As ocular dryness and glaucoma are more prevalent with increasing age, understanding how the tear film affects tonometry is important. The present study aims to understand the impact that changes in the tear film have on intraocular pressure (IOP), corneal hysteresis, and corneal resistance factor measurements.
MethodsCross-sectional research was conducted and 37 patients were assessed. The tear film lipid layer and the non-invasive break-up time (NIBUT) were evaluated using the Tearscope Plus (Keeler, Windsor, UK). Dry eye symptoms were evaluated using the Ocular Surface Disease Index (OSDI) questionnaire. IOP was measured using rebound tonometry and the Ocular Response Analyzer (ORA, Reichert). Corneal biomechanical properties were measured using ORA.
ResultsIt was found that an increase in the IOP measured with the iCare was directly correlated with the subclass that evaluated symptomatology associated with environmental factors (r = 0.414, p<0.05, Spearman). Goldmann-correlated IOP (IOPg) and Corneal-compensated IOP (IOPcc) values were statistically significantly different between the various interferometric patterns (p<0.05). It was also found that an increase in the corneal biomechanical properties measured with ORA was directly correlated with the overall scores obtained when using the OSDI and some of its subclasses.
ConclusionsTear film interferometric patterns were shown to have some impact on the IOP measured using ORA. The IOP measured with iCare seems to be related to the symptomatology obtained from OSDI. Corneal biomechanical properties were related to the OSDI total score and some of its subclasses. An increase in symptomatology was associated with an increase in the measured biomechanical properties of the cornea.
Currently, glaucoma is the leading cause of irreversible blindness in the world, affecting about 80 million people.1 Glaucoma is characterized by progressive dysfunction and loss of retinal ganglion cells and their axons.2–4 The main risk factors are increasing age, high intraocular pressure (IOP), and genetic predisposition.2–6
Today there are several methods available for measuring the IOP in the clinical practice, but they only provide an estimate of the IOP. Manometry is still the only method that can accurately measure the IOP.7 The purpose of tonometry is to obtain accurate IOP measurements with minimal invasion of the ocular surface. There are transpalpebral, applanation, indentation, rebound, and contour tonometers, with the Goldmann applanation tonometry being considered the gold standard for clinical use.6
Currently, most tonometers perform the IOP estimation through the corneal surface, and consequently, through the tear film. Rebound tonometry is still widely used clinically, and despite its very short duration and small area of corneal contact, it has been shown to have an impact on the tear film stability, which may have implications when estimating the IOP.8 Some tonometers, such as the ORA, measure the IOP and the biomechanical properties of the cornea using a quick air pulse and an infrared electro-optical system to monitor the deformation of the cornea.9 These tonometers also have an automatic centering system to ensure that the measurements are taken over the central surface of the cornea. Both systems work by interpreting light signals reflected on the tear film.
The ocular surface is very complex, comprising various epithelial and glandular structures and tissues such as the cornea, the bulbar conjunctiva, and the lacrimal and accessory glands. The main accessory lacrimal glands secrete the tear film that covers, protects, and lubricates the ocular surface thus allowing visual health and clear vision.10 The tear film is the interface between the corneal epithelium and the external environment. Even though it is only 3 µm in thickness, it has a very complex composition containing water, electrolytes, mucins, and a variety of proteins and lipids,11,12 with studies describing the presence of more than 1500 proteins.13 The model of the tear film structure is still evolving, but currently, evidence points to a hydrated mucous layer covered by lipids that move over the epithelial glycocalyx.12,14
According to the Dry Eye Workshop II (TFOS DEWS II), launched by the Tear Film & Ocular Surface Society, dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface.15 It is estimated that there are approximately 2.3 billion people worldwide with dry eye according to the TFOS DEWS II diagnostic criteria.16,17
As age is known to be associated with ocular dryness and is a major risk factor for glaucoma, it is necessary to understand how the tear film affects the tonometry measurement. The present study aims to understand the impact that the tear film have on IOP and corneal biomechanical properties measurement.
