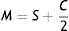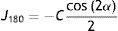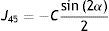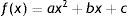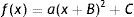To determine whether the initial characteristics of individual patterns of peripheral refraction relate to subsequent changes in refraction over a one-year period.
Methods54 myopic and emmetropic subjects (mean age: 24.9±5.1 years; median 24 years) with normal vision were recruited and underwent conventional non-cycloplegic subjective refraction. Peripheral refraction was also measured at 5° intervals over the central 60° of horizontal visual field, together with axial length. After one year, measurements of subjective refraction and axial length were repeated on the 43 subjects who were still available for examination.
ResultsIn agreement with earlier studies, higher myopes tended to show greater relative peripheral hyperopia. There was, however, considerable inter-subject variation in the pattern of relative peripheral refractive error (RPRE) at any level of axial refraction. Across the group, mean one-year changes in axial refraction and axial length did not differ significantly from zero. There was no correlation between changes in these parameters for individual subjects and any characteristic of their RPRE.
ConclusionNo evidence was found to support the hypothesis that the pattern of RPRE is predictive of subsequent refractive change in this age group.
Determinar si las características iniciales de los patrones individuales de la refracción periférica guardan relación con los cambios subsiguientes de la refracción, durante un periodo de un año.
MétodosSe reunió a 54 sujetos con miopía y emetropía (edad media: 24,9±5,1 años; mediana: 24 años) con visión normal, sometiéndoles a una refracción subjetiva no ciclopéjica. Se midió también la refracción periférica a intervalos de 5 grados, sobre los 60 grados centrales del campo visual horizontal, al igual que la longitud axial. Al cabo de un año, se repitieron las mediciones de la refracción subjetiva y de la longitud axial a los 43 sujetos que se hallaban disponibles para la realización del examen.
ResultadosEn consonancia con los estudios previos, los grandes miopes tendieron a reflejar una hiperopía periférica relativa superior. Sin embargo, se produjo una variación considerable entre los sujetos, en cuanto al patrón del error refractivo periférico relativo (RPRE) a cualquier grado de la refracción axial. Dentro del grupo, los cambios medios de la refracción axial y la longitud axial al cabo de un año no difirieron significativamente de cero. No se produjo correlación entre los cambios de estos parámetros en los sujetos, y cualquier característica del RPRE.
ConclusiónNo se halló evidencia alguna que apoyara la hipótesis de que el patrón del RPRE es predictivo de un cambio refractivo subsiguiente en este grupo de edad.
Early measurements of the distribution of ametropia usually showed that a relatively broad distribution in early life became markedly narrower and peaked near emmetropia by the age of about 8 years.1,2 Although the distribution became more skewed beyond this age, most young adults remained near-emmetropic e.g. Weale.3 However, in contrast to these earlier results, recent decades have seen a marked, progressive increase in myopia through childhood in some parts of the world, particularly Asia e.g. Lin et al.4 This has led to a search for the factors that may influence the development and progression of myopia, in the hope that strategies to inhibit or retard progression may be introduced.
Various possible causative factors have been proposed, including genetics (for review5), near work (for review6), outdoor activities,7 lighting,8 and concentration of vitamin D in the blood.9 A further possibility is peripheral refraction. Comparisons of the patterns of relative peripheral refractive error (RPRE) of human eyes indicate that, on average and in contrast to emmetropes and hyperopes, myopes have a relatively hyperopic defocus in the periphery e.g. Mutti et al.10 Largely on the basis of the study of late-onset myopia in trainee pilots by Hoogerheide et al.11 it has been postulated that these differences arise because relative peripheral hyperopia has a causative effect for the development of axial myopia.12–14 This suggestion is apparently supported by animal studies, which show that the emmetropisation process is visually guided and may involve both central15,16 and local effects,17–19 and that peripheral refraction can guide emmetropisation even in the absence of an axial image.20 These ideas have led to trials in humans of contact and spectacle lenses designed to reduce peripheral hyperopia, in the hope that this might in turn reduce the rate of myopia progression: a minimal, but significant reduction in progression has been found.21–23 On the other hand, some authors24 have suggested that the findings of Hoogerheide et al.11 may have been misinterpreted and that the different patterns of relative peripheral refractive error (RPRE) associated with different refractive groups are the result of the ametropia rather than its cause.25
In view of these contradictory opinions, the aim of the present study was to measure the initial peripheral refractive errors along the horizontal meridian in a group of young adults, and their axial refractive error at baseline and twelve months later. It was hoped that this would allow the relationship, if any, between an individual's initial peripheral refractive parameters and any changes in central refractive error over a one-year time interval to be determined, so that any parameter holding promise of offering a predictive effect for myopisation could be identified. In particular, the hypothesis that a hyperopic RPRE is a precursor to myopic change could be tested. While usually progression of myopia has ceased by early adulthood26 it was expected that, in a population consisting of undergraduates and postgraduates undergoing intensive studies, some cases of late-onset myopia or myopia progression might occur e.g.11,27–33
MethodsFifty-four subjects (20 male and 34 female) were recruited. The majority of subjects were undergraduate or postgraduate students. The age of the subjects ranged between 19 and 38 years (mean: 24.9±5.1 years; median 24 years). All subjects were free of any ocular pathology and could achieve a visual acuity of 6/6 partially or better when corrected. There were 32 myopes (spherical equivalent from −9.63D to −0.63D; mean −3.46±2.35D; median −1.38D) and 22 emmetropes (spherical equivalent from −0.50D to +0.50D; mean −0.03±0.36D; median −0.19D). For all the parameters, only the right-eye data were analysed. The mean ages of the myopes and emmetropes were 25.3±5.5 and 24.5±4.5 years, respectively.
Eleven (∼20%) patients could not be followed up after one year, because they had left the Manchester area or could not be contacted, leaving 25 of the original myopes and 18 of the emmetropes. The mean ages of these remaining myopes and emmetropes were 25.4±5.9 years and 24.7±4.7 years, respectively. The initial refractions were −0.01±0.37D in these emmetropes and −3.38±2.08D in the myopes.
The study followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants after the nature of the study and possible consequences of the study had been explained. The project protocol was approved by the Senate Committee on the Ethics of Research on Human Beings of the University of Manchester.
The subjects were first seen between February and March 2010. At that time non-cycloplegic subjective refraction and peripheral refraction were performed. Axial length was also measured, since it was expected that any refractive change with time might also involve change in this parameter.27,29,33
Subjective refraction was performed to an accuracy of ±0.25 DS and ±0.25 DC to obtain maximum plus giving best visual acuity. The cylindrical component, if existent, was found using a cross-cylinder. To refine the spherical component at the end of the routine, the duochrome test was used.
