Silicone oil is used as endotamponade following vitreoretinal surgery to maintain the retina reattached when indicated. This study investigates the hypothesis that silicone oil causes insulation effects on the retina by affecting its response to light.
MethodsElectrophysiological responses to a flash stimulus were recorded using full-field electroretinography (ERG) and visual evoked potentials (VEP). Recordings were performed in 9 patients who underwent surgery for retinal detachment, before (1–2 days) and after (2–3 weeks) silicone oil removal (SOR) in both the study and the control eye. Flash ERG and VEP recordings were performed according to the ISCEV standard protocol.
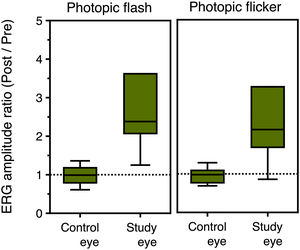
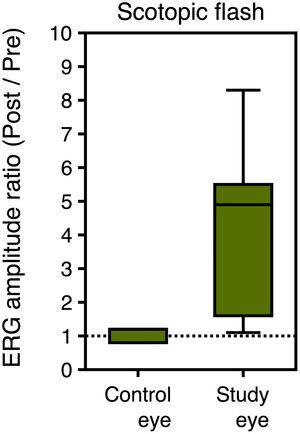
ResultsStatistically significant differences were found in the study eye in the amplitudes of the ERG responses and their corresponding ratios, i.e. the amplitude after SOR over the amplitude before SOR, in all conditions tested. No differences were observed in the control eye. The mean ratio of photopic ERG response was 3.4 ± 2.4 for the study and 1.0 ± 0.3 for the control eye (p<0.001). The mean ratio of ERG flicker response was 3.1 ± 2.4 and 1.0 ± 0.3, respectively (p = 0.003). Scotopic flash ERG ratio was 5.0 ± 4.4 for the study and 1.3 ± 0.6 for the control eye (p = 0.012). No differences were observed for the amplitude and latency of flash VEP response after SOR.
ConclusionsSilicone oil causes a reduction in flash ERG responses; no effect was found on flash VEP responses. ERGs in eyes filled with silicone oil should not be considered representative of retinal functionality, in contrast to VEPs, which are not affected by silicone oil presence.
As a clinical manifestation, retinal detachment has always been a challenge for retina specialists around the world. The primary purpose in therapy is the creation of a solid chorioretinal adhesion at the site(s) of retinal break(s), and silicone oil is often used as an endotamponade. The idea was first introduced in 1962 by Cibis,1 and since then, it has been one of the preferable materials in demanding cases of retinal detachment.2 Silicone oil's physical and chemical properties have given the vitreoretinal surgeon a vital tool to cope with traumatic retina injuries, proliferative diabetic retinopathy (PDR), giant retinal tears, and proliferative vitreoretinopathy (PVR).3 The main difference between silicone oil and other tamponade materials (e.g. gases) is the necessity of a second surgery to remove it from the vitreous cavity when the retina is securely reattached.
To be effective as an internal tamponade, the silicone oil needs to isolate aqueous humour from the retinal surface. Specific gravity, buoyancy, viscosity, and interfacial tension influence this function.4 An essential characteristic of silicone oil is its optical clarity which permits visual function of the operated eye and optical access to the fundus for imaging and functional tests.
In their general industrial use, silicone oils are primarily used as lubricants, thermic fluid oils, or hydraulic fluids. They are excellent electrical insulators and, unlike their carbon analogs, are non-flammable. An electrical insulator is a material in which electric current does not flow freely. The atoms of the insulator have tightly bound electrons which cannot readily move. Their use as electrical insulators is widely known in Material Science.5
Visual electrophysiology has been broadly used for evaluating the integrity of the visual system and in the differential diagnosis of diseases, using specific protocols, such as the electroretinogram (ERG) and the visual evoked potentials (VEPs).6,7 Flash ERGs are easily recorded in eyes filled with silicone oil due to their optical clarity. However, it has been reported that ERG recordings might be unreliable in the presence of silicone oil due to its insulating effect.8 In the current study, in addition to ERGs, we implement VEP recordings to determine both the ocular integrity and visual function in eyes that underwent silicone oil insertion. Measurements were evaluated before and after silicone oil removal (SOR) surgery to assess whether silicone oil, used as a tamponade following a retinal detachment surgery, interferes with pre- and post-retinal function.