MethodsThe tear film lipid layer and the non-invasive break-up time (NIBUT) were evaluated using the Tearscope Plus (Keeler, Windsor, UK). Dry eye symptoms were evaluated using the Ocular Surface Disease Index (OSDI) questionnaire. IOP was measured using rebound tonometry (iCare TA01l, iCare, USA) and the Ocular Response Analyser (ORA; Reichert Ophthalmic Instruments, Buffalo, NY). Corneal biomechanical properties were measured using ORA. Only the IOP values measured with the iCare tonometer were considered, whilst the Corneal-compensated IOP (IOPcc), the Goldmann-correlated IOP (IOPg), the Corneal Hysteresis (CH), and the Corneal Resistance Factor (CRF) values measured with the ORA were considered.
First, subjects filled out the OSDI questionnaire, followed by the assessment of the tear lipid layer and NIBUT. Then, the IOP and corneal biomechanical properties were measured. The order of the tests was chosen to minimize the potential impact of the previous test on the results of the following, with the more invasive tests being performed last.
Statistical analysis was performed only on the data from the right eye of 37 subjects. The right eye was chosen for analysis because both eyes were assessed for NIBUT and interferometric pattern with both eyes open, and the left eye was assessed last, which could have resulted in changes in the parameters due to extended exposure to air.
This work was carried out at the Clinical and Experimental Optometry Research Lab (CEORLab), in the Physics Center of Minho and Porto Universities (CF-UM-UP), School of Sciences, University of Minho, Braga, Portugal.
The study protocol was reviewed and approved by the Ethics Subcommittee for Life and Health Sciences of the University of Minho. Following the guidelines of the Declaration of Helsinki, all participants signed an Informed Consent form before the experimental procedure and after being explained all the objectives and procedures of the study.
Volunteers, predominantly young adults, were recruited from the population of the University of Minho. Excluded participants included: contact lens users, participants using any ocular or systemic medication with ocular impact and participants with ocular pathology or who had been submitted to ocular surgery.
Experimental procedureInitially, the volunteers filled out a questionnaire to obtain subjective data on ocular dryness symptomatology. The questionnaire completed was the OSDI which was designed to quickly obtain a subjective assessment of eye irritation symptoms consistent with dry eye syndrome and their impact on vision. The OSDI consists of 12 questions rated on a scale of 0 to 4, where 0 indicates no symptoms and 4 indicates the most severe symptomatology. The OSDI questions can also be grouped into three subscales: vision (6 questions), ocular symptoms (3 questions), and environmental triggers (3 questions).17 The vision subscale measures the impact of dry eye on vision, the ocular symptomatology subscale measures the symptomatology, and the environmental factors subscale measures the impact of the environment on dry eye. The OSDI score is calculated using the following formula: OSDI = [(sum of scores for all questions answered) X 25] / (total number of questions answered). There is a scale of 0 to 100, with a score of 0 to 12 being considered normal; 13 to 22, mild dry eye; 23 to 32, moderate dry eye; and 33 to 100, severe dry eye.18
The OSDI has good reliability, validity, sensitivity, and specificity, allowing the diagnosis and determination of the severity of any form of dry eye, as well as conducting a rapid assessment of irritation and impact on visual functions related to dry eye.18 The lipid layer and the NIBUT were evaluated using the Tearscope Plus (Keeler, Windsor, UK). The Tearscope uses a cold cathode light, which minimizes eye dryness during the procedure and can be attached to a slit lamp. Exposing the lipid layer to appropriate light results in the generation of an interferometric fringe pattern. The interferometric patterns were classified as open meshwork, closed meshwork, wave, amorphous, and color fringe according to the classification of Guillon et al., which provided an estimate of the thickness of the lipid layer.19–21 The NIBUT was measured three times in each eye and the mean was used in the analysis. The patient was instructed to blink three times and then to keep the eye open for as long as possible. The time elapsed between the last blink and the appearance of the first distortion point was measured.
