We investigated the dynamics of accommodative and pupillary responses to random-dot stereograms presented in crossed and uncrossed disparity in six visually normal young adult subjects (mean age=25.8±3.1 years). Accommodation and pupil measures were monitored monocularly with a custom built photorefraction system while subjects fixated at the center of a random-dot stereogram. On each trial, the stereogram initially depicted a flat plane and then changed to depict a sinusoidal corrugation in depth while fixation remained constant. Increase in disparity specified depth resulted in pupil constriction during both crossed and uncrossed disparity presentations. The change in pupil size between crossed and uncrossed disparity conditions was not significantly different (p>0.05). The change in pupil size was also accompanied by a small concomitant increase in accommodation. In addition, the dynamic properties of pupil responses varied as a function of their initial (starting) diameter. The finding that accommodation and pupil responses increased with disparity regardless of the sign of retinal disparity suggests that these responses were driven by apparent depth rather than shifts in mean simulated distance of the stimulus. Presumably the need for the increased depth of focus when viewing stimuli extended in depth results in pupil constriction which also results in a concomitant change in accommodation. Starting position effects in pupil response confirm the non-linearity in the operating range of the pupil.
Investigamos la dinámica de las respuestas acomodativa y pupilar a los estereogramas de puntos aleatorios (RDS) que se presentaron en disparidad cruzada y no cruzada en seis sujetos jóvenes adultos con visión normal (edad media= 25,8±3,1 años). Se supervisaron monocularmente las respuestas acomodativa y pupilar con un sistema de foto-refracción desarrollado para tal fin, mientras los sujetos fijaban la vista en el centro de un estereograma de puntos aleatorios. En cada prueba, el estereograma representaba inicialmente un plano liso, representando a continuación una ondulación sinusoidal en profundidad, mientras que la fijación permanecía constante. El incremento de la profundidad debido a la disparidad dio lugar a una constricción de la pupila durante las presentaciones de disparidad cruzada y no cruzada. El cambio del tamaño pupilar en las situaciones de disparidad cruzada y no cruzada no resultó significativamente diferente (p>0,05). El cambio del tamaño pupilar se vio también acompañado de un pequeño incremento acomodativo concomitante. Además, las propiedades dinámicas de las respuestas pupilares variaron en función de su diámetro inicial (de partida). El hallazgo del incremento de las respuestas acomodativa y pupilar con la disparidad, independientemente del signo de la disparidad retiniana, sugiere que dichas respuestas fueron impulsadas por la profundidad aparente, en lugar de deberse a los cambios en la distancia simulada media del estímulo. Presumiblemente, la necesidad de un incremento de enfoque al visionar los estímulos ampliados en profundidad deriva en una constricción pupilar, que deriva a su vez en un cambio acomodativo concomitante. Los efectos de la posición de partida sobre la respuesta pupilar confirman la no linealidad del rango operativo de la pupila.
The pupil is an important oculomotor system that is affected by a diverse set of stimuli including changes in retinal luminance,1,2 sudden changes in stimulus motion,3 emotional4 and cognitive factors,5 grating stimulus parameters and color.6,7 In addition, changes in accommodation8,9 and/or vergence10 have also been shown to result in pupil constriction (miosis) via the near triad.11 Functionally, the constriction of the pupil limits the amount of light entering the eye, reduces optical aberrations (until the eye becomes diffraction limited) and improves the depth of focus. These latter changes also have strong effects on the performance of other oculomotor systems. For example, pupil constriction improves the depth of focus of the eye and this reduces the demand for precise accommodation.12 Pupil responses to optical blur, changes in ambient luminance and fusional vergence eye movements have also been shown to exhibit a strong nonlinearity in their response characteristics depending on the pupil's initial diameter. Specifically, pupil miosis is larger in amplitude when initiated from intermediate starting diameters (4–5mm) compared to larger or smaller diameters.13,14 It has been suggested that this effect reflects a non-linearity within the iris motor plant.
