To compare macular thickness obtained using two different modes of image acquisitions with Cirrus HD-OCT 5000.
MethodsPatients with diabetes were recruited and macular thickness were obtained using optical coherence tomography (OCT) mode and optical coherence tomography angiography (OCTA) mode. The OCT mode involved a Macular Cube (512×128 pixels) centred on the fovea covering a 6 × 6 mm2 macular region. The OCTA acquisition involved scanning of a 6 × 6 mm² scan (350×350 pixels) centred on the fovea. Data was exported and compared according to the Early Treatment Diabetic Retinopathy Study (ETDRS) subfields. Fixation deviation was defined as the deviation of the foveal point from the grid center in the OCT scan and OCTA scan.
ResultsEighty-six diabetic patients were recruited had similar macular thickness in all ETDRS subfield except the superior outer sector. The 95 % limits of agreement between the two modes were within 9.7μm to -9.0μm. It took longer to complete each OCTA mode (median of 7.4 s) than the OCT mode (median time of 5.8 s) (Wilcoxon test, p < 0.001), but OCTA generated a smaller fixation deviation (median 68.8μm) than the OCT mode (median 103.0μm) (Wilcoxon test, p = 0.014).
ConclusionsImproved fixation in OCTA compared with OCT was evident, likely because of the faster scanning speed and higher sampling density of OCTA. Macular thickness was found similar. There appears no requirement to obtain macular thickness measurements using a separate OCT mode. This approach can reduce patient chair time, improve patient comfort, and streamline the clinical workflow.
Since the launch of the first commercial model two decades ago, optical coherence tomography (OCT) imaging has changed the clinical practice of the ophthalmic field. OCT allows for non-invasive, reproducible, and quantitative evaluation of the retinal layers in vivo at the optical microscopic level. The normative data obtained with OCT devices enhances the interpretation of the measurement results, hence explaining the increase in the number of academic publications on this topic.1 Optical coherence tomography angiography (OCTA) is an advancement over OCT that is used to evaluate ocular diseases involving hypoxia such as retinal vessel occlusion,23 neovascularization such as wet age-related macular degeneration (AMD),45 or both hypoxia and neovascularization such as diabetic retinopathy.67 OCTA is also superior to OCT in guiding anti-vascular endothelial growth factor therapy in wet AMD.8 It offers improved sensitivity and specificity compared with gold-standard fluorescence angiography in diagnosing wet AMD.910 Over the last decade, the number of studies using OCTA has dramatically increased.11
Some practitioners use conventional OCT for measuring retinal thickness and OCTA for retinal vasculature. For example, Sacconi et al.12 used Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) to obtain macular thickness and Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA) to obtain the vessel density of the superficial and deep capillary plexuses. Cirrus HD-OCT measures the macular thickness and presents the results on a standard Early Treatment of Diabetic Retinopathy Study (ETDRS) grid. In another study, Winegarner et al.13 measured macular thickness by using an older version of Cirrus OCT together with Optovue OCTA (RTVue XR Avanti, Optovue, Inc., Fremont, CA, USA) to evaluate retinal vasculature. Some practitioners use different modes of the same OCTA device to separately evaluate thickness and vasculature. For example, Yoon et al.14 and Shin et al.15 used Cirrus HD-OCT but switched to the OCT mode to measure macular thickness after acquiring retinal vasculature measurements by the OCTA mode. These approaches may increase patient chair time and disrupt the clinical workflow. Alternatively, utilizing a single mode from a single device to examine retinal thickness and vasculature could be more time-saving and convenient. Some OCTA devices measure the retinal thickness during OCTA acquisition. For instance, Yang et al.2 used Optovue OCTA 3 × 3 mm Angio Retina mode to obtain both the central macular thickness and retinal vasculature.