IOP was measured in millimeters of mercury (mmHg) using the tonometer iCare TA01I (IOP iCare) and the ORA (corneal-compensated IOP (IOPcc) and Goldmann-correlated IOP (IOPg)). When measuring with the iCare TA01I, care was taken to perform the measurement at the central surface of the cornea. With the ORA, the process of centering the device with the central surface of the cornea was automatic. Three successful acquisitions with the rebound tonometer were considered, each involving averaging the outcome of six readings (which implied a total of eighteen measures). Three measurements were taken in each eye with ORA. Measurements with a waveform score < 3.7 may indicate an unreliable signal22 and, for that reason, were repeated. The mean across measures used for the statistical analysis.
Intra-participant measures were collected over a short period of time, while inter-participant measures are alternated. Comparisons considering the participants’ sex were also performed. Measurements were collected during June and July, between 8:18 am and 9:36 pm, with 80% of the measures done between 10 am and 4 pm.
StatisticsAccording to the Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop II (DEWS II), the minimum sample size required to detect a clinical difference of 5 s in NIBUT using the Tearscope Plus is 33 participants.17
Statistical analysis was performed using version 22.0 of IBM®SPSS® Statistics software (SPSS Inc, Chicago, IL). The normality of the variables was assessed using the Kolmogorov-Smirnov test. For normal distributions, the following were used: the independent samples t-test, to see if there were statistically significant differences between two subgroups of a variable, and the one-way ANOVA, to see if there were statistically significant differences between several subgroups of a variable. For non-normal distributions, the following were used: the Mann-Whitney U test, to see if there were statistically significant differences between two subgroups of a variable, and the Kruskal-Wallis H test, to see if there were statistically significant differences between several subgroups of a variable. For comparison of the methods of measuring the IOP, the paired-sample t-test was used. To evaluate the independence of nominal and ordinal variables, the Chi-square test was used. Correlations were performed by the Pearson test if the sample had a normal distribution; otherwise, the Spearman correlation was used.
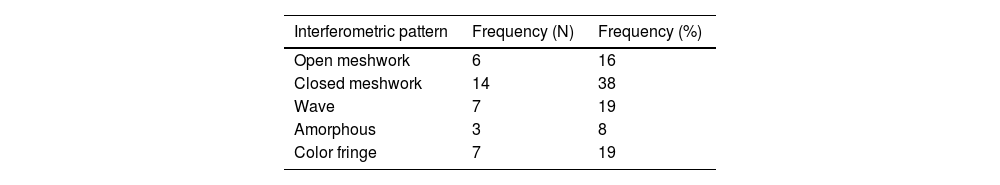
ResultsThirty-seven right eyes from 37 patients were evaluated. Of the 37 patients, 20 were female (24.30 ± 6.49 years old) and 17 were male (24.59 ± 5.9 years old), with an average age of 24.4 ± 6.3 years. No statistically significant differences were found when comparing the age differences between men and women. (p = 0.619). The mean OSDI score obtained was 8.68±10.42. When comparing the OSDI scores between men and women, the differences found were not statistically significant (p = 0.729). As shown in Table 1, the most frequent interferometric pattern was the closed meshwork, found in 38% of cases and the least frequent was the amorphous pattern, found in 8% of cases. The interferometric pattern was found to be independent of sex and there were no significant correlations between the interferometric patterns and age (p = 0.157). The NIBUT was on average 17.74±20.40 s and it was found that 27% of the participants had a NIBUT lower than 10 s. No statistically significant differences were found in NIBUT values between men and women (p = 0.311).