For peripheral refraction an open-field autorefractor Shin-Nippon SRW 5000 (Ajinomoto Trading Inc., Tokyo, Japan) was used. This has been shown to be repeatable for central and peripheral refraction.34,35 Participants fixated on targets (Maltese crosses) along the horizontal meridian that were placed at 5m in 5° steps from 30° nasal to 30° temporal retina. The order of target fixation was randomised. Subjects were asked to turn their head to fixate each target and five consecutive measurements were taken. Relative peripheral refraction was computed as the difference between mean spherical equivalent in primary gaze and mean spherical equivalent in each peripheral gaze. A Bland–Altman comparison of the subjective results for central refraction with those from the autorefractor showed close similarity (mean difference±standard deviation in mean sphere −0.23±0.53D).
For axial length measurement the LenStar 900 (Haag-Streit, Koeniz, Switzerland) was used. It has been shown that the LenStar is a reliable instrument for axial length measurements.36–39 The instrument was aligned using the image of the patient's eye on the computer monitor. Subjects were asked to blink just prior to measurements being taken. Blinking or loss of fixation were detected automatically by the instrument and in this case the measurements were repeated. The instrument takes 16 consecutive scans per measurement without the need for realignment and five measurements were taken as recommended by the manufacturer. The internal software calculated the mean of these five readings. The axial length measurements were performed only on 48 subjects (which included 30 myopes and 18 emmetropes).