MethodsParticipantsParticipants were recruited from the outpatient retina clinic of the Ophthalmology Department of the University Hospital. Surgery for retinal detachment and subsequent SOR was performed in the Ophthalmology Clinic. All of them were treated for retinal detachment in one of their eyes, with silicone oil as a tamponade. Patients with previous retinal pathology were excluded from the study. Nine patients (7 males) were included with a mean age of 63±11 years old. ERG and VEP recordings were performed 1 to 2 days before SOR surgery and 2 to 3 weeks following SOR to allow for recovery from surgery. The eye that underwent surgery served as the study eye while the other was used as control. Informed consent was obtained from all participants after they received a detailed written description of the nature of the study. The study was conducted in adherence to the tenets of the Declaration of Helsinki according to a research protocol approved by the Research Ethics Committee of the University Hospital.
Recording procedureInitially, flash VEPs measurements in photopic conditions (background luminance of 30 cd/m2) were recorded; each eye in every patient was measured separately. Subsequently, after 5–10 min, flash ERGs were recorded under the same photopic conditions with simultaneous measurements for both eyes. Finally, ERG responses were recorded in scotopic conditions, following 20 min of adaptation in the dark. Pupils from both eyes were dilated using tropicamide 1 % and phenylephrine 5 % drops, instilled into the lower conjunctival fornix 30 min prior to the recordings. Both ERG and VEPs were recorded using the computerized Primus 2.5 system (Tomey, Germany) that incorporated the ISCEV Standards,9,10 utilizing Ganzfeld stimulation with a maximum flash intensity of 3.5 cd s /m2. Data were sampled at a rate of 1000 Hz, constrained by online band-pass filtering between 0.3 Hz and 300 Hz, and a gain of 5000 K. Artifactual signals (e.g., blinks) were automatically removed.
Flash VEPs recordingsFull-field flash VEPs were obtained from the patients' study and control eyes. Stimulus strength was set at 3 cd s/m2 at 2 Hz frequency. The active electrode was placed at a distance from the occiput equal to 10 % of the perimeter of the skull (position Oz), while the reference electrode was placed at a distance from the nasal bone equal to 30 % of the perimeter (position Fz). In addition, a third electrode, placed on the forehead, served as ground (position Fpz). At least 16 sweeps were recorded for each condition.
Flash ERG recordingsThe full-field light-evoked ERG was recorded using corneal silver-nylon thread DTL electrodes (DTLplus, Diagnosis LLC, Lowell, USA) draped across the limbus as an active electrode. A 9 mm silver-silver chloride (Ag-AgCl) electrode (Biosense Medical, Chelmsford, UK) mounted at the ipsilateral outer canthus, used as a reference, while the earth electrode was placed on the forehead.
Recordings were performed first in the light-adapted state (with a 5 min light adaptation at a background luminance of 30 cd/m2) and then in the dark-adapted state, following a period of 20 min adaptation in the dark. Three ERG responses were recorded: (a) photopic flash responses (average response of 3 consecutive events of 1 Hz frequency and 3 cd s/m2 intensity each), (b) photopic steady-state responses (average response of 30 sweeps) with flashes presented at a rate of 30 stimuli per second (30 Hz) and an intensity of 3 cd s/m2, (c) scotopic bright flash responses (average response of two consecutive events of 0.3 Hz frequency and 3 cd s/m2 intensity).
The amplitude / implicit time of ERG b-waves in the three ERG responses and the amplitude / latency of flash VEP P100 peak were analysed and compared before and after SOR. In addition, the ratio of responses, i.e. the amplitude after SOR over the amplitude before SOR were calculated. Normality was evaluated using the Shapiro-Wilk test. Statistical significance was assessed though paired-sample t tests for the absolute responses and the non-parametric Mann–Whitney U test at p<0.05 for the ratio of responses.