It was found that the mean IOP measured with iCare was 16.44±3.42 mmHg, the mean IOPg value was 13.72±2.95 mmHg, and the mean IOPcc value was 13.90±2.86 mmHg. Differences with statistical significance were found between the IOP measured with the iCare and the IOPg (iCare–IOPg) (p<0.001) and the IOPcc (iCare-IOPcc) (p<0.001). No differences with statistical significance, were found between IOPg and IOPcc (p = 0.533). IOP measured with iCare was moderately correlated with IOPg (r = 0.541, p<0.001, Pearson) and reasonably correlated with IOPcc (r = 0.345, p<0.05, Pearson). The IOPg and IOPcc were strongly correlated (r = 0.845, p<0.0001, Pearson).
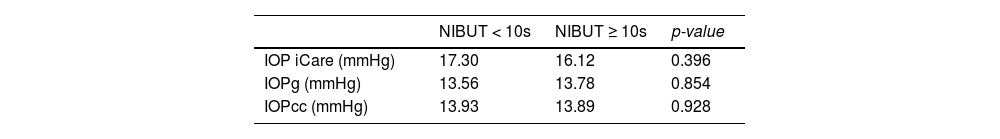
As shown in Table 2, the IOP measured with the iCare and the ORA (across IOPg and IOPcc values) for patients with NIBUT lower or higher than 