It has been reported that pupil responses can be elicited by disparity in dynamic random-dot stereograms. Li et al.15,16 studied pupil responses to dynamic random-dot stereograms (DRDS) that changed from depicting a flat surface to a sinusoidal corrugation in depth (appearing in uncrossed retinal disparity) using an infrared pupillometer. In all three subjects, there was transient constriction of the pupil concomitant with the change in disparity. The pupillary response was characterized by a long reaction time (∼500ms) and was not accompanied by a change in vergence.16 The constriction was not apparent with monocular viewing of one half-image of the DRDS and, under binocular viewing, its magnitude increased with increase in the spatial-frequency and amplitude of the corrugation. Since there were no changes in either blur or luminance, the dependence on the disparity-defined form led the authors to suggest that ‘stereo information’ drove the pupil constriction although they did not speculate whether this might serve any behavioral purpose.15,16
When such step changes in retinal disparity are introduced in a random-dot stereogram (RDS), the perception changes from a flat surface without depth to a surface with stereoscopic apparent depth. The direction of the apparent depth depends on the sign of retinal disparity. Crossed disparities depict depth in front of a fixation plane and uncrossed disparities depict depth extending beyond the fixation plane. The magnitude of the simulated depth increases with increase in the disparity in the RDS. Introducing a mean uncrossed or crossed disparity simulates increases or decreases (respectively) in the mean distance as well as the depth of the stimulus. This apparent distance change could induce proximal accommodation, even if fixation distance were held constant, assuming spatial pooling of the retinal stimulus to accommodation.17,18 While earlier studies recorded changes in pupil size, measurements of accommodation were not attempted. It is possible that, due to the commonalities in the neural control, pupil responses to stereoscopic stimuli such as RDS could be accompanied by concomitant changes in accommodation. It is also possible that the oculomotor system works to achieve more accurate accommodation in response to a large range of apparent depth, resulting in an increase in the accommodative response to partially or fully offset the typical accommodative lag for the stimulus demand or the increased depth of focus from pupil constriction could decrease the demand for accurate accommodation. In either case, it would be expected that such changes in ocular focus would be modest in the interest of maintaining a clear perception of the object seen in depth (which of course remains optically in the plane of the display regardless of disparity).
To obtain a better understanding of these issues, we investigated the dynamics of pupil and accommodation responses to either crossed or uncrossed disparities presented within a random-dot stereogram. To the best of our knowledge, both accommodation and pupil responses to cyclopean stimuli (such as RDS) have not been reported before. While Li et al.15,16 studied only pupil responses to uncrossed retinal disparities; we investigated accommodation and pupil responses to both crossed and uncrossed disparity. Furthermore, our experimental paradigm was improved on the earlier work by using a mirror stereoscope display, which avoids the cross-talk artifacts associated with the time-sequential shutter glasses used in the experiments by Li et al.15,16
MethodsSubjects and experimental set-upSix adult subjects (Mean age=25.8±3.1 years) participated in the study. All subjects had a best-corrected visual acuity of at least 6/6 in each eye and normal binocular vision. The experimental procedure was conducted with the understanding and consent of the subjects and followed the tenets of the Declaration of Helsinki. The random-dot stimuli were generated on a G4 Power Macintosh computer using Python 2.3. Stereoscopic half images were displayed on a pair of computer monitors (Clinton DS2000HB, 14.25″×10.7″), one for each eye, placed at 60cm set and viewed through pellicle beam splitters (03 PBS 003, Melles Griot, USA) in a Wheatstone stereoscope arrangement. The monitors were calibrated and set to a resolution of 1024×768 at 100Hz. A custom built eccentric photorefraction system provided measures of accommodation and pupil responses at a sampling rate of 100Hz. The photorefractor consisted of a CCD firewire camera (EC 750, Prosilica Inc., Canada), which imaged the right eye from a distance of 70cm, and an infrared illuminator LED array (OP290A, Optek Technologies, TX, USA), which provided eccentric illumination of the eye due to its vertical offset from the center of the camera aperture. The photorefractor recorded a video file (292×263 pixel resolution, 8 bit monochrome image) which captured the dynamic changes in pupil size and accommodation. The video file was exported to custom software (Dynamic Photorefraction System) which provided a calibrated output of pupil size and accommodation.19