After a year (March 2011) the same patients, when available, were seen again for subjective refraction and axial length measurement.
Data analysisSubjective refraction and autorefractor refractions in terms of sphere (S), cylinder (C) and axis (α) were converted into vector components by the following formulas40:
Each of the three components was fitted against the retinal eccentricity, x degrees, with a second-order polynomial function using OriginPro 8 (OriginLab Corporation, Northampton, MA, USA). Data affected by the optic disc were disregarded for the fitting. The polynomial fit formula was:
The formula approximates the RPRE data as the sum of a parabola centred on the axis, where in the case of M a positive coefficient a represents relative peripheral hyperopia, and a straight line, where a non-zero linear coefficient b means that the fit is no longer symmetrical about the visual axis. If required, Eq. (4) can be transformed to represent a shifted parabola of the form
where B=b/2a and C=(c−b2)/4a2. The extreme value of the parabola then lies at x=−B, y=C.Field angles are taken as negative for the nasal retina. If relative values are used, the constant c approximates to zero. Differences between the myopes and emmetropes were assessed using one-way analysis of variance.
Values are provided as means±standard deviations.
Refractive error change was characterised as the difference between second visit spherical equivalent and first visit spherical equivalent (one-year visit minus baseline) using subjective refraction data. Therefore negative values describe a change towards minus, i.e., progression of myopia. For axial length measurements, a positive difference between the second and first visit indicates an axial elongation.
All statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The conservative Bonferroni adjustment was applied for multiple comparisons.
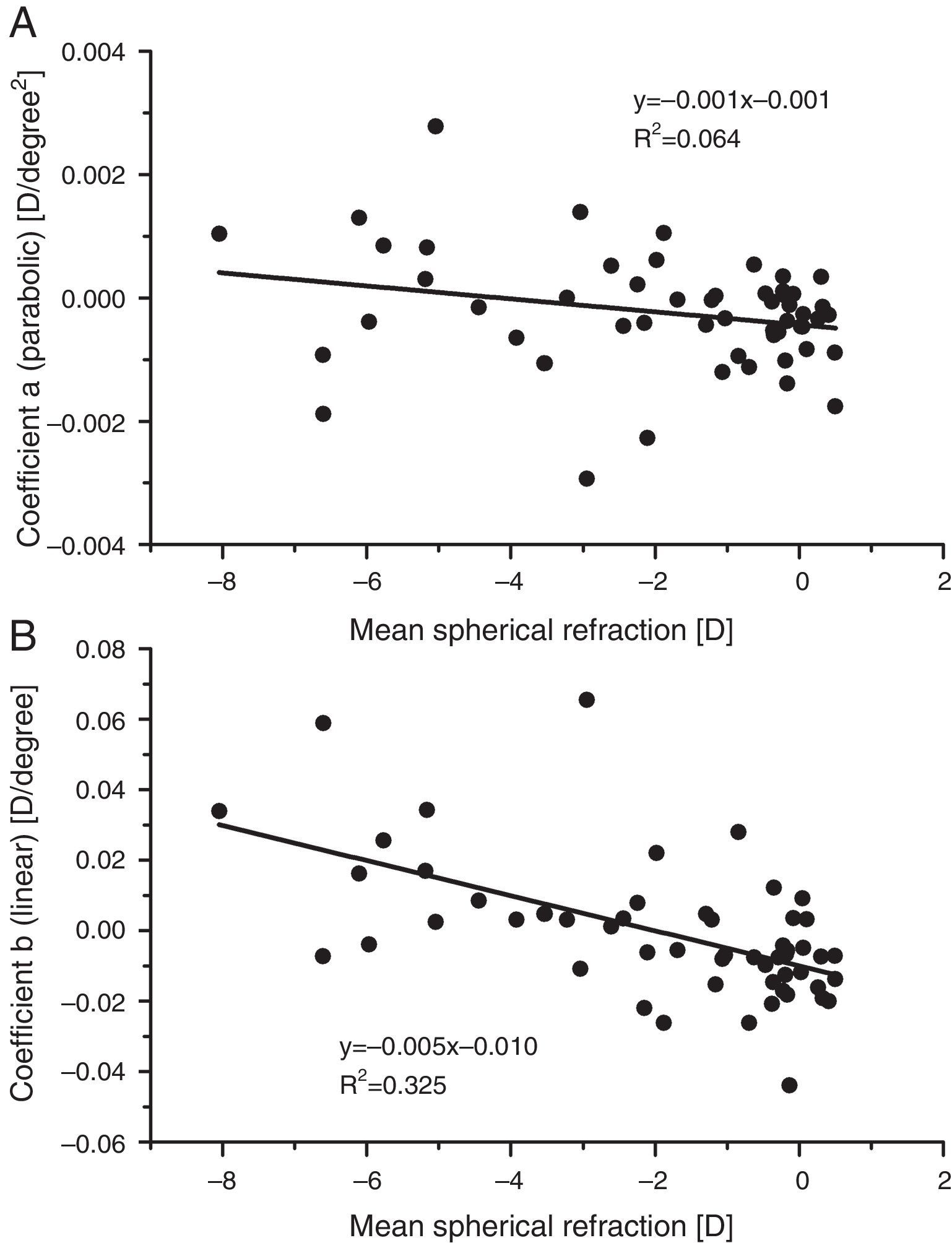
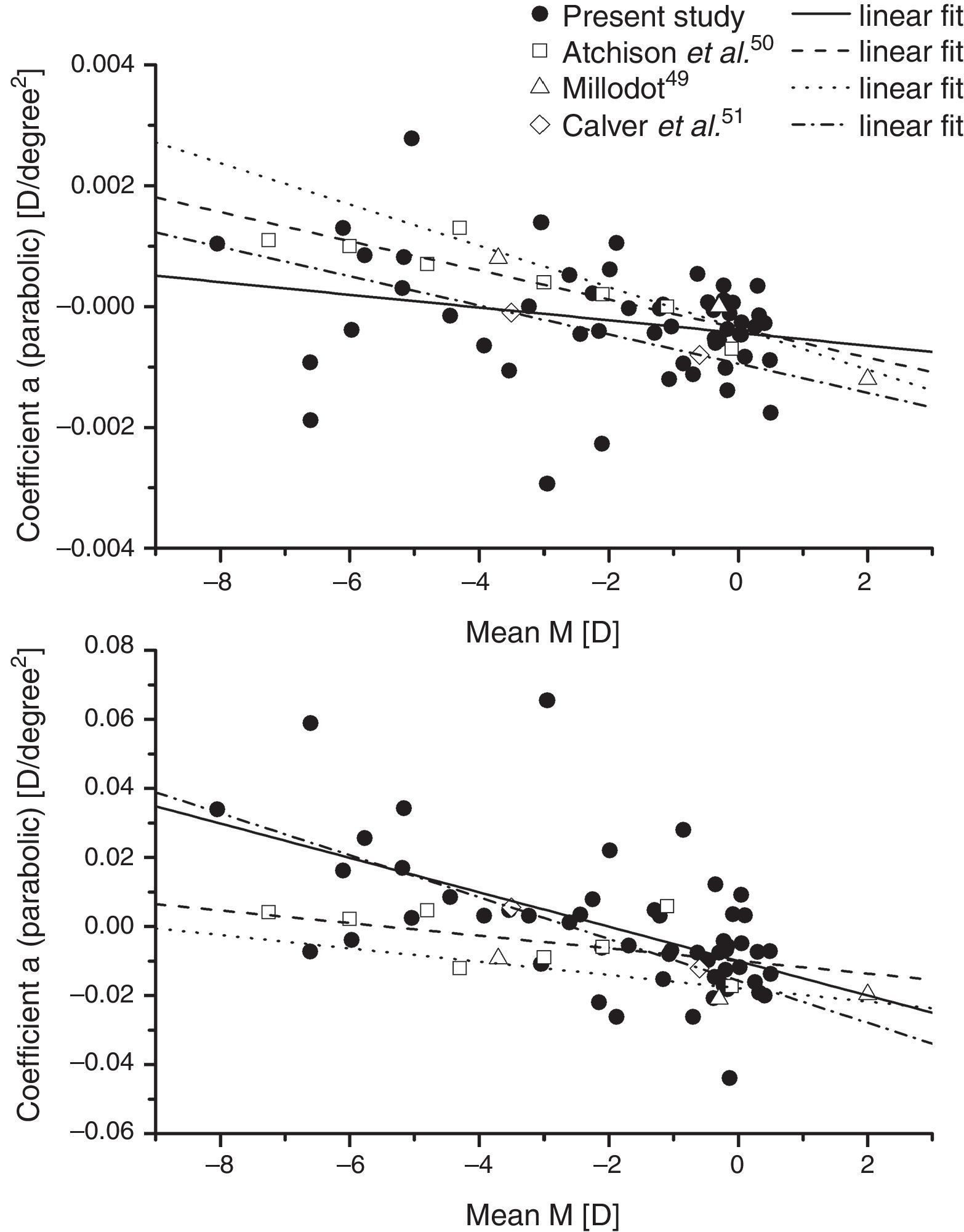
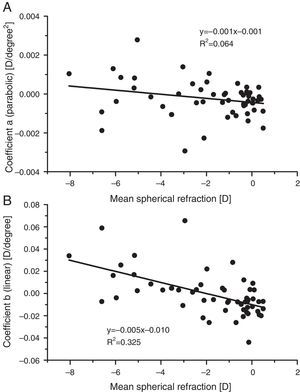
ResultsBaseline measurementsPeripheral refractive errorsFig. 1 shows the values of the coefficients a and b (Formula (4)) plotted as a function of the axial mean sphere M for each subject. There is considerable inter-subject scatter in both parameters. However, although there is a weak trend towards relative hyperopia in the periphery (positive a) as the axial myopia increases, the slope of the regression line is not significant. The nasal/temporal asymmetry, as indicated by coefficient b, appears to change significantly with the central refraction (p<0.001).
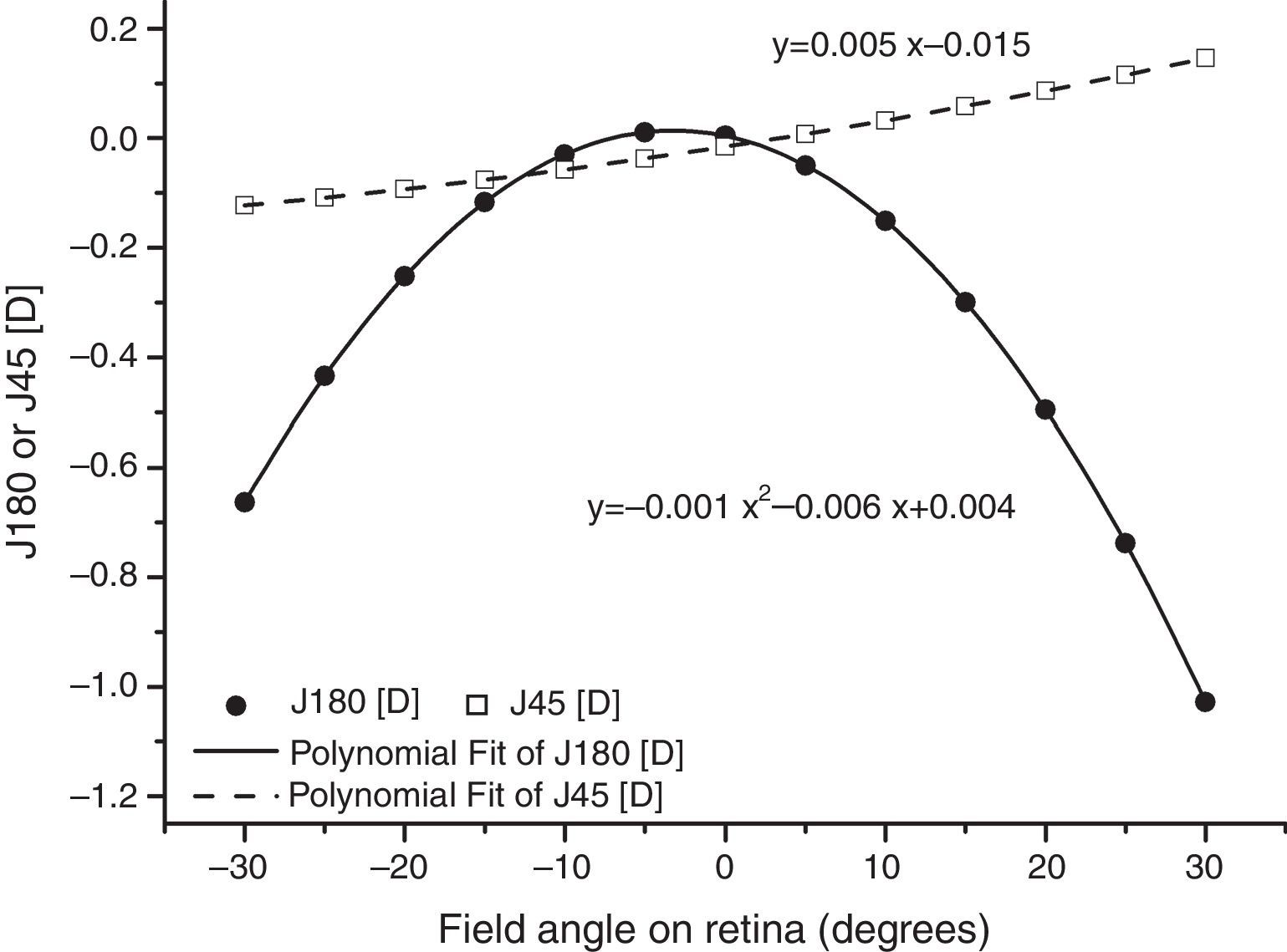
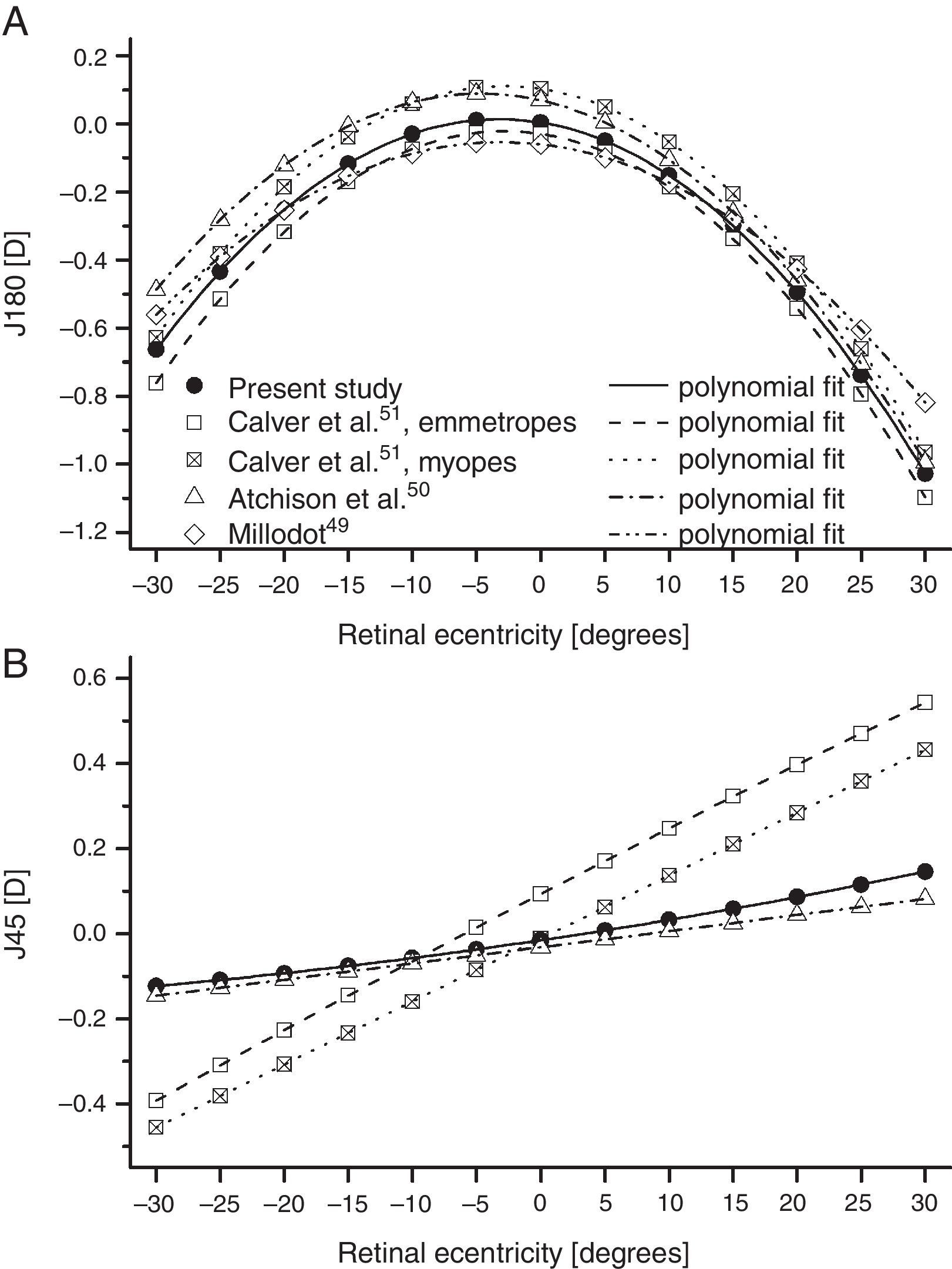
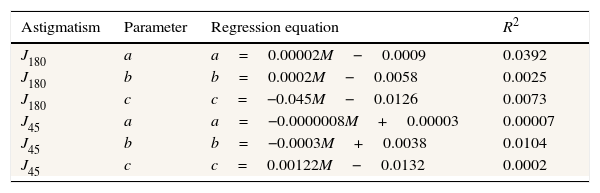
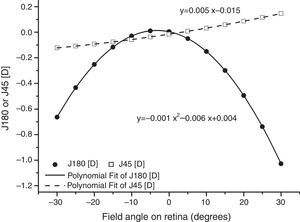
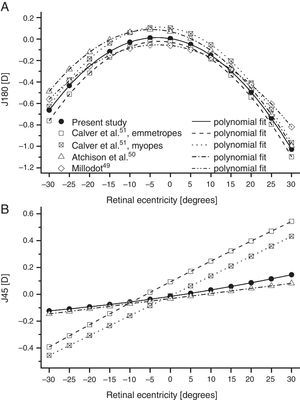
As found by earlier authors,41 the J180 and J45 components of the RPRE showed no systematic dependence on the axial mean spherical error M (see regression line data in Table 1) and data were therefore pooled across all subjects. J180 became increasingly negative in the peripheral field and J45 changed almost linearly with field angle from negative values on the nasal retina to positive values on the temporal retina (Fig. 2). The extreme value of J180 was displaced slightly nasally from the visual axis.