10 s showed differences with no statistical significancy. No statistically significant differences were also found when relating the NIBUT values and the OSDI scores.
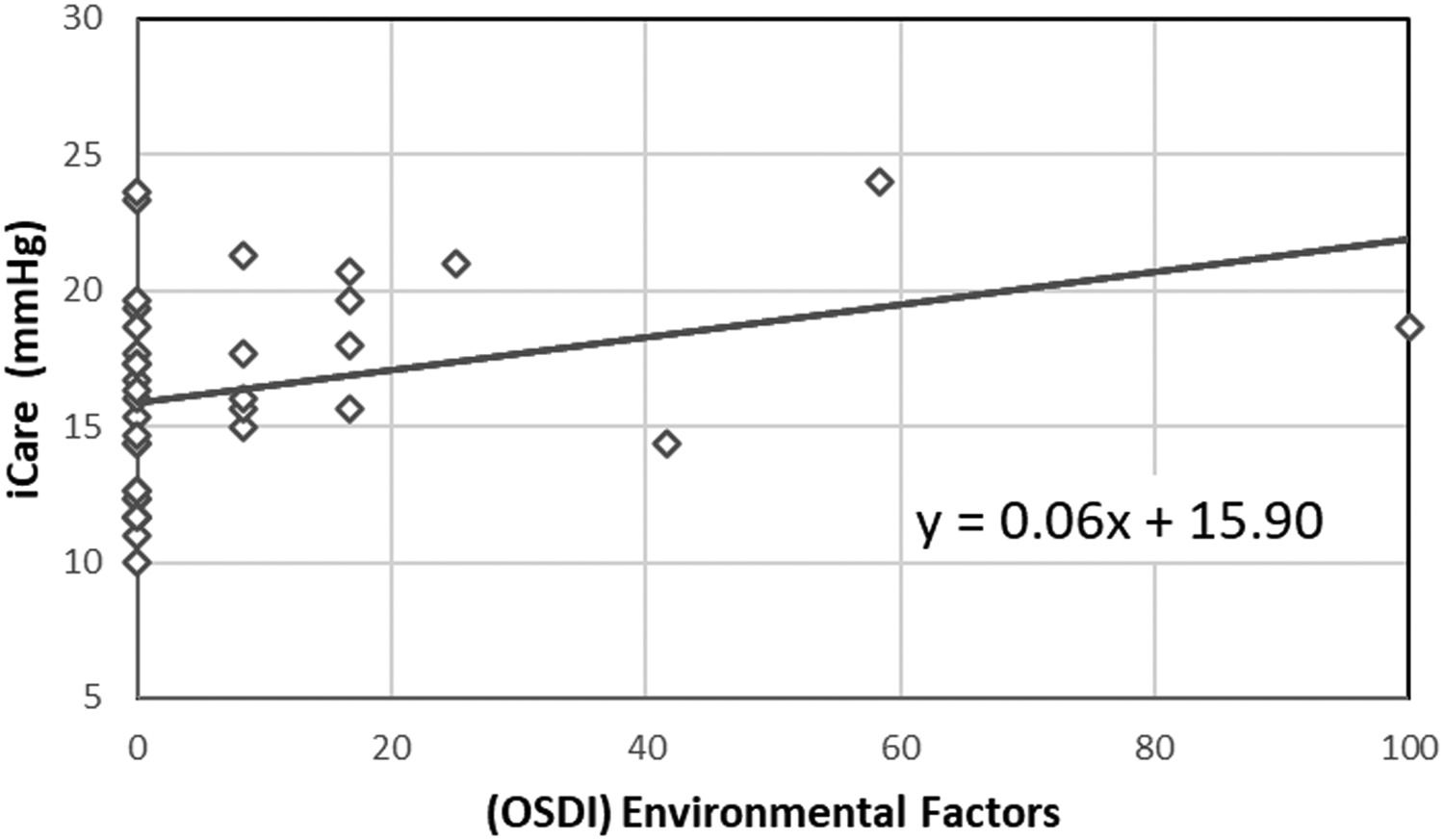
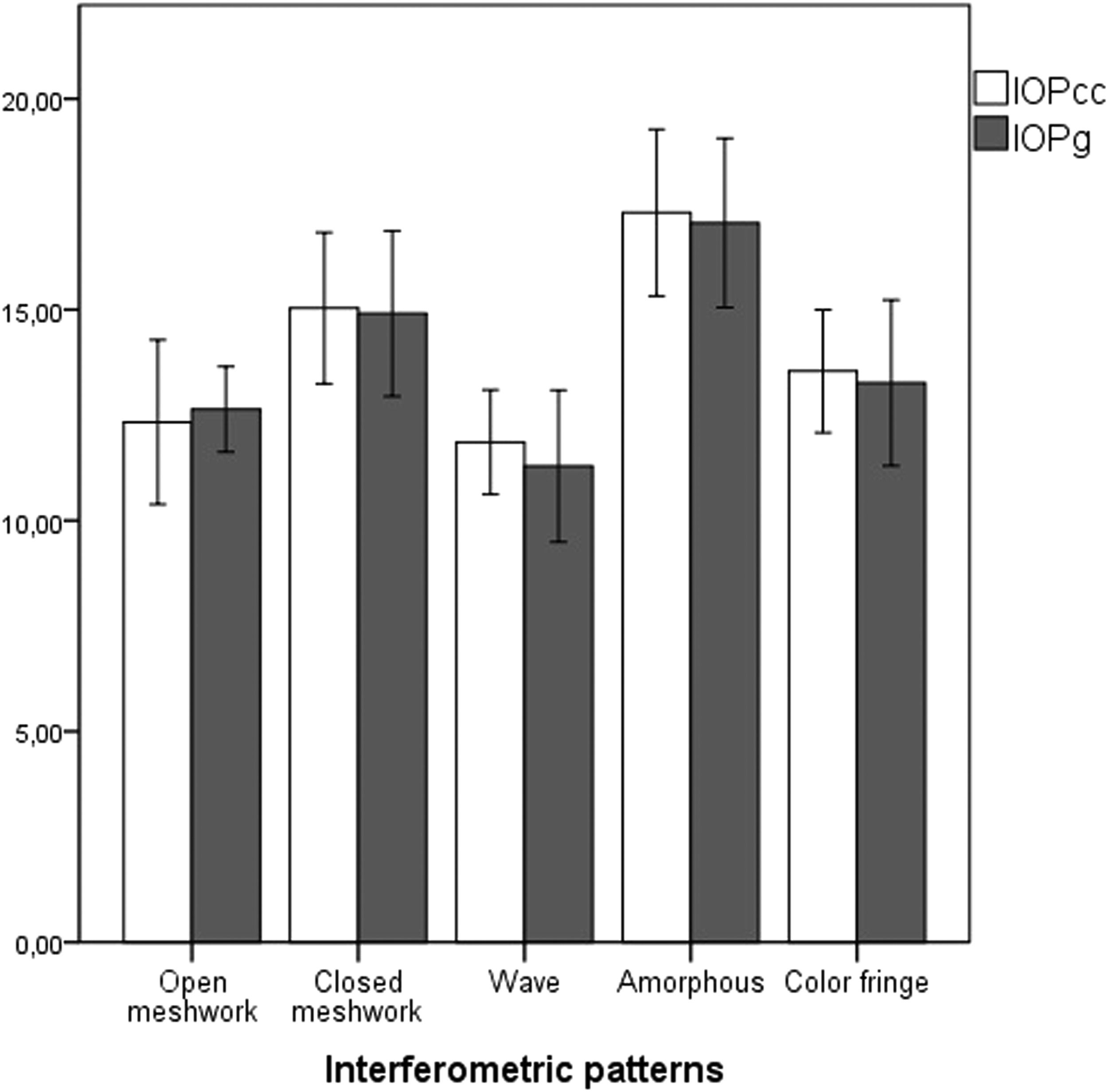
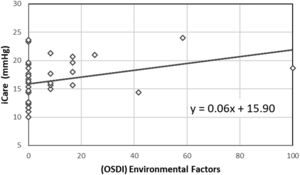
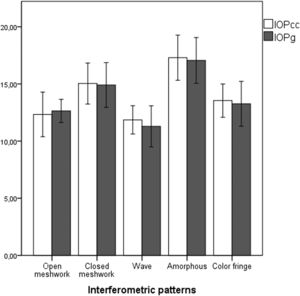
Comparing the IOP measured with iCare (p = 0.820) and the OSDI results (p = 0.098) across the different patterns returned no statistically significant differences. However, a moderate correlation between the IOP measured with iCare and the environmental factors subscale of the OSDI was found (r = 0.414, p<0.05, Spearman). In Fig. 1, it is possible to see the correlation between OSDI Environmental Factors subscale and the IOP measured with iCare. The black line represents a linear regression to the