.
In summary, we have demonstrated that it is possible to use commercially available CL as delivery systems for a molecule that induces tear secretion. Our experiments demonstrate that the delivery of Ap4A is slower and the effect last longer with non-ionic lenses than ionic lenses but in vitro experiments do not always reflect what happens in vivo, suggesting that apart from in vitro approaches in vivo experiments are necessary to fully evaluate the potential use of CL as devices for drug delivery.
Conflict of interestThe authors do not have any financial interest on the materials and instruments used in this study.