Regression line fits to individual data for the second-order fitting parameters a (D/deg2), b (D/deg) and c (D) for J180 and J45 plotted against the axial mean spherical error M (D) (N=54). No R2 value reaches significance.
| Astigmatism | Parameter | Regression equation | R2 |
|---|---|---|---|
| J180 | a | a=0.00002M−0.0009 | 0.0392 |
| J180 | b | b=0.0002M−0.0058 | 0.0025 |
| J180 | c | c=−0.045M−0.0126 | 0.0073 |
| J45 | a | a=−0.0000008M+0.00003 | 0.00007 |
| J45 | b | b=−0.0003M+0.0038 | 0.0104 |
| J45 | c | c=0.00122M−0.0132 | 0.0002 |
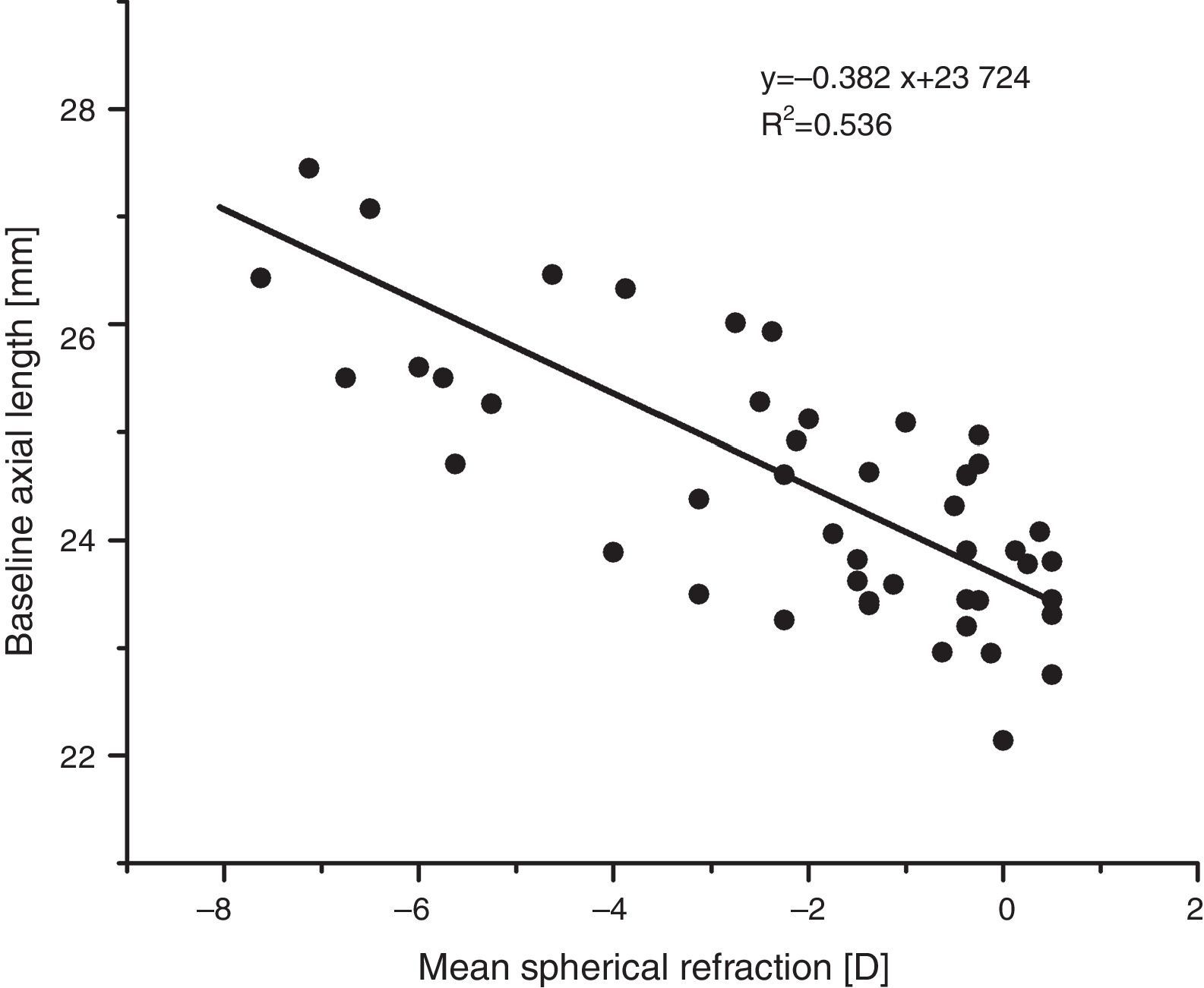
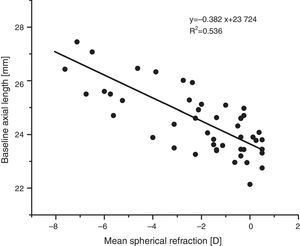
As expected, e.g. Atchison et al.42, axial length increased with increasing myopia (regression line fit: axial length (mm)=−0.39x+23.68, p<0.001, see Fig. 3).
Progression during one yearThe mean subjective refractive error changes within one year were −0.04±0.29D in myopes (range −1.00D to +0.50D), 0.12±0.37D in emmetropes (range −0.75D to +0.75D), and −0.03±0.33D (95% confidence limits −0.13 and +0.07D) in all eyes. Axial length changed by +0.01±0.07mm in myopes (range −0.14mm to 0.12mm), +0.02±0.07mm in emmetropes (range −0.10mm to 0.13mm), and 0.02±0.07mm in all eyes. Changes in refractive error and axial length did not differ significantly from zero in either myopes or emmetropes, or differ significantly between myopes and emmetropes (F(1,41)=2.35; p=0.13; F(1,35)=0.03, p=0.87, respectively).