data, with an equation y = 0.06x + 15.90. Statistically significant differences in IOPg and IOPcc between the different interferometric patterns were also found (p<0.05). Fig. 2 shows the variation in the IOP obtained across the various interferometric patterns considered.
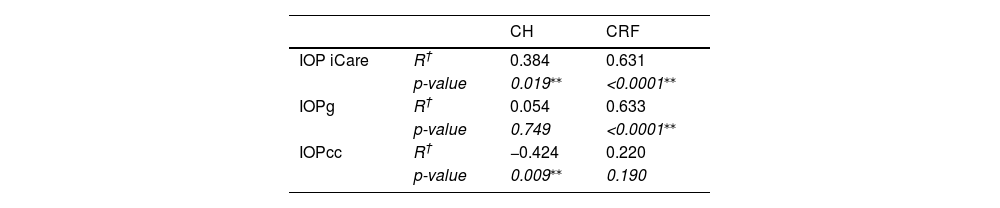
A CH of 10.95±1.31 and a CRF of 10.35±1.39 was found. There was a strong correlation between CH and CRF (r = 0.789, p<0.0001, Pearson). Table 3 shows the correlations of corneal biomechanical properties with the different IOP measurement methods.
Correlations (R representing the Pearson coefficient) of IOPs obtained using the iCare, the IOPg, and IOPcc obtaining using the ORA, with corneal biomechanical (CH and CRF) properties obtained using the ORA. p-values indicates the statistical significance of the correlation.
