Postural intraocular pressure (IOP) variations have been found to be higher in glaucoma, especially in normal tension glaucoma. Higher IOP variation is also associated with greater field defects and thinner retinal nerve fiber layer. Air-puff tonometer has been found to be feasible to determine postural IOP variations. This study investigated if rebound tonometry can pick up such IOP changes.
MethodsFifty-four young adults, one eye randomly selected, had their IOP measured randomly by Pulsair EasyEye tonometer (Keeler Ltd, UK) and iCare rebound tonometer (Tiolat, Helsinki, Finland), in sitting followed by supine and finally sitting postures. IOP was measured after resting for 15min in each posture. Masked practitioners were involved. Repeated measures analysis of variations followed by post hoc tests were used to compare the IOP findings. Postural IOP changes were measured and compared between tonometers.
ResultsIOP significantly varied with postures (p<0.001). The two tonometers had similar IOP findings in each posture (p>0.05). Rebound tonometer gave a slightly higher IOP in the first sitting posture (difference=0.42±2.23mmHg), but provided a lower IOP in the supine posture (difference=−0.66±2.58mmHg) and the second sitting posture (difference=−0.11±2.24mmHg). Supine IOP was measured 3.10mmHg (SD 2.35mmHg) higher by Pulsair but only 2.02mmHg (SD 2.18mmHg) higher by iCare. This difference was significant (paired t-test, p<0.01).
ConclusionsPostural IOP variations were slightly lower when measured by iCare compared to Pulsair. Rebound tonometry is useful to screen for postural changes in IOP.
Se ha comprobado que las variaciones de la presión intraocular (PIO) por cambios posturales son superiores en el glaucoma, especialmente en el glaucoma de tensión normal. Una mayor variación de la PIO se asocia también a unos mayores defectos de campo y a un menor grosor de la capa de fibras nerviosas de la retina. La tonometría de no contacto ha resultado ser de utilidad para la determinación de las variaciones de la PIO debidas a cambios posturales. El objetivo del presente estudio fue investigar si la tonometría de rebote era capaz de monitorizar dichos cambios de la PIO.
MétodosSe midió la PIO de cincuenta y cuatro adultos jóvenes, seleccionando un ojo al azar, utilizando el tonómetro Pulsair EasyEye (Keeler Ltd, RU) y el tonómetro de rebote iCare (Tiolat, Helsinki, Finlandia), en posición sentada, luego supina y finalmente sentada. La PIO se midió tras un descanso de 15 minutos en cada postura. Examinadores enmascarados participaron en dichas medidas. Se empleó un análisis de medidas repetidas de las variaciones, seguido de los correspondientes tests post-hoc, para comparar los hallazgos tonométricos. Se compararon también las medidas de PIO obtenidas con cada tonómetro.
ResultadosLa PIO varió considerablemente entre las diferentes posturas (p<0,001). Los dos tonómetros proporcionaron resultados similares de PIO en cada postura (p>0,05). El tonómetro de rebote mostró una PIO ligeramente superior en la primera postura sentada (diferencia=0,42±2,23mmHg), pero mostró una PIO inferior para la postura supina (diferencia=−0,66±2,58mmHg) y la segunda postura sentada (diferencia=−0,11±2,24mmHg). El incremento de la PIO en posición supina fue de 3,10mmHg (SD 2,35mmHg) con el tonómetro Pulsair, pero sólo de 2,02mmHg (SD 2,18mmHg) con el tonómetro iCare. Esta diferencia resultó ser significativa (prueba de t pareada, p<0,01).
ConclusionesLas variaciones de la PIO debidas a cambios posturales fueron ligeramente inferiores al medirse utilizando el tonómetro iCare, en comparación con Pulsair. La tonometría de rebote es útil para monitorizar las variaciones de la PIO por cambios posturales.
Intraocular pressure (IOP) is usually clinically measured in a sitting position. It varies from normal physical and physiological alterations, such as postural change.1 IOP has been found higher in supine position, elevated further in prone2 and head-down positions.3–5 Glaucoma patients demonstrated a higher postural IOP elevation,1,6 especially at night time.7
Higher postural IOP elevation has been found to correlate with greater and faster visual field progression in primary open angle glaucoma8 and normal tension glaucoma.9,10 It may also lead to a thinner retinal nerve fiber layer thickness.11 Although Goldmann applanation tonometry (GAT) is the gold standard in measuring IOP, it has to be mounted to a slit-lamp biomicroscope. Perkins tonometry uses GAT principle and has the advantage of its portable design for measuring IOP at different positions.12
Non-contact tonometry using air-puff shares its advantages of portability and non-invasive nature. It is good for IOP screening.13 Recently, Jorge et al.14 reported that air-puff tonometry is sensitive enough to pick up postural IOP variations. Rebound tonometry is also portable and non-invasive with good performance compared with air-puff tonometry and GAT.15 It is reproducible when applied in school children.16 Only simple training is required for healthcare practitioners and parents to use it.17,18 Self-administration is possible so patients can monitor their IOP at home.19–21 Rebound tonometry compared reasonably well with Perkins tonometry when used in supine position.22
This study investigated if rebound tonometry could detect postural IOP variation as effectively as air-puff tonometry can.
Materials and methodsHealthy subjects were recruited from student population of the School of Optometry. All students studying optometry had comprehensive eye examination including tonometry and dilated fundus examination. Eligible subjects were those with good general and ocular health. Exclusion criteria included history of corneal abnormality, ocular trauma, and astigmatism more than 3.00D. Contact lens wearers were asked to cease lens wear at least one day before data collection. All procedures followed the Declaration of Helsinki. The protocol was reviewed and approved by the Ethics Committee of the Hong Kong Polytechnic University. Informed consent was obtained from the subjects prior to the measurements.
One eye was randomly selected for this study. After sitting and resting for 10min, subjects had their IOP measured by an air-puff non-contact tonometer (Pulsair EasyEye, Keeler Instruments, Inc, Broomall, PA) and a rebound tonometer (iCare, Tiolat, Helsinki, Finland) in a random order. They laid flat at a supine position for 15min followed by IOP measurements with the same devices, the sequence of which was randomly assigned. The final IOP was measured by the two devices after another 15min of rest at sitting position. Two masked practitioners took the IOP measurements using each device. A third practitioner was assigned to record the IOP findings.
For Pulsair, three measurements were taken and the mean was used for analysis.13,23 For iCare, the recommended procedures were followed in which six readings were taken continuously and the final IOP displayed with a letter P. Whenever an error sign appeared, a new set of readings was taken.24,25 The iCare tonometer used in the current study was the classic model which is designed to be used in an upright position (patient at the sitting position). In order to measure IOP at the supine position, subject was required to turn the head to the side opposite to the selected eye.22 That means when the right eye was measured, the subject at supine position had to turn the head to the left so the right eye was at a higher level than the left eye. The manufacturer has recently launched a new model which allows downward measurement at supine position.
Central cornea thickness (CCT) was measured, with three consecutive readings taken on the selected eye using a specular microscope (Topcon SP2000P, Topcon, Tokyo, Japan) for potential IOP adjustment if initial sitting IOP was high. Subjects came to our clinic for the above measurements between 5pm and 8pm. All the measurements were completed within 1h.
Statistical analysesQuantitative variables were expressed as mean±standard deviation. Results were tested for normality using the Shapiro–Wilk test. IOP findings were compared using two-way repeated measures analysis of variance (ANOVA) with positions (initial sitting versus supine versus final sitting) and devices (iCare versus Pulsair) as factors, followed by post hoc analysis using the Bonferroni test. Statistical analysis was performed using SigmaPlot version 11 (Systat Software, Inc.). Significance was set at 5%. Agreements between iCare and Pulsair (1.96 times standard deviation of the difference) at each position were compared.26
ResultsFifty-four subjects (30 males and 24 females) were recruited with a mean age of 21.43±2.02 years (ranging from 17 to 26 years). It involved 32 right and 22 left eyes. Mean spherical equivalent refraction was −3.78±2.86 D, spherical power was −3.33±2.78 D and cylindrical power was −0.89±0.85 D. Initial sitting IOP measured by Pulsair ranged from 9 to 21mmHg. Two subjects had IOP of 21mmHg and their CCT was 604.0 and 617.7μm, respectively.
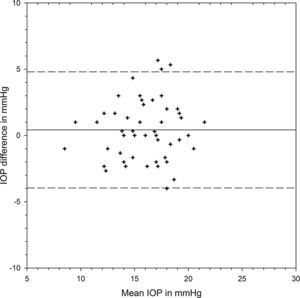
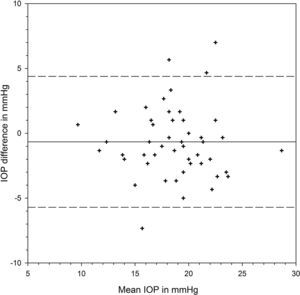
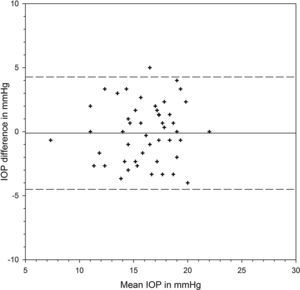
Table 1 shows the IOP findings from each device at different positions and their differences. There was significant variation from positions (F=73.613, p<0.001) but the two devices provided similar IOP results (F=0.253, p=0.617). There was significant interaction between positions and devices (F=4.029, p=0.021). At each position, the two devices shared similar IOP results (Bonferroni t-tests, p>0.05 for all three pair comparisons). IOP was measured slightly higher by iCare than Pulsair at the initial sitting position, with the 95% limits of agreement (LoA) varying from −3.96 to 4.80mmHg (Fig. 1). There was significant IOP rise from sitting to supine positions measured by each device (Bonferroni t-tests, p<0.001). Postural IOP elevation was 2.02±2.18mmHg from iCare and 3.10±2.35mmHg from Pulsair. The difference in IOP elevation was significant between iCare and Pulsair (paired t-test: t=2.954, p=0.0047). The 95% LoA between the two devices at supine position were −5.71 and 4.39mmHg (Fig. 2). Fig. 3 is the Bland and Altman plot of the agreement between the two devices at the final sitting position.
Intraocular pressure (IOP) measured by iCare and Pulsair at different positions.
| iCare | Pulsair | Difference | Significance | |
| Initial sitting (mmHg) | 16.26±3.03 | 15.84±2.83 | 0.42±2.23 | p>0.05 |
| Supine (mmHg) | 18.28±3.64 | 18.94±3.73 | −0.66±2.58 | p>0.05 |
| IOP elevation (mmHg) | 2.02±2.18 | 3.10±2.35 | −1.08±2.66 | p=0.0047* |
| Final sitting (mmHg) | 15.83±3.17 | 15.94±2.92 | −0.11±2.24 | p>0.05 |
Table 2 shows the number of subjects with different IOP elevations. For rebound tonometry, thirty-four subjects (61%) did not have IOP change or IOP elevated 2mmHg or less. Twenty-one subjects (39%) had IOP elevation greater than 2mmHg. On the other hand, 37 subjects (69%) had IOP elevated by more than 2mmHg using Pulsair. Comparing the two devices, 36 subjects (67%) had greater IOP elevation measured by Pulsair. Among them, nineteen subjects (out of 36) had difference greater than 2mmHg with the two devices.
Number of subjects with postural intraocular pressure (IOP) elevation determined by iCare and Pulsair.
| Number of subjects | |||||
| Negative | 0–2mmHg rise | >2 to 4mmHg rise | >4 to 6mmHg rise | >6mmHg rise | |
| iCare | 6 | 27 | 17 | 2 | 2 |
| Pulsair | 5 | 12 | 21 | 11 | 5 |
| Pulsair minus iCare | 18 | 17 | 13 | 4 | 2 |
“Negative” for iCare/Pulsair refers to supine IOP<initial sitting IOP. “Negative” in Pulsair minus iCare refers to greater IOP elevation determined by iCare.
Although the 95% LoA between iCare and Pulsiar at the two sitting positions were similar, iCare IOP was slightly lower than Pulsair IOP at the final sitting position. Initial sitting IOP from both iCare and Pulsair was significantly correlated with CCT (iCare: correlation coefficient r=0.6263, p<0.001; Pulsiar: r=0.471, p<0.001).
DiscussionPrata et al.1 conducted a comprehensive review on postural IOP elevation. A greater IOP increase should be expected from eyes with glaucoma (1.6–23.1mmHg IOP rise) when changing from sitting to supine positions. In eyes with primary open angle glaucoma, a higher postural IOP elevation was associated with a greater visual field defect.8 Similar findings were reported in normal tension glaucoma.10 Kiuchi et al.10 monitored the visual field deterioration in normal tension glaucoma patients for an average of 4.6 years (2.5–7.5 years). A greater postural IOP elevation was associated with a faster visual deterioration. This functional change could be due to anatomical variation. For example, Mizokami et al.11 used time-domain optical coherence tomography and found that retinal nerve fiber layer thickness was thinner in eyes with greater postural IOP elevation. These eyes also had greater visual field defects. Postural IOP variation may be used to predict glaucoma progression.9–11 The association between IOP elevation and functional change is conceivable as one-third of our time is spent on sleeping. If IOP increases significantly when lying flat, the ganglion cell fibers have to tolerate high IOP fluctuation continuously in every 24-h cycle. However, postural IOP variation is not commonly measured in clinical practice.
Portable tonometer such as Perkins is commonly used for nocturnal study because measurement can be conducted at supine position.12 Otherwise, patients have to be seated for tonometry.27 Although Perkins is portable, topical anesthesia could be inconvenient for nocturnal study. Apart from air-puff tonometry, rebound tonometry might be an alternative. Rebound tonometry was introduced by Kontiola.28 It compared reasonably well with GAT, over-estimation varied from 0.5mmHg to 2.6mmHg.29,30 Since it is easy to use, portable and non-invasive, it is good for screening children.31 Both air-puff and rebound tonometers performed similarly in initial sitting position (Table 1) as reported before.32
We found a slightly higher postural IOP elevation from Pulsair than Jorge et al.14 They took 2 series of sitting IOP in order to investigate the repeatability of Pulsair. Their study therefore involved 2 sitting and 1 supine IOP measurements. The supine IOP was randomly assigned as the first, second or the third measurement in the series. Supine IOP was compared with the mean of the two sitting IOP. To simplify the experimental protocol, our supine IOP was always measured between the two sitting IOPs. The resting time of 15min at each position was to ensure stabilization of IOP.2,11 Rebound tonometry demonstrated less postural IOP elevation in the current study (Table 2). This may be due to poor agreement between the two devices. From the Bland and Altman plots (Figs. 1–3), the 95% LoA were similar at the two sitting positions (8.8mmHg) and comparable to previous study.32 However, the 95% LoA were up to 10.1mmHg at the supine position. The difference in postural IOP variations between devices may be due to the limitation of our rebound tonometer at supine position. In performing rebound tonometry at supine position, the subject had to turn the head opposite to the side of measurement because the tonometer could not function when facing downward. For example, when the right eye was measured, turning the head to the left side resulted in a higher position of the right eye compared with the heart. The eye position was different than when using Pulsair. The eye would be at the same height as the heart. It is conceivable to get a higher IOP from Pulsair. Malihi and Sit33 found that IOP of the lower positioned eye at lateral decubitus position was usually higher than the fellow eye. The difference between the two eyes was around 1mmHg. The latest model of rebound tonometry could be applied at a downward position. We would postulate a higher postural IOP rise measured from this new model.
We found significant association between rebound tonometry and CCT, similar to previous studies.34–36 This is unexpected as corneal contact in rebound tonometry is minimal. Chui et al.25 found that the confounding effect of CCT on rebound tonometry was significant only if corneal biomechanical properties were not taken into consideration. Effect of CCT on rebound tonometry became insignificant in multiple regression analysis. On the other hand, non-contact tonometry is influenced by CCT more than GAT is.37,38
There were some limitations of the study. Firstly, we have mentioned about different eye positions at supine position when using Pulsair and iCare tonometers. It would be ideal if IOP could be measured with both instruments facing downward. Secondly, we did not include applanation tonometry such as Perkins in our study. This was to reduce the experimental time as well as avoiding repeated use of topical anesthesia. Only adopting a shorter resting time should applanation tonometry be feasible without repeated topical anesthesia.5
High postural IOP elevation could be a risk factor for the development of glaucoma. Advising patients to sleep with head rise such as using a pillow could effectively reduce the IOP elevation.39 Previous studies reported that air-puff tonometer is sensitive enough to pick up postural IOP elevation. Postural IOP elevation could also be identified by rebound tonometry. Similar study is warranted using the latest model of rebound tonometer.
Financial disclosureThe authors have no proprietary or commercial interest in any materials discussed in this paper.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study was supported by the Development of Niche Areas Funding on “Glaucoma Research – A programme Approach” J-BB76 from The Hong Kong Polytechnic University. A poster was presented in the American Academy of Optometry annual meeting in October 2012 at Phoenix, USA.