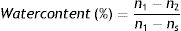The physicochemical stability, thermal and water plasticizing effect on transport properties of contact lenses (CL) were analyzed to verify its capacity to maintain the original properties after being dehydrated and rehydrated.
MethodsTwo daily disposable (nesofilcon A and delefilcon A) and two monthly CL (comfilcon A and lotrafilcon B) were used. Measurements of refractive index (RI), water content (WC), chemical structure and thermal properties were taken: new (N), after dehydration (D) and rehydrated (R). RI and WC were accessed using a digital automated refractometer (CLR-12-70). Chemical structure was evaluated by a Fourier Transformed Infrared Spectroscopy (FTIR-ATR) and Differential Scanning Calorimetry (DSC) with a calorimeter (Mettler Toledo DSC-821).
ResultsThe FTIR spectrum of N, D and R was quite similar for all CL, with higher differences observed in the transmission between 3500–3000cm−1 due to the hydroxyl group (OH). After dehydration and rehydration, there were no significant changes in the chemical structure. RI and WC of the CL rehydrated did not vary significantly from the initial CL (p>0.05) and thermal properties also confirm that the behavior did not change. It was observed that the glass-transition temperature decrease with increased WC.
ConclusionNo significant alterations were observed in the physicochemical structure of the materials after dehydration and rehydration showing a good stability of their components. The strong water plasticizing effect in the silicone hydrogel lens materials improves flexibility and chain mobility and may should be considered for other biomedical applications.
Se analizaron la estabilidad fisicoquímica, las propiedades térmicas y el efecto plastificante en las propiedades de transporte de las lentes de contacto (LC), para verificar su capacidad de mantener sus propiedades originales tras la deshidratación y rehidratación.
MétodosSe utilizaron dos lentes de contacto desechables diarias (nesofilcon A y delefilcon A) y dos mensuales (comfilcon A y lotrafilcon B). Se realizaron medidas del índice de refracción (IR), contenido de agua (CA), estructura química y propiedades térmicas: nuevas (N), tras la deshidratación (D) y tras la rehidratación (R). Se accedió a IR y CA utilizando un refractómetro digital automatizado (CLR-12-70). La estructura química se evaluó mediante Espectroscopía de infrarrojos con transformada de Fourier (FTIR-ATR) y Calorimetría diferencial de barrido (DSC) con un calorímetro (Mettler Toledo DSC-821).
ResultadosEl espectro FTIR de N, D y R fue bastante similar para todas las LC, observándose mayores diferencias en la transmisión entre 3500–3000cm−1 debido al grupo hidroxilo (OH). Tras la deshidratación y rehidratación no se produjeron cambios significativos en la estructura química. Los valores IR y CA de las LC rehidratadas no variaron significativamente con respecto a la LC inicial (p>0,05), y las propiedades térmicas confirmaron también que el comportamiento no experimentó cambio alguno. Se observó que la temperatura vidrio-transición disminuía al incrementarse WC.
ConclusiónNo se observaron alteraciones significativas en la estructura fisicoquímica de los materiales tras la deshidratación y rehidratación, reflejando una buena estabilidad de sus componentes. El fuerte efecto plastificante del agua en los materiales de las lentes de hidrogel de silicona mejora la flexibilidad y la movilidad de la cadena, debiendo considerarse esta circunstancia para otras aplicaciones biomédicas.
Contact lenses materials are abundant in the biomaterials world and their evolution has been subject of great research and development. Biomaterials namely polymers for the production of contact lenses are chain macromolecules, in which the connections between the monomers are made through polymerization processes and should have some important physical and chemical properties. Hydrogels are hydrophilic polymers with a specific three-dimensional structure that have a high water absorption and retention capacity due to the hydrophilic functional groups connected to the main structure.1 Poly(2-hydroxyethyl methacrylate) (pHEMA) was the original hydrophilic lens monomer and remains today the most commonly used hydrophilic monomer in the conventional soft contact lens field although silicone-hydrogel lenses have become widespread on new fits, due to their higher oxygen permeability.2 Of particular importance in siloxane hydrogels is the use of bulky polysiloxane containing monomers, the most common being those with the tris-(trimethylsiloxysilyl) (TRIS) moiety.3 The majority of siloxane hydrogel patents use N-vinyl pyrrolidone (NVP) and dimethylacrylamide (DMA). Polysiloxanes and most hydrophilic polymers are typically immiscible and when given the opportunity, they will phase separate. Phase separation occurs when cohesive interactions between chemically identical or similar molecules are stronger than the adhesive interactions between chemically different molecules.3
Not only with the intention of evaluating the stability of contact lenses polymers for their normal use, it is also important to study their behavior for other future applications and to understand the conditions in which they can undergo significant changes in their physicochemical properties. Papas et al.4 made a review of recent patent submissions in contact lens field and the results show that the main areas where contact lenses may be applied include drug delivery, visual augmentation and biosensing.