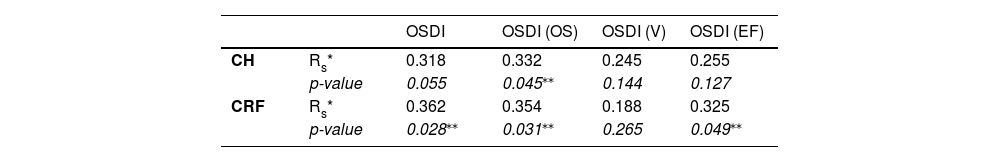
No statistically significant differences were found in the corneal biomechanical properties between sex. The correlations (and its statistical significance) between corneal biomechanical properties and scores on the OSDI test and its subclasses can be seen in Table 4.
Correlations (R, representing Spearman coefficient) of corneal biomechanical properties obtained using the ORA with the OSDI total score and its subscales. OS- Ocular symptomatology; V- Vision; EF - Environmental Factors.
The purpose of this study was to evaluate how the tear film affects tonometry and corneal biomechanics measurements. The iCare TA01I and the ORA were used to measure the IOP, the ORA was used to measure the corneal biomechanics, the Tearscope plus was used to analyze the tear film characteristics, and the symptoms quantification was done using the OSDI questionnaire.
Regarding the analysis of symptomatology through the quantification of symptoms with the OSDI questionnaire, the mean OSDI score obtained in this study (8.68±10.42) was in agreement with the score obtained by Shery et al. in a similar age group. The same study found an OSDI higher than 12 in 34% of the cases, within the same values obtained in this work.23 No statistically significant differences were found in the IOP values measured with the iCare and those obtained with the ORA (either considering the IOPg or the IOPcc) between sex. The results are in agreement with Yassin and Al-Tamimi, who also found no statistically significant differences in IOP between men and women.24
No statistically significant differences were found between the IOP measured with iCare and the IOP measured with the ORA, both IOPg and IOPcc, for each degree of the OSDI questionnaire: normal, smooth, moderate, and severe, respectively. Although no correlation between the IOP and the total OSDI score was found, a correlation between the IOP measured with iCare and the subclass that evaluated symptomatology associated with environmental factors was found. The participants who reported more ocular discomfort associated with environmental factors (9.01±19.87), such as wind, dry places, and air-conditioning, had the highest IOP values measured with iCare, as can be seen by the positive correction between the OSDI “Environmental Factors” subclass score and the IOP measured with iCare (r = 0.414, p<0.05, Spearman). Since the environmental factors is the more sensitive subclass in detecting any degree of dryness than other subclasses and the OSDI total score,18 it is possible that subclass may be the only related to the IOP measured with iCare, due to the sample being very young and having few symptoms.
The prevalence of interferometric patterns in this study was close to that of Maissa and Guillon for people under 45 years of age.25 IOPg and IOPcc values had statistically significant differences across the various interferometric patterns and these differences can be seen in Fig. 2. A poor-quality tear film can have a negative optical and visual impact, and the ORA records a reflected infrared signal on the cornea26 and consequently on the tear film, specifically in the lipid layer, to monitor central corneal flattening. Different thicknesses of the lipid layer may provide different interferometric patterns that can induce different reflections of the infrared signal, thus causing different IOP values. The ORA also uses a centering optical system to measure the IOP as centrally as possible, and these different lipid layer thicknesses can also induce changes in this system that may also account for the changes found in the IOP with different lipid patterns. Since iCare does not use any centering optical system for IOP measurement, it is probably the reason no such correlation was found with the interferometric patterns.