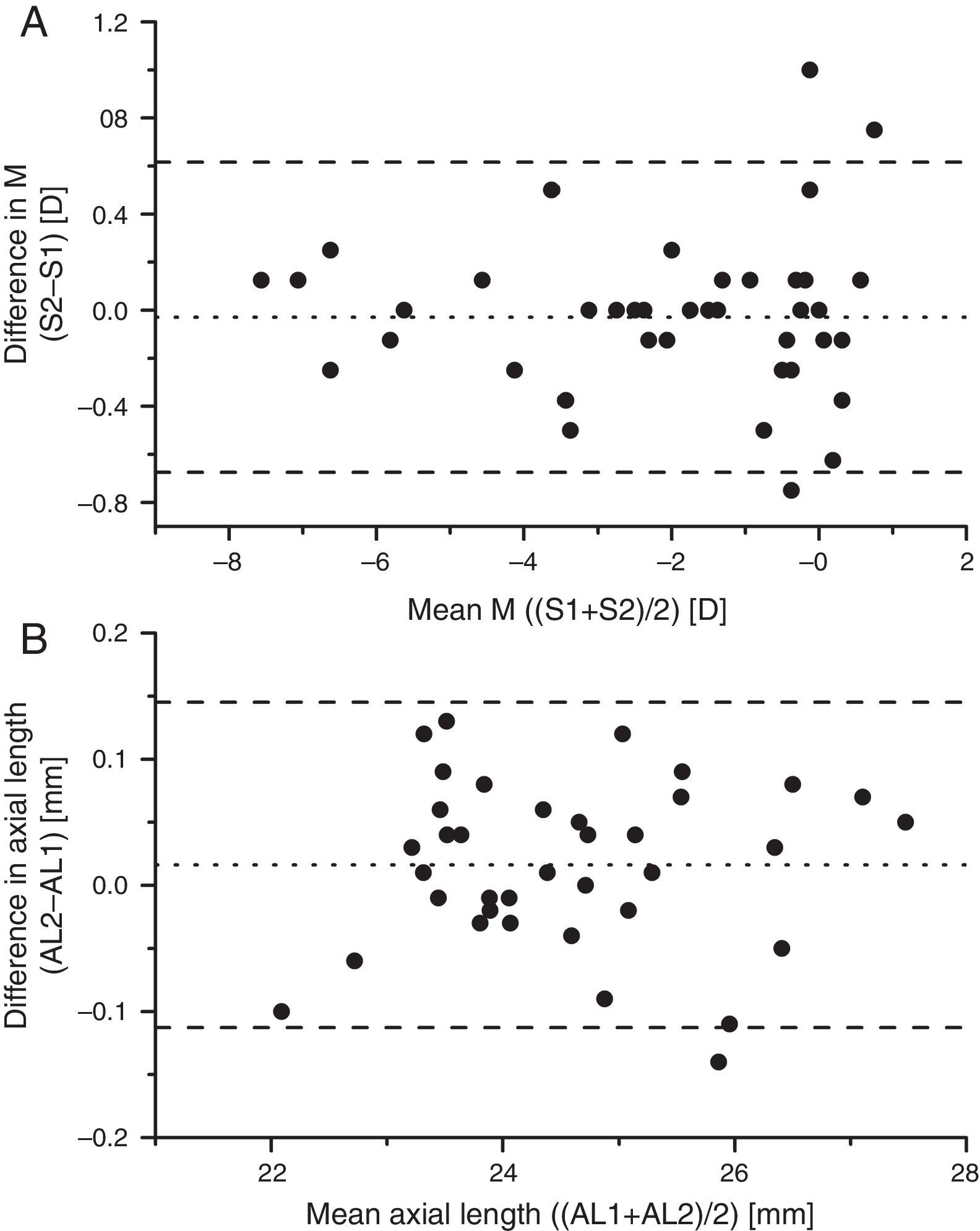
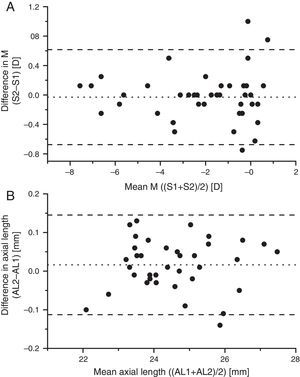
Fig. 4 shows Bland–Altman plots of the changes in spherical equivalent as a function of the mean of the baseline and one-year values of axial M for each of the 43 subjects who completed the study, and for the change in axial length as a function of the mean axial length. In both cases the data give no evidence for any systematic changes in either the myopes or the emmetropes. The observed scatter of the differences in Fig. 4 is broadly explicable in terms of the limited reliability of subjective refraction for a single refractionist,43 around 0.25D, e.g. Zadnik et al.,44 and of the LenStar's estimates of axial length (standard deviation of intersessional difference in lengths 0.03mm, Verkicharla et al.45).
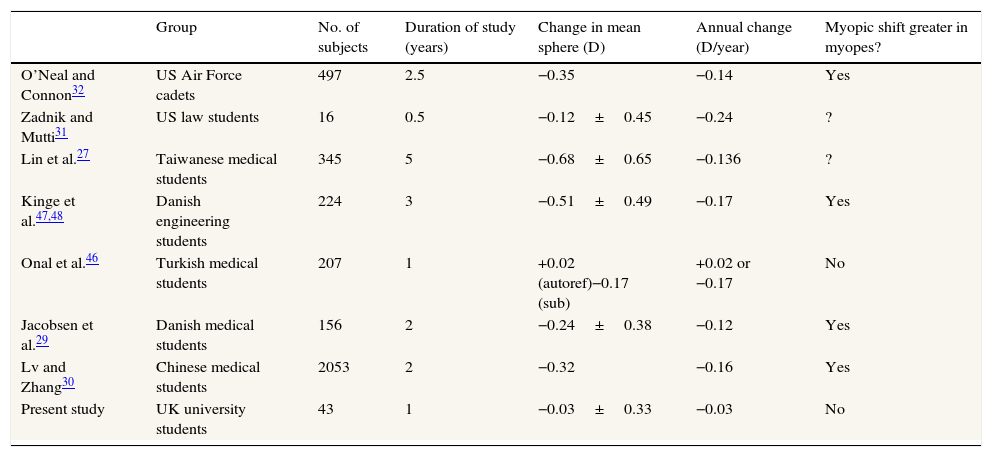
DiscussionThe study failed to find any evidence of refractive or axial length change in the group as a whole, or in the emmetropic and myopic subgroups. This was perhaps surprising. Table 2 shows the results of various longitudinal studies of refractive changes occurring in student groups broadly similar to our own. Most show a myopic change of about 0.1–0.2D/year, which tends to be higher in subjects who are initially myopic. The only exception is the study of Onal et al.46 where cycloplegic autorefraction showed no significant change, although subjective non-cycloplegic refraction showed an average −0.17D/year myopic change.