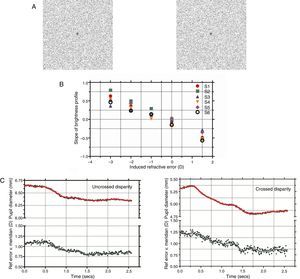
StimuliThe stimuli were random-dot stereograms with a fixation point in the center. The RDS subtended 5.71° and with a dot density of 15% black pixels on a white background (Fig. 1A). The stereoscopic half-images were generated using MATLAB (R2006b, Mathworks Inc, USA) and Adobe Photoshop 7.01 (Adobe Inc., USA). During the experiment, the RDS changed from depicting a zero disparity flat surface to a stationary 0.5cpd, 30 arcmin peak disparity, sinusoidal corrugation in depth. The sinusoidal corrugation was offset from fixation so that it appeared to extend in front of the fixation plane for crossed disparity trials and to extend behind the fixation plane for uncrossed disparity. In both conditions, the fixation point in the center of the RDS remained at the plane of the computer monitors and at a peak (for the uncrossed case, with the remainder of the corrugation lying beyond the screen plane) or trough (for the crossed case) of the corrugation. Thus, in all trials, the vergence required to fixate the target remained at the screen distance for both the flat and corrugated stereograms (Fig. 1A). The nearest point on the uncrossed corrugated stimulus and the farthest point on the crossed corrugated stimulus laid at the screen plane.
(A) Stereo half images presented to each eye shown in crossed disparity (with cross-eyed fusion). Subjects were instructed to maintain fixation on the center dot while the stimulus changed from a flat presentation to a stereoscopic corrugation. (B) Photorefractor calibration showing the slope of the brightness profile across the pupil as of function induced refractive error (+1.5 to −3D). Linear regression was used to define an individual calibration equation for each subject which was used to convert slope of the brightness profile to refractive error in the vertical meridian (accommodation). (C) Dynamic pupil and accommodation responses during uncrossed and crossed disparity demands. The raw accommodation (shown by filled circles) and pupil responses (shown by filled squares) were filtered to remove noise artifacts and the resulting smoothed profile is shown as a solid green and red line, respectively. The start and end coordinates of the two responses were identified using a velocity threshold criterion and the ratio of pupil change to accommodation was determined and compared. Note that the scales for two pupil plots for crossed and uncrossed disparity were offset, albeit with the same intervals, due to differences in starting pupil size for the example traces.
Pupil and accommodation data from the photorefractor were analyzed and calibrated using custom software. For the pupil, a scale factor was used to convert pixels (in the image) to millimeters. A metal rule was placed in the plane of the pupil and imaged using the photorefractor. From these images the number of pixels per mm was estimated visually for each mm interval over 1cm on the rule. This resulted in 10 measures which were then averaged to yield one final spatial calibration factor. For the image resolution used in this study, the spatial calibration factor was 16.9 pixels per millimeter.
The procedure for accommodative calibration using the photorefractor was similar to previous investigations.19–21 Briefly, the subjects held an infrared glass filter (R-72 Hoya Optics, USA) in front of one eye (right) which blocked visible light while the other eye (left) fixated on a high contrast target set at a distance of 65cm. Ophthalmic lenses (1D steps for a range of +1.5 to +3.5D) were introduced in front of the right eye to induce refractive errors while the photorefractor recorded the brightness profile across the pupil in the right eye. The rate of change of pupil brightness (slope) was then plotted as a function of the induced refractive error (D) and this provided the calibration equation for each subject. Fig. 1B shows the calibration data for all the subjects.