The Zeiss PLEX Elite 9000 (Carl Zeiss Meditec, USA) is a research-use swept-source OCT device that enables both retinal thickness and vasculature to be obtained through OCTA acquisition.1617 The Cirrus HD-OCT 5000, a commercial model from the same company, also measures retinal thickness on the basis of OCTA acquisition. However, the Cirrus HD-OCT 5000 does not display the thickness results on a screen and requires practitioners to export the results manually. When using the Cirrus HD-OCT 5000, the OCT mode runs at 27 kHz, whereas the OCTA mode runs at 68 kHz. The OCT Macular Cube mode uses 512×128 pixels to scan a 6 × 6 mm2 macular region. However, the OCTA mode has a pattern that consists of 350×350 pixels. Macular thickness results may vary because different scanning speeds and resolutions are used with both modes (OCT and OCTA). A high scanning speed may improve fixation stability.
In this study, we compared the macular thickness results obtained when applying the OCT and OCTA modes of the same device to patients with diabetes. We hypothesized that fixation would be more stable when using the higher-frequency OCTA mode and that macular thickness measurements would thus differ between the two modes.
MethodsThis study was a collaborative observational study among the Department of Family Medicine and Primary Health Care, Kowloon West Cluster, Hospital Authority (HA) of Hong Kong; Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong; Department of Rehabilitation Sciences, and School of Optometry of The Hong Kong Polytechnic University.
The participants were patients with type II diabetes aged 45 to 70 years. All participants were recruited by physicians from HA hospitals. In addition, all participants were able to fully understand the information provided. Informed consent was obtained from each participant before any of the ophthalmic assessments (described in the following) were initiated. This study was performed according to the tenets of the Declaration of Helsinki and was approved by the institutional review boards of all the participating units which included Clinical Research Ethics Committee of CUHK (reference no. 2017.562) and PolyU Institutional Review Board (reference no. HSEARS2017005001).
Patients with diabetes had the general health conditions regularly monitored by physicians. The inclusion criterion was a glycated hemoglobin (HbA1c) level ≥ 6.5 %. The exclusion criteria included eye diseases affecting the macula such as age-related macular degeneration or glaucoma, a history of previous ocular surgery or treatment, or other metabolic disorders.
Ophthalmic assessmentsAll participants were examined by an optometrist who was masked to history of diabetes medication, and laboratory test results. Objective refraction was performed using an auto-refractor (ARK-510A, Nidek Co., Ltd.) followed by subjective refraction. Best-corrected visual acuity at distance (BCVA) was measured using Snellen notation and converted to the logMAR scale. Axial length was measured using an ocular biometer with partial coherence interferometry (Nidek AL-Scan, Nidek Co. Ltd., Gamagori, Japan). Six valid readings were obtained with a signal-to-noise ratio ≥ 2.50 in each measurement. One drop of 1 % tropicamide was applied to each eye for mydriasis after pre-mydriatic examinations, which included intraocular pressure (IOP) and central corneal thickness measurements using a non-contact tonometer (Nidek NT-530P, Nidek Co. Ltd., Gamagori, Japan), and an evaluation of the anterior chamber angles using the Van Herick technique and slit-lamp biomicroscopy.
Upon successful pupil dilation, digital fundus photographs (one centered on the optic disc and one centered on the fovea) were obtained for each eye to categorize the severity of diabetic retinopathy (DR) according to the ETDRS grading system. Subsequently, fundus biomicroscopy was performed to detect ocular abnormalities. All ophthalmic assessments were performed for both eyes. Finally, all ocular outcomes, including refractive error, BCVA, IOP, DR severity, as well as the most recent ocular health conditions, were reported to the participants.
Optical coherence tomography (OCT) and Optical coherence tomography angiography (OCTA)All OCT and OCTA images were acquired using a Cirrus HD- OCT 5000 device, version 11.0.0.29946, (Carl Zeiss Meditec, Dublin, CA). It is a commercially available spectral-domain OCT device that uses a superluminescent diode laser with a wavelength of 840 nm. The sequence of OCT and OCTA scans was arranged in a randomized manner. One eye was randomly selected for the OCT and OCTA measurements.