Results of previous studies of the changes in mean spherical refraction over time in student groups.
| Group | No. of subjects | Duration of study (years) | Change in mean sphere (D) | Annual change (D/year) | Myopic shift greater in myopes? | |
|---|---|---|---|---|---|---|
| O’Neal and Connon32 | US Air Force cadets | 497 | 2.5 | −0.35 | −0.14 | Yes |
| Zadnik and Mutti31 | US law students | 16 | 0.5 | −0.12±0.45 | −0.24 | ? |
| Lin et al.27 | Taiwanese medical students | 345 | 5 | −0.68±0.65 | −0.136 | ? |
| Kinge et al.47,48 | Danish engineering students | 224 | 3 | −0.51±0.49 | −0.17 | Yes |
| Onal et al.46 | Turkish medical students | 207 | 1 | +0.02 (autoref)−0.17 (sub) | +0.02 or −0.17 | No |
| Jacobsen et al.29 | Danish medical students | 156 | 2 | −0.24±0.38 | −0.12 | Yes |
| Lv and Zhang30 | Chinese medical students | 2053 | 2 | −0.32 | −0.16 | Yes |
| Present study | UK university students | 43 | 1 | −0.03±0.33 | −0.03 | No |
It is possible that our negative findings were influenced by the characteristics of our subject group and their pattern of activity: in particular, the mean age of our group (about 25 years) was somewhat older than those in the earlier studies. More subjects, a longer follow-up period and, possibly, the use of cycloplegia might have led to the observation of significant mean refractive change over time. Nevertheless all of our data show considerable scatter and therefore it is possible that significant changes might have occurred in a few individuals, so that more detailed examination of the data is justified.
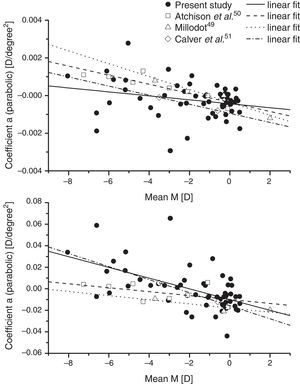
The data on baseline mean sphere component, M of the relative peripheral refraction appear to be in reasonable accord with earlier data when analysed in a similar way. Fig. 5 shows our linear fits for the coefficients a and b for the mean-sphere RPRE in comparison with those derived from fits of averaged data over the central 60° of field from three earlier studies.49–51 All the studies suggest that there is a general trend towards relative peripheral hyperopia (i.e. a positive coefficient a) in high myopes, and that there may be systematic changes in the asymmetry of the RPRE as the ametropia varies, with the peripheral hyperopia increasing more rapidly with field angle on the temporal retina. The same studies also agree that differences in the peripheral J180 and J45 values across refractive groups are small (Fig. 6) with the observed behaviour suggesting slight misalignment between the visual and optical axes.52 Although the data from earlier studies in Fig. 5 represent averages over groups of subjects (each data point representing a total of about 20 eyes for Millodot,49 groups of 7–32 for Atchison et al.,50 and groups of 10 for Calver et al.51), it is evident that, as found in the early work of Ferree et al.,53 considerable inter-subject differences occurred within the groups.
Presents data for the coefficients of second-order fits to the mean-sphere RPRE along the horizontal meridian compared with those from the work of earlier authors.49–51 In each case the mean results of earlier authors over the central 60° of horizontal field have been fitted with equations of the form y=ax2+bx+c.
Mean least-squares second-order polynomial fits for relative J180 and J45 as a function of retinal eccentricity along the horizontal meridian from various studies. Although the original mean data were expressed in relative terms, with an axial value of zero, the fits to the data have values slightly different to zero on axis. Millodot49 and Atchison et al.50 pooled their results over all ametropias, and Millodot assumed that the principal meridians of the peripheral astigmatism were always horizontal and vertical (i.e. that J45=0).
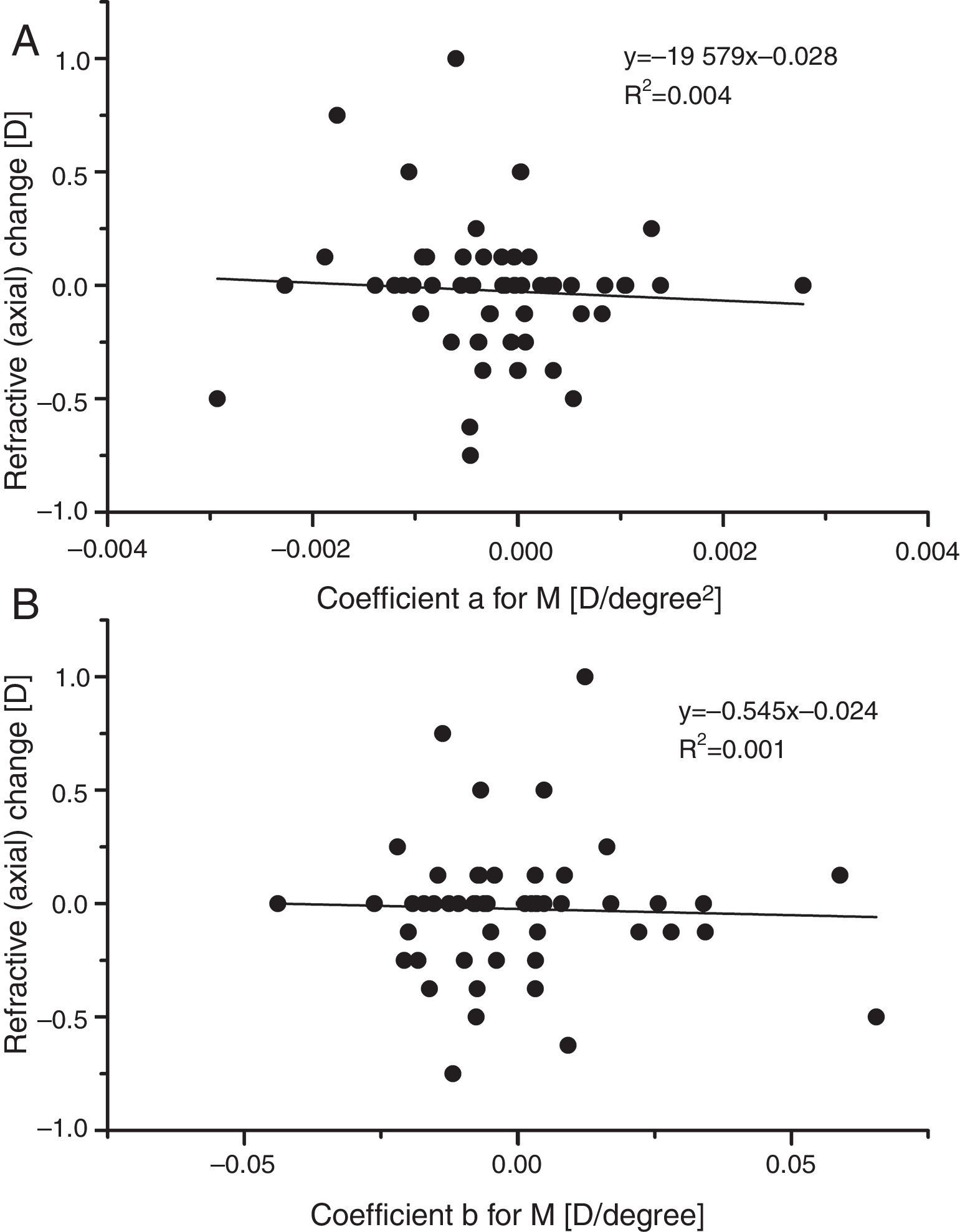
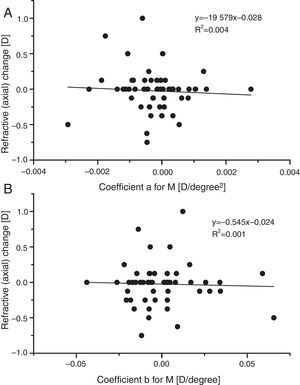
In a search for any indication that mean sphere RPRE was in any way predictive of the very small changes in axial M observed over one year, the refractive changes recorded in the present study were plotted against the fitting coefficients a and b for baseline peripheral M (Fig. 7). There was no indication of any association. The hypothesis that a hyperopic RPRE (i.e. a +ve value of a) would make an individual more susceptible to myopic change is not confirmed. Similarly, it does not appear that departures of the RPRE from symmetry about the line of sight (coefficient b) are predictive of myopic change.