ProcedureFollowing calibration, subjects were seated at the apparatus with head restrained with a chin and forehead rest. On each trial, they were required to fixate the central fixation point. Initially the correlated RDS appeared as a flat surface without depth and after a random time interval of 2–3s the flat RDS changed to a sinusoidal corrugation in depth. During this transition, the subjects continued to maintain fixation at the central fixation point in the RDS. The photorefractor recorded the accommodation and pupil responses from the right eye during this stimulus transition as a video file at a sampling rate of 100Hz.
AnalysisOnce the raw accommodation and pupil responses were obtained from the photorefractor software, an analysis algorithm was used to determine the response amplitude and peak velocity. Complete details on this analysis algorithm can be found elsewhere.21 Briefly, the raw accommodation/pupil position were differentiated using a two-point differentiator22 and then the velocity and position responses were filtered/smoothed using a ten-point FFT low-pass filter. The start and end co-ordinates of the response to change in apparent depth were then identified using a velocity threshold criterion. This criterion has been frequently used to determine the start and end coordinates of oculomotor responses like saccades, vergence and accommodation.23–25 For example, the response onset for accommodation was defined as the first point where velocity exceeded 0.5D/s and continued to do so (in a consistent direction) for the next 100ms. An opposite/inverse criterion where velocity fell below 0.5D/s and continued to do so for the next 100ms was used to define the response end. The response onset and end points identified by this procedure were also visually inspected to ensure accuracy and consistency. A similar criterion of 0.20mm/s was used for estimating the start and end points for the pupil responses. The difference between the start and end point measures was defined as the response amplitude and this was individually determined for both pupil and accommodation responses. Also, the ratio of the amplitude of pupil change to accommodative change was determined separately for crossed and uncrossed disparity conditions. In addition, for the pupil response, the peak velocity (mm/s) was plotted as a function of amplitude (main sequence) and as a function of the initial (starting) pupil diameter.
ResultsFig. 1C shows typical changes in pupil diameter and accommodation after the stimulus changed from a flat surface to a sinusoidal corrugation in depth (for both crossed and uncrossed disparity). Under both disparity conditions, there was a pupil constriction and this change in pupil size was also accompanied by a concomitant increase in accommodation. The mean amplitude of the pupil and accommodation change were 0.28±0.12mm and 0.23±0.09D, respectively, for step changes in uncrossed disparity and 0.41±0.36mm and 0.29±0.19D, respectively, for step changes in crossed disparity. The magnitude of the change in pupil size or accommodation was not significantly different between crossed and uncrossed disparity (paired student t-test, p>0.05) but was significantly greater than zero (p<0.05). The mean ratio of the change in pupil size to change in accommodation was 1.39mm/D and 1.43mm/D for uncrossed and crossed disparity, respectively. The ratio was not statistically different between the two disparity types (paired student t-test, p>0.05).
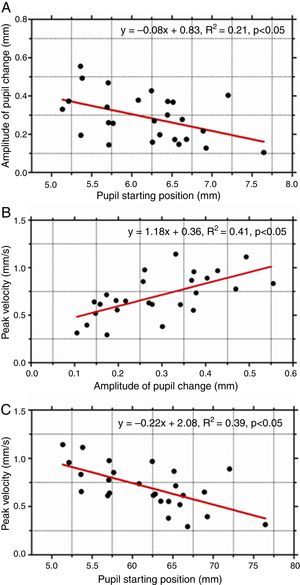
As pupil changes were similar regardless of the type of retinal disparity, the responses to both crossed and uncrossed disparity were pooled and three separate plots were generated to examine the effect of starting diameter of the pupil (initial pupil diameter at response onset) on response dynamics. The amplitude of the pupil constriction was plotted as a function of starting diameter of the pupil (Fig. 2A). In addition, the peak velocity of the pupil responses was plotted as a function of response magnitude (Fig. 2B) and as a function of initial pupil position (Fig. 2C). The results show there was a significant effect of the starting pupil diameter on response dynamics. Amplitude of pupil constriction and its velocity varied inversely as a function of starting pupil diameter suggesting larger and faster pupil constrictions were associated with smaller starting pupil diameters. Also, the main sequence of pupil showed a linear increased in peak velocity of the pupil responses as a function of response amplitude.