The mean value of the NIBUT found in this study was of 17.74±20.40 s and the prevalence of NIBUT lower than 10 s (27%) was within the same values obtained by Maissa and Guillon in people under 45 years of age.25 No statistically significant differences in the NIBUT were found between men and women. These results are in agreement with Borrelli et al., who also found no differences between the two sexes.27
Regarding the analysis of tear quality and its influence on IOP, there were no statistically significant differences in the IOP measured with iCare, and ORA, both IOPg, and IOPcc, across volunteers with the NIBUT greater or lower than 10 s (Table 2). Although there are no studies or other information that correlates the tear film properties to possible changes in IOP values, it was expected that there would be differences in the measured IOP between these two groups, predicting higher IOPs associated with the NIBUT<10 s group and lower IOPs associated with the NIBUT>10 s group, however, this has not been confirmed. Not finding any correlation between NIBUT and the measured IOP may have been due to the sample used being too homogeneous, with most of the NIBUTs greater than 10 s and the values lower than 10 s being very close to 10 s, thus limiting the analysis.
One of the aims of this work was to find out whether the two corneal biomechanical properties were correlated with tear film parameters, and signs and symptoms that may be indicators of dry eye. Some corneal biomechanical properties were related to the OSDI total score and some of its subclasses (Table 4). The subclass “Vision” was unrelated to the measured CRF and CH. This may have happened because the condition related to the symptomatology found may not have been severe enough to produce changes in the measured CRF and CH. The total OSDI score was only related to the measured CRF (Table 4). The same is true for the eye symptoms and vision subclasses that are related to the measured CRF, with only the ocular symptoms subclass being related to the measured CH. It was expected that the biomechanical properties of the cornea would be related to the OSDI score, as found by Satitpitakul et al.28 This was not found on this study, probably because a large percentage of the participants had a normal score. The correlation of the CH with the OSDI was very close to significance (p = 0.055), supporting this assumption. Long et al. and Satitpitakul et al. concluded that dry eye might be associated with more compliant corneas.28,29 Long et al. indicated that this may be associated with the superficial ocular damage caused by dry eye.29 However, present work found high corneal deformation resistance associated with a higher OSDI score. Even though it is not clear what tear or environmental properties are mostly correlated with the OSDI scores, results found here seem to indicate that participants who scored higher in the ocular symptoms subclass had higher values of corneal biomechanical properties. This may have happened because the population used in this work was low-symptomatic, that is, probably without any damage at the level of the corneal epithelium. This data suggests that when there is symptomatology without signs, the cornea may be more resistant to deformation, but when there is damage to the corneal epithelium, the opposite may occur, and the cornea may become more flexible. There were no statistically significant differences in corneal biomechanical properties between participants with the NIBUT greater than and lower than 10 s, and there was also no correlation between the corneal biomechanical properties and the NIBUT. This may have happened because, to detect differences, the sample should contain participants with more extreme NIBUT values, such as in cases of dry eye, which was one of the limitations of this study. Age may also have been a factor in the absence of these differences since a low-quality tear film is associated with older people, not found in this study, which may also be limitation. Satitpitakul et al. also found no correlation between the biomechanical properties of the cornea and the tear film break-up time.28 It can be concluded that the NIBUT had no impact on the biomechanical properties of the cornea measured in the age range considered in this work.
ConclusionsThe tear film has been shown to have some impact on IOPs measured with ORA, however, further study would be needed to define this impact. IOP measured with iCare has been shown to have some correlation with the symptomatology obtained from the OSDI questionnaire. Although the IOP measured by the various methods was not related to the NIBUT, the tear film was not completely neutral when measuring IOP. Further study would be needed to understand this impact in a larger population and with different tear film, age, and ocular surface conditions. Corneal biomechanical properties were related to the OSDI total score and some of its subclasses. An increase in symptomatology was associated with an increase in the biomechanical properties of the cornea.
FundingThis work was supported by the Portuguese Foundation for Science and Technology (FCT) in the framework of the Strategic Funding UIDB/04650/2020.
Authors' contributionsFDS. The conception and design of the study, acquisition of data, analysis and interpretation of data and drafting the article. JMML. Drafting the article and revising it critically for important intellectual content. ML. The conception and design of the study, drafting the article and revising it critically for important intellectual content.