Physicochemical properties represent a significant concern in the design and quality control of soft contact lenses materials. The parameters of soft contact lenses may alter during wear as a result of the material dehydration.5 Changes in the water content may be related to changes in the structure and molecular dynamics of the material. Refractive index and water content are closely linked in soft hydrophilic materials. As changes in refractive index may be a sign of changes in the water content of lens materials,6 this observation may allow the evaluation of the contact lenses ability to maintain their water content after been dehydrated and rehydrated.7
The biocompatibility of the material is essential for the ocular health.8 While all soft lenses can modify dimensions to some extent on-eye, those that best maintain their physicochemical properties provide the least change in visual acuity and physiologic response during wear. Regardless the process of microbial contamination and also optical quality, parameters that were not studied here, the question remains, can the materials recover some of their characteristics?
Numerous efforts have been made to improve physical and chemical properties to avoid adverse reactions on the ocular surface and increase comfort during wear.9 An important characteristic of contact lens materials is their ability to maintain its physical properties after exposure to different external conditions. The application of the Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) technique has been used to study a direct qualitative and quantitative investigation of protein adsorption on hydrophilic polymers10 but, up to our knowledge, no studies were found in what concerns chemical stability of contact lenses.
In this work, it was evaluated the glass-transition temperature (Tg) and thermal behavior using Differential Scanning Calorimetry (DSC) technique. The purpose of the presented work was the study of the physicochemical stability of contact lenses materials and thermal properties by Attenuated Total Reflectance-Fourier Transform Infrared, refractometry and Differential Scanning Calorimetry after the materials have been subjected to a total dehydration and subsequent hydration.
Materials and methodsContact lensesIn this work, four contact lenses produced using the same method of manufacture (cast molding), with different composition of monomers and water content, were used in this study: two daily disposable contact lenses (nesofilcon A and delefilcon A) and two monthly contact lenses (comfilcon A and lotrafilcon B). All contact lenses studied in this work have a power of −3.00 diopters and their properties are detailed in Table 1. Two lenses from each material were used in all experiments.
Characteristics of the contact lenses used in the study.
| USAN | Nesofilcon A | Delefilcon A | Lotrafilcon B | Comfilcon A |
|---|---|---|---|---|
| Manufacturer | Bausch & Lomb | Alcon Vision Care | Alcon Vision Care | CooperVision |
| Commercial name | Biotrue ONEday | Total1 | Air Optix™ Aqua | Biofinity™ |
| Water Content (%) | 78 | ≥80 (surface) 33 (bulk) | 33 | 48 |
| DK/t (−3.00 D) | 42 | 156 | 138 | 160 |
| Central thickness (mm) | 0.10 | 0.09 | 0.08 | 0.08 |
| Main monomers | pHEMA, PVP;NVP | Si-Hy, DPMC | DMA;TRIS; siloxane macromer | M3U, FMM, TAIC, IBM, nMNVA, NVP, HOB |
| Refractive index (−3.00 D) | 1.374a | 1.420a | 1.421a | 1.405b |
USAN: United States Adopted name.
DK/t: oxygen transmissibility (units: X 10−9(cm/s) (ml O2/ml.mm Hg)).
Si-Hy: Silicone Hydrogel PVP: Polyvinylpyrrolidone; mPDMS: Polydimethylsiloxane monofunctional; DMA: N,Dimethylacetamide; HEMA: Hydroxyethyl methacrylate; EGDMA: Ethylene glycol dimethylacrylate; TRIS: propyltris(trimethylsiloxy)silane; NVP: N-Vinylpyrrolidone; TAIC: 1,3,5-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione; IBM: isobornil methacrylate; HOB: 2-hydroxyethyl-methacrylate; NMNVA: N-methyl N-vinyl acetamide; PVP: Polyvinyl pyrrolidone.
Data collected from manufacturers and FDA reports.
All properties were measured in three different states: new out of blister (N), dehydrated (D) at room temperature during 12h and after this period of time the lenses were rehydrated (R) for 12h in a 0.9% NaCl solution with neutral pH.