(A) The amplitude of pupil response plotted as a function of initial (starting) pupil diameter. Peak velocity of pupil responses as a function of response amplitude (B) and starting position (C). Amplitude of pupil constriction was significantly lower for larger initial starting pupil diameters. Also, such responses were significantly slower.
This experiment provides a more complete description of pupil and accommodation dynamics in response to a sudden change in apparent stereoscopic depth. We confirm that disparity-specified depth changes introduced in random-dot stereograms elicit pupil constriction15,16 and also found a previously unreported small but concomitant increase in accommodation for both crossed and uncrossed disparity stimuli. We extend the earlier studies by measuring the dynamic properties of accommodation and pupil responses for both crossed and uncrossed disparity demands. The motor responses had the same sign regardless of the sign of stimulus disparity.
For introduction of both crossed and uncrossed disparity into the stimulus, pupil size decreased and accommodation increased. This suggests that the responses were driven by the apparent depth signal in the fused stimulus and not by the simulated distance. Normally, retinal disparity signals the range of depth over the target stimulus (RDS in this study). A large disparity range within the target would signal a need for a greater depth of field in order to maintain clarity and avoid defocus especially at the proximal or distal ends of the depth interval. A decrease in pupil size would be an effective optical strategy to deal with this need as it provides clear focus over an extended depth interval. Note that in the RDS stimuli used, depth was signaled solely by disparity – in the current experiment fixation remained stable at the plane of the monitor and all stimuli were optically at this distance. Thus, unlike natural stimuli, there was no actual demand for increased depth of field associated with the disparity. Pupil constriction would not reduce blur in the stereoscopic stimulus and this lack of feedback may explain the transient nature of the pupil response.
The concomitant changes in accommodative response observed in this study may be due to commonalities in the neural control of accommodation and pupil responses underlying the near triad. The motor impulses for both responses originate in the Edinger–Westphal nucleus, a subdivision of the oculomotor nucleus, located in the midbrain. The Edinger–Westphal nucleus contains first-order parasympathetic neurons which synapse in the ciliary ganglion while the second-order neurons travel from the ganglion to the globe, eventually innervating the ciliary muscle and sphincter pupillae, thus causing accommodation and constriction, respectively.26 Although one might expect that an increase in accommodation would cause the retinal image to defocus, it should be noted that the magnitude of the accommodative response during both crossed and uncrossed disparity presentation was well within the normal depth of focus interval.27–29 In addition, the accommodative system is known to exhibit a physiological lag in its response for a given stimulus demand30 and based on age, refractive error of the subjects and the experimental set-up used in this study, a physiological accommodative lag of up to 0.5D could be expected.31 Hence, the small increase in accommodation following the onset of the disparity stimulus would be expected to decrease the physiological lag and increase the accuracy of accommodation. Hence, the changes in accommodation observed in this study would not be expected to adversely affect the clarity of the image.
It should be noted that Li et al.15,16 excluded accommodation changes. In their study, only pupil size and ocular vergence were monitored continuously. Hence, it is not known if accommodation changed during binocular conditions since it was not measured. Their basis for excluding a role for accommodation was based on observations obtained when the stereoscopic images were viewed monocularly. As accommodation is active monocularly, it was concluded that the lack of change in pupil responses under these conditions provides evidence against a role of accommodation. However, this is an incorrect presumption. Under monocular conditions, the disparity signal is absent and hence there is no stereoscopic depth to drive the pupil response. In other words, the apparent depth signal which triggers a change in the pupil size is absent under monocular conditions and hence, it is not surprising that pupil constriction was not observed monocularly by Li et al.15,16 On the other hand, in our study, both accommodation and pupil responses were monitored using the photorefraction system. During both crossed and uncrossed disparity presentations, accommodation and pupil showed a consistent change.