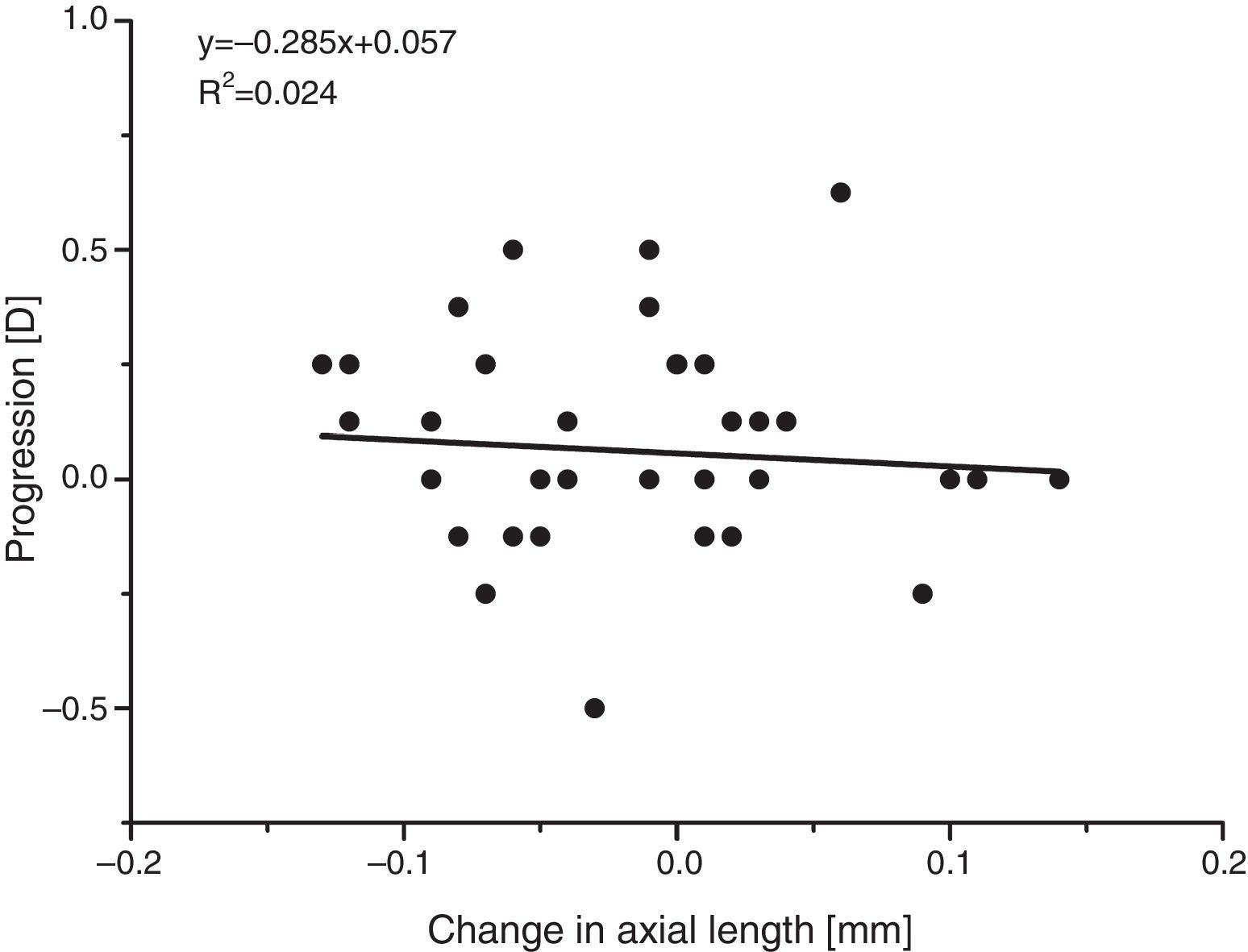
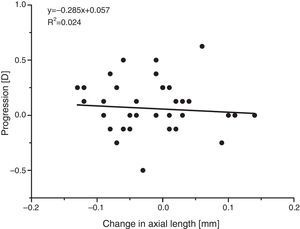
The apparent absence of significant refractive or axial length changes is supported by comparing the individual changes in the two parameters. Since most myopic change is likely to be due to an increase in axial length,27,29 with an increase in length of around 1/3mm producing an increase in myopia of about 1D, a plot of the change in refraction against the change in axial length should have a negative slope of about 3D/mm. There is no evidence for such a relationship (see Fig. 8), again suggesting that the minor differences observed arose mainly from the limited reliability of the measurements.
In fact, as already noted, the changes in mean sphere refraction observed over one year are comparable with those to be expected on the basis of studies of the reliability of the results of subjective refraction.
It should be emphasised that our failure to find any link between the pattern of RPRE and subsequent refractive change applies only to the young adult group studied. Clearly it would be of interest to carry out a similar study on children, for whom not only may progression rates be much higher but also the causes of the myopic change may differ from those in young adults.54
ConclusionsFor the young-adult age-group studied, although baseline measurements of RPRE were in accord with many earlier studies in showing that the RPRE tended to become more hyperopic with increasing myopia, measurements of changes in axial refraction over a period of one year showed no correlation with any aspect of the RPRE. This may suggest that a hyperopic RPRE is a result of axial elongation rather than its cause.25
Conflicts of interestThe authors have no conflicts of interest to declare.