Fourier transform infrared spectroscopyTo obtain information of the structure and chemical linkages of the materials, the Fourier transform infrared spectroscopy technique coupled to an attenuated total reflectance accessory was used (FTIR-ATR 8 JASCO 4100) in the range between 4000 and 600cm1 using 32 scans and a nominal resolution of 8cm−1. This technique is based on the fact that the chemical linkages of the elements have specific vibration frequencies that correspond to the energy levels of the molecule.
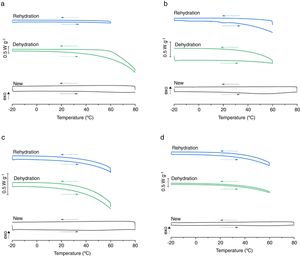
Water content and refractive indexThe refractive index was obtained with a digital automated refractometer (CLR 12-70, Index Instruments, Cambridge, UK).7 In order to increase reliability, three measurements per lens were performed and the mean value was considered for the analyses. The water content was estimated through an expression (Eq. 1), based in BS ISO 10339.1997 which relates the water content to the refractive index of the lens obtained directly from the refractometer.
Where n1, n2, and ns are the refractive index of the dehydrated polymer, the contact lenses and the solution in which the lenses were in, respectively.Differential scanning calorimetryDifferential scanning calorimetry measurements were performed in a Mettler Toledo DSC 821e calorimeter coupled to a cooling accessory (Labplant cryosta). The differential scanning calorimetry analyses were conducted over a temperature range of −20 to 80°C, at a heating rate of 2.5°C.min−1, under argon purge (50mL.min−1). These samples were subsequently cooled from 80 to −20°C, at a rate of 2.5°C.min−1. A second heating program was applied. Approximately 2.00mg of all lens were sealed in standard 40μL aluminium crucibles (Mettler Toledo). These assays were performed using as reference an empty crucible.
In order to evaluate the glass transition temperature, it was necessary to perform separate measurements at a temperature range (10–30°C) at a rate of 10°C.min−1 and more quantity of the sample was used.
ResultsTwo types of hydrogels were analyzed: a conventional hydrogel and a silicone-hydrogel. Despite the differences between these two types, as well as, in the composition of the different lenses studied (Table 1), the results obtained in the reflectance-Fourier transform infrared spectroscopy spectrum were very similar and can be observed in Fig. 1.
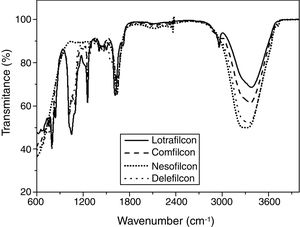
Several are the changes that can occur in a polymer after its natural conditions and therefore Fig. 2 (a–d) present the attenuated total reflectance-Fourier transform infrared spectroscopy for each new contact lenses (N), after dehydration (D) followed by the rehydration procedure (R).
As fluctuations in the water content may be related to modifications in the structure and molecular dynamics of the material, the refractive index and water content of the studied lenses were evaluated under the same conditions previously mentioned and mean values of refractive index and water content for each contact lenses are shown in Table 2. Although there are some differences between the nominal values (given by the manufacturer) and those obtained in this study, all measurements (new and rehydrated lenses) were made using the same technique and the values compared with each other and not with the manufacturer's value. No significant changes were observed on these parameters when the contact lenses were rehydrated (p>0.05).
Mean values of refractive index and water content and statistical significance.
| RI (N) | RI (R) | p | WC (N) (%) | WC (R) (%) | p | |
|---|---|---|---|---|---|---|
| Nesofilcon A | 1.3726±0.0003 | 1.3729±0.0003 | 0.285 | 78.37±0.21 | 78.03±0.38 | 0.180 |
| Delefilcon A | 1.4267±0.0006 | 1.4275±0.0007 | 0.180 | 24.10±0.57 | 23.45±0.64 | 0.180 |
| Lotrafilcon B | 1.4218±0.0001 | 1.4219±0.0002 | 0.593 | 28.57±0.15 | 28.20±0.10 | 0.109 |
| Comfilcon A | 1.4045±0.0005 | 1.4056±0.0004 | 0.109 | 47.9±0.20 | 47.27±0.15 | 0.109 |
RI (N): refractive index (new lens); RI (R): refractive index (rehydrated lens); WC (N): Water content (new lens); WC (R): Water content (rehydrated lens).
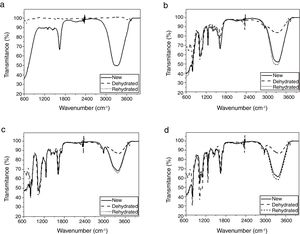
The differential scanning calorimetry cooling and heating thermograms of lenses in the temperature range from −20 to 80°C are shown in Fig. 3 (a–d). For the first heating/cooling program no endothermic melting peaks are observed in the differential scanning calorimetry curves of lenses. A second program shows the onset endothermic peak at about 60°C. It is assumed that the onset observed peaks are associated with the melting of the solid phase of water.
In this work, the glass-transition temperature of the lenses and its association with their water content is presented in Table 3.
DiscussionChanges in contact lens materials originated by repeated application and removal, environmental conditions and dry areas caused by exposure to the environment, can cause permanent deterioration and result in loss of some important features necessary to have a good clinical performance and yet to be considered for other non-conventional applications. Since contact lenses materials chemistry affects both surface and bulk characteristics, and a basic understanding of the way in which materials behave and differ in their ability to withstand dehydration, it is interesting to see how these materials react and behave under external conditions.
Although the main and greater use of contact lenses is for refractive compensation, they have also raised interest as therapeutic devices for delivery of ophthalmic drugs.11 Soft contact lenses have the ability to absorb some drugs and prolong the retention and residence time of drugs on eye, releasing them into tear film.12 An ideal soft contact lenses -based ophthalmic drug delivery system would display some significant capabilities as moisture retention characteristics and also stability during preservation and transportation.13 Hydrogels are water-swollen polymeric materials and due to fast drugs release rate, many strategies are developed to modify the conventional contact lenses for extended ophthalmic drug delivery.13–15
The extent of dehydration and the time necessary to reach equilibrium depends of some factors, that can include the polymer water content,16,17 lens thickness18 and the atmosphere conditions (temperature and relative humidity).19
Although the lenses studied are from three different manufacturers and with different compositions, as can be seen by observation of Fig. 1, the main bands from the attenuated total reflectance-Fourier transform infrared spectra of all new contact lenses are quite similar indicating that the monomers present in the structure belong to analogous materials. Main differences can be observed in the transmission between 3500–3000cm−1 due to hydroxyl groups (OH) which is in agreement with the water content of each contact lens (Table 1). Higher transmittance is observed for the delefilcon A (33–80%) and nesofilcon A (78%) followed by comfilcon A (48%) and lotrafilcon B (33%). Differences were also observed in the transmission around 1000cm−1 due to SiOSi groups belonging to silicone-hydrogel materials that all the lenses studied have with the exception of nesofilcon A (Fig. 1).20
After dehydration process an appearance of a complete destruction of the chemical structure of each lens seems to occur as almost no bands are present in the attenuated total reflectance-Fourier transform infrared spectra (Fig. 2 (a–d)). This may indicate that chemical scission occurred. After the rehydration process it was observed that none of the lenses presented significant changes on chemical structure, as no modifications were observed either on the wavenumber, as well as, in the bands intensity, when comparing to the attenuated total reflectance-Fourier transform infrared spectra present in Fig. 2(a–d).
As previously shown, a hydrogel subjected to a relatively small deformation, less than 20%, will fully recover to its original dimension in a rapid way.1 The chemical structure of the hydrogel can affect the swelling behavior as hydrogels containing hydrophilic groups swell to a higher degree compared to those containing hydrophobic groups.1 This was not confirmed in the present study, as all the silicone-hydrogel contact lenses behaved similar as the pHEMA based contact lenses and recovering its original water content (Table 2) after been totally dehydrated. It is possible to produce very stable high water content (>70%) soft contact lenses as the flexible behavior and stiffness of hydrogels is also ruled by polymer arrangement which apparently does not change after dehydration and rehydration process.
Although the relationship between water content and refractive index of silicone-hydrogel materials could follow a different behavior than conventional hydrogels,21 in this study it was verified that the higher water content, the lower refractive index for both materials conventional (nesofilcon A) and Si-Hy (lotrafilcon B and comfilcon A). The exception was the values obtained for delefilcon which is a silicone-hydrogel lens with low water content in bulk and with an extremely high surface water content. The effect of this particularity results in what the manufacturer defines as a water gradient across the lens, with a 90μm hydrophobic core of 33% water content and 6μm thick hydrophilic surface of approximately 80% water content on each side.22
In the present study, the initial refractive index values measured with the automated refractometer of the lenses was close to the nominal values presented in Table 1 and the refractive index of the lenses dehydrated and rehydrated did not vary significantly from the new contact lenses (p>0.05, Table 2). Although hydrogel lenses, particularly those that present a high water content, demonstrated higher levels of dehydration,19 in the present study, as predicted by the values of the refractive index, the results revealed only a small decrease in water content after been rehydrated (nesofilcon A: −0.34%, delefilcon A: −0.65%, comfilcon A: −0.63% and lotrafilcon B: −0.37%) that were not statistically significant (p>0.05, Table 2) and would have no impact on the lenses performance. This shows that despite the total dehydration of the materials, they can recover its water content due to the hydrogel high water absorption and retention capacity.1 Nesofilcon A is a high water, traditional hydrogel lens with a block copolymer surfactant into a lens fabricated from pHEMA and N-vinylpyrrolidone monomers.23 This lens has 78% water content and a surface that preserves constant water by delaying its evaporation.23 Comfilcon A lenses is a silicone–hydrogel material using Aquaform technology. This fabrication method uses a longer silicate chains, which converts into a lesser silicone content make the lens more flexible and better wetting. It also keeps the water in its interior, minimizing dehydrating.24,25 Lotrafilcon B has an ultrathin (25nm), continuous, hydrophilic plasma coating with high RI as surface treatment which forms a permanent thin continuous hydrophilic surface. Lotrafilcon B is now marketed as “Aqua” with an additional wetting agent, poli(vinil pirrolidona)-dimethylaminoethyl methacrylate, added to the lens packaging before sterilization to increase surface wettability.26,27
The differential scanning calorimetry of the lenses shows no freezing water and according to literature this fact promotes salt rejection, with a reasonably high total water content and maximise water flux.28 The melting of water frozen in lenses typically exhibits two broad endotherm peaks, one centred at ≈0°C and one extending down to about ≈−33°C 29–31 Although somewhat controversial,32 the area under these two peaks, suitably scaled by the heat of fusion of pure water, gives the mass of free water in the lenses. The remaining water in the gel matrix is considered tightly bound to the polymer network and nonfreezable, presumably through hydrogen bonding with polar groups along the polymer chains.29 It is postulated that no freezing water is responsible for the majority of the plasticization in the contact lens.33
The retained water within the lenses, affects many properties of the component polymers, such as glass-transition temperature. Normally, the glass-transition temperature is dependent upon the amount of moisture in a sample, as moisture not only lowers the temperature at which the transition occurs but also broadens the range over which it is seen.34 Change of the glass-transition temperature can affect many factors for on-eye lens performance, including comfort, on-eye lens movement, and water transport, ions and oxygen in the lens.34 In this work, it was obtained the glass-transition temperature of the lenses and correlation with their water content (Table 3). In these experiments the glass-transition temperature was difficult to obtain and a higher mass of lenses were used to identify this thermal event. Transport properties in lenses are largely determined by the relaxation modes of the polymer host matrix. Lower values of glass-transition temperature indicate that the local chain segment motion of the host polymer in the lenses system is less constrained by intermolecular interactions. In polymers, typically, the transport properties increase when the glass-transition temperature of the material decrease. As we can see in Table 3, in this case, demonstrate that for lower water content, the lens has higher glass-transition temperature. The value of the glass-transition temperature, is much lower for the delefilcon A. Thus, this phenomenon allows the largest water plasticization for delefilcon A. The plasticization is attributed to the increasing free volume and lubrication provided as the water swells the polymer and disrupts polymer-polymer hydrogen bonding.33
Low water content soft contact lenses have approximately the same amount of bound water as those with much higher water content.29 The strong water plasticizing effect the rubber-to-glass transition and can promote salt dissociation leading to an increase of the number of mobile ions and therefore to an increase of the transport properties. The plasticization is also able to increase the amorphous phase content and thus improve the water transport, ions and oxygen in the contact lenses. In addition, the plasticization has a significant influence on the mechanical properties of the contact lenses and also may be on the permeability to drug delivery.
Some of the techniques used in this work are not very common in studies with contact lenses but they have proven to be a useful tool to be used in contact lenses studies mainly in those in which it is necessary to obtain information on its chemical composition, thermal and water plasticizing effect on transport properties of the materials.35
In conclusion, although silicone-hydrogel lens materials represent a different class of contact lenses and behave completely different from conventional hydrogels, in this study no changes were observed in the chemical structure of any of the materials under the experimental conditions used and all contact lenses recovered their water content. Equally interesting is the strong water plasticizing effect in the silicone-hydrogel lens materials, suggesting decrease in intermolecular forces along polymer chains, which improves the flexibility and chain mobility and may be considered for the application of drug delivery systems.
Conflict of interestThere are no conflicts of interest to declare.
The authors acknowledge support from the Portuguese Foundation for Science and Technology in the framework of the Strategic Funding through the Chemistry Research Centre (UID/QUI/0686/2016) and the Physics Research Centre (UID/FIS/04650/2019) of University of Minho.