The third aspect of the near triad is convergence. One of the limitations of the present study is that the vergence response of the eyes was not continuously monitored. Although, stimulus vergence remained constant throughout the experiment and the subjects were instructed to carefully maintain the fixation, small errors in vergence state (i.e., fixation disparity) cannot be ruled out. In this case, the synergistic links of the near triad could have also triggered a change in pupil size. However, it is unlikely that this could produce the pattern of results we found in this study for two reasons. First, as noted above, Li et al.15,16 monitored vergence with similar stimuli and found no consistent vergence changes. Second, one would expect any stimulus driven vergence response to reflect the sign of the stimulus disparity. The fact that our accommodative and pupil responses were of the same sign regardless of disparity suggests that they were not elicited by vergence error.
A reviewer asked us to speculate on the possible results of this experiment in subjects with central scotomas. Depth of field increases with eccentricity reflecting the variation in visual acuity across the visual field.32 Similarly, the effectiveness of defocus blur as an accommodative stimulus should also decrease with eccentricity as acuity declines (and effective depth of focus increases).33 Consistent with this prediction, White and Wick34 found that accommodation response to defocus blur was reduced in juvenile macular degeneration (Stargardt's disease). The degree of reduction was generally greatest in those with the poorest acuity. Interestingly, when provided with proximal and binocular cues to accommodation, the subjects responded with more accurate accommodation than with defocus blur alone. Thus, we might speculate that this increased sensitivity to cues other than defocus blur might produce a larger response to binocularly disparate stimuli in observers with central scotomas compared to our normal subjects. Duane35 reported that patients with small, faint central scotomas often present with signs resembling accommodative insufficiency and our results suggest that these signs might be reduced in the presence of stereoscopic cues. Conversely, due to the decreased acuity in the periphery, the demand for increased depth of focus with increased depth in the stimulus might be reduced producing a smaller effect in central field loss than we found in normal subjects. Determining the effects of disparity on pupil size and accommodation in subjects with central field loss must be resolved by experimental research. The functional implications of these findings need to be investigated further to understand if clinically, the drive for increased accommodation, concomitant increase in depth of field and conflict between the accommodation and vergence systems might cause difficulties in viewing stereoscopic displays and movies in normal subjects and in subjects with binocular vision anomalies.
Finally, another important result of this study was that the dynamic characteristics of the pupil responses, specifically amplitude and peak velocity, appear to depend on the initial (starting) pupil diameter. Amplitude of pupil constriction was significantly less when responses started from larger pupil diameters. Such responses were also significantly slower. While these dynamic properties of pupil responses have been reported for other stimuli such as blur, changes in ambient light levels and also for accommodative and fusional vergence responses,14 our results have extended this finding to pupil responses driven by apparent depth in stereoscopic stimuli. Together, the findings suggest that the starting position or initial state of the iris motor plant has a strong influence on the final pupil response regardless of the nature of the sensory stimulus. This commonality suggests that the source of this non-linearity could be attributed to the iris motor plant.
ConclusionApparent depth in random-dot stereograms produces dynamic pupil and accommodation responses. While the magnitudes of these changes are small, the responses have the same sign regardless of the sign of the horizontal retinal disparity. When viewing stereoscopic images, the visual system appears to employ a strategy to control ocular focus within the apparent depth interval by decreasing pupil size and increasing depth of focus.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study was funded by Natural Sciences and Engineering Research Council of Canada (RA) and Canadian Institutes of Health Research (CIHR) Strategic Training Grant in Vision Health Research (RS). The authors would like to thank the two anonymous reviewers who provided constructive comments that helped improve the manuscript.