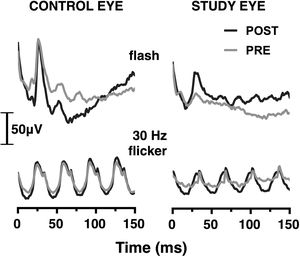
ResultsERG in photopic conditionsCharacteristic waveforms of grand-averaged photopic flash and flicker ERG responses before and after SOR surgery are depicted in Fig. 1. Paired sample t-test analysis demonstrated a significant increase in the average ERG flash amplitude in the study eye following the SOR, from 25.4 ± 19.3 to 60.1 ± 30.7 μV (p = 0.001). No difference was found in the ERG amplitude in the control eye (103.1 ± 32.9 μV and 101.8 ± 40.9 μV in the first and second session, respectively, p = 0.89). Similarly, the mean amplitude of the ERG flicker response for the study eye improved from 17.5 ± 12.1 to 38.1 ± 20.4 μV (p = 0.009), while no change was observed for the control, i.e., the average amplitude was 81.1 ± 16.7 μV and 78.3 ± 27.5 μV in the first and second session, respectively, (p = 0.74).
Fig. 2 presents box plots of the ERG amplitude ratio for the study and control eyes in photopic flash and flicker conditions. The average (±SD) amplitude ratio for the study eyes was 3.4 ± 2.4 and 3.1 ± 2.4 for the photopic flash and flicker conditions, respectively. On the other hand, no difference was observed for the control eyes, with the corresponding values being 1.0 ± 0.3 and 1.0 ± 0.3. Mann–Whitney U test revealed a statistically significant difference between control and study eyes in both conditions (p<0.001 and p = 0.003, respectively).
ERG in scotopic conditionsThe mean value of the scotopic flash ERG response for the study eye was 52.4 ± 44.2 μV and to 182.3 ± 105.2 μV, before and after the removal of the silicone, respectively (see Fig. 3). The difference was highly statistically significant (p = 0.008). The corresponding values for the control eye were 217.1 ± 79.9 μV and 264.0 ± 77.2 μV, with the difference being statistically insignificant (p = 0.266).
Moreover, the post/pre amplitude ratio of the scotopic ERG flash responses was 1.3 ± 0.6 in the control eye and 5.0 ± 4.4 in the study eye, with the difference being statistically significant (p = 0.012) (see Fig. 4). No differences were observed for the implicit times in any ERG response before and after SOR.
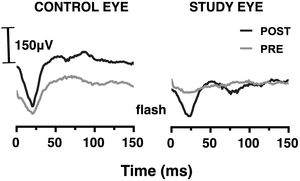
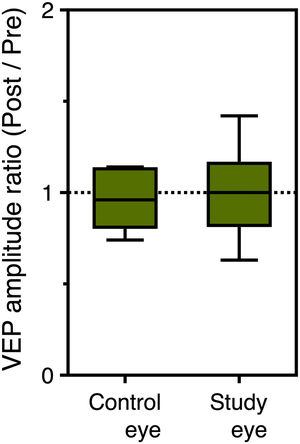
VEPs in photopic conditionsThe mean amplitude of the VEP flash response for the study eye was 17.0 ± 5.7 μV before SOR and 16.4 ± 11.0 μV after SOR; the difference was not statistically significant (p = 0.710). For the control eye, the corresponding values were 21.2 ± 11.3 μV and 19.4 ± 10.4 μV (p = 0.477). No statistically significant differences were found between the two eyes. In addition, the mean latency of the VEP response for the study eye was 112.1 ± 10.7 ms and 112.9 ± 9.4 ms before and after SOR (p = 0.890). The corresponding values for the control eye were 108.9 ± 9.5 ms and 109.6 ± 10.5 ms (p = 0.872). P100 post/pre-SOR amplitude ratio of the VEP response was found 0.99±0.40 and 0.98±0.31 for the control and the study eye, respectively (p = 0.965) (see Fig. 5). The corresponding difference in P100 latency was 3.1 ± 4.1 ms and 3.9 ± 3.9 ms (p = 0.983).
DiscussionSince the introduction of silicone oils as endotamponade materials, several concerns have been raised amongst ophthalmologists about their biosafety.2 Contradictory results have been published regarding histologic and electrophysiological findings following vitreoretinal surgery using silicone oil.11 Even though complications such as cataract formation, keratopathy, and secondary glaucoma have been well postulated,12,13 the question about toxicity effects remains.14,15 In this context and due to the possible insulating effect of silicone oil, questions are raised about the appropriate timing for electrophysiological testing in silicone oil-filled eyes. In the current study, we recorded the ERG and VEPs in eyes operated for retinal detachment, before and two to three weeks after SOR. We hypothesized that if silicone oil acts as an insulating material, ERG amplitudes would be reduced by its presence and increased after its removal. We found a significant increase in ERG amplitudes in all conditions in the study eyes in a short period of two to three weeks following SOR, while no change was observed in the control eyes. On the other hand, both flash VEP P100 amplitude and latency were similar before and after SOR surgery, in both control and study eyes. VEP recordings correspond to a gross electrical response generated by neurons at the primary visual cortex in response to flash stimulation. Thus, it is not surprising that VEPs are not influenced by the presence of endotamponade material in the vitreous cavity, which does not interfere with signal transmission between the retina and the visual cortex.6,10
The absence of any effect on flash VEP offers strong support to the silicone-oil insulation hypothesis, with the presence of silicone oil leading to an underestimation of retinal electrical activity. Silicone oils are a group of polymer compounds and hydrophobic monomers form silicone oxygen bonds with a higher refractive index (1.405) compared to vitreous and water.16 Therefore, is expected that the speed of sound when passing through a vitrectomized eye with a silicone oil tamponade is much slower than in an eye with vitrectomy alone or without vitrectomy.17
Multiple studies have been carried out to determine whether the silicone oil causes toxicity to the retina or just insulates it. Azarmina et al.18 used flash ERG in photopic and scotopic conditions and showed that ERG amplitudes and BCVA are increased after SOR after an adequate retention period, necessary for photoreceptors recovery. Supporting data can also be found in earlier literature, when Frumar et al.19 described changes in the amplitude of the ERG before and after SOR. However, conditions for the ERG recordings, e.g., stimulus parameters, recording conditions, state of adaptations, were not stated, so direct comparisons could not be made. Moreover, the insulation effect of the silicone oil was shown once more by Ozaki et al.20 who found significantly reduced a- and b- wave amplitudes before SOR compared to the fellow control eye.
It is essential to note that electrical activity in operated eyes, although increased by about a factor of two after SOR, remained significantly lower compared to control eyes. This confirms previous findings, using full field,21 multifocal22 and cone-specific23 ERGs, reporting reduced electrophysiological responses, which may be attributed to retinal detachment, which is rapidly followed by degeneration of the photoreceptors, affecting initially the outer segments and later the receptor cell bodies.24 There is some evidence, though, that electrical activity in the retina might be reversible after successful retinal detachment surgery, especially in “macula-on” eyes.23 However, some degree of silicone oil toxicity cannot be totally excluded. Clinical studies have recently discussed the gravity and mechanical pressure of the silicone oil on the retina, as a mechanism that could influence retinal blood flow, resulting in secondary changes in the retina.25 Retinal hypoxia and alteration of the retinal oxygen supply have also been described, demanding additional evidence regarding the interference of the physical properties of silicone oil with the retina.26 However, b-wave amplitudes of full-field 30 Hz flicker ERGs were found reduced at six months follow-up in patients who underwent pars plana vitrectomy with gas endotamponade compared with normals.27 Similarly, a decrease in flash ERG amplitude in vitrectomized eyes, even after successful scleral buckle surgery, was shown by Kim et al., when compared with that in the fellow non-vitrectomized eyes.28
Following SOR we observed a significant variability in ERG responses between patients. This variability might be due to differences in optical parameters of the eye, such as refractive error / axial length, the dilated pupil size, and lens opacity, as well as between subject differences in the extend of retinal detachment and the duration of silicone oil tamponade.29–31 Due to the limited number of participants in the current study, such analysis could not be performed. In addition, no valid correlations between electrophysiology findings and visual function could be accomplished. These should be further investigated in a future work.
In any case, based on the study findings, and due to the insulation effects of silicone-oil, ERG recordings should always be performed after SOR surgery. Clinicians should not ignore the fact that silicone acts like a barrier to the electrical current generated by the retinal neurons. On the other hand, VEP recordings do not seem to be affected by silicone oil and can be accurately performed in silicone oil-filled eyes when it is clinically indicated for differential diagnosis.
In conclusion, silicone oil exerts an insulation effect when used as endotamponade; as a result, ERG measurements are not reliable during its presence and should be performed after its removal. On the contrary flash VEPs are not affected by the presence of silicone oil and can be reliably used in silicone oil filled eyes.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.