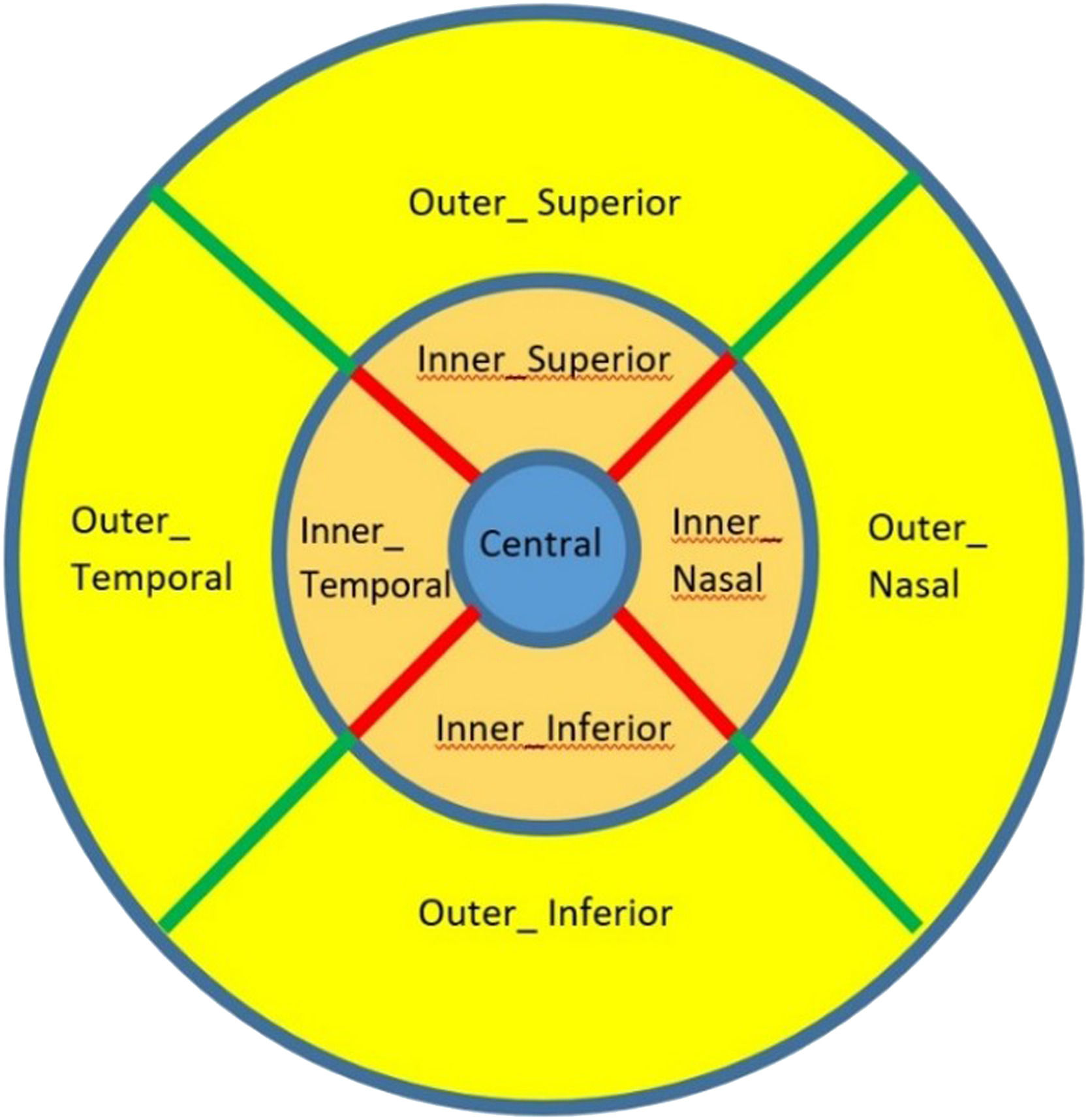
All participants had their maculae scanned with both OCT and OCTA modes. The OCT mode involved a Macular Cube (512×128 pixels) centred on the fovea covering a 6 × 6 mm2 macular region. This was equivalent to a transverse resolution of 11.7 µm horizontally and 47.2 µm vertically. The scanning speed of this mode was 27,000 A-scans/sec. The macular thickness (from the inner limiting membrane to the retinal pigment epithelium) was presented on a screen in an ETDRS grid format. The ETDRS grid consists of three rings: a central circular subfield with a diameter of 1 mm around the fovea, an inner parafoveal ring with a diameter of 3 mm, and an outer perifoveal ring with a diameter of 6 mm. The inner and outer rings were composed of 4 quadrants: superior, inferior, temporal, and nasal (Fig. 1).
OCTA acquisition involved scanning of a 6 × 6 mm² scan (350×350 pixels) centred on the fovea. This was equivalent to a transverse resolution of 17.2 µm both horizontally and vertically. The scanning speed in this mode was 68,000 A-scans/sec.
The FastTrac™ retinal tracking system was activated to minimize motion artefacts during both OCT and OCTA image acquisitions. Images with a signal strength < 7, or with considerable artefacts or vessel discontinuity were excluded. The measurement results were exported using a proprietary XML exporter provided by the manufacturer. The macular thickness results were presented in an ETDRS grid format in different Excel worksheets for OCT and OCTA image acquisitions.
Statistical analysisData distribution was examined using the Kolmogorov–Smirnov test. The macular thickness results obtained using OCT and OCTA scanning were compared. Parametric tests (paired t-test and unpaired t- test) and non-parametric tests (Wilcoxon signed rank test and Mann- Whitney test) were performed according to the normality results. Data are presented as mean ± standard deviation (SD) for parametric tests and as median (interquartile range, IQR) for non-parametric tests. Difference in macular thickness between the OCT and OCTA scan measurements, as well as their 95 % limits of agreement (LoA) with different ETDRS subfields, were studied. All statistical analyses were performed using SPSS version 26.0 (IBM-SPSS, Chicago, IL).
Fixation deviation (FD) was defined as the deviation of the foveal point from the grid center in the OCT scan (512×128 pixels) and OCTA scan (350×350 pixels). The exported file included the location (x, y-coordinates) of the thinnest foveal point. At the x, y-coordinates of 256, 64, and 175, 175, the fovea was at the centre of the OCT 512×128 scan and the OCTA 350×350 scan, respectively. The distance from the thinnest foveal point to the center of the scan pattern, in micrometer (μm), was calculated as the hypotenuse of the deviated x, y-coordinates.
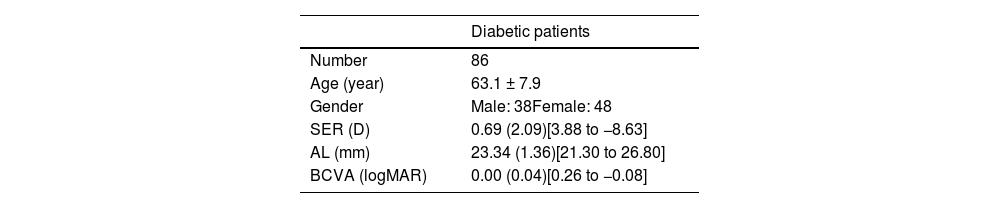
ResultsA total of 86 patients with diabetes were recruited. Table 1 shows their demographic information. Only 5 patients with diabetes had DR, four of which were mild and one of which was moderate. All five patients with mild to moderate DR exhibited normal macular structure, and no abnormalities were detected. Additionally, there were no segmentation errors observed.
Information of diabetic patients. Results are presented in mean ± standard deviation, or mean (interquartile range) with [range].
| Diabetic patients | |
|---|---|
| Number | 86 |
| Age (year) | 63.1 ± 7.9 |
| Gender | Male: 38Female: 48 |
| SER (D) | 0.69 (2.09)[3.88 to −8.63] |
| AL (mm) | 23.34 (1.36)[21.30 to 26.80] |
| BCVA (logMAR) | 0.00 (0.04)[0.26 to −0.08] |
SER: spherical equivalent refraction; AL: axial length; BCVA: best corrected visual acuity.
Significant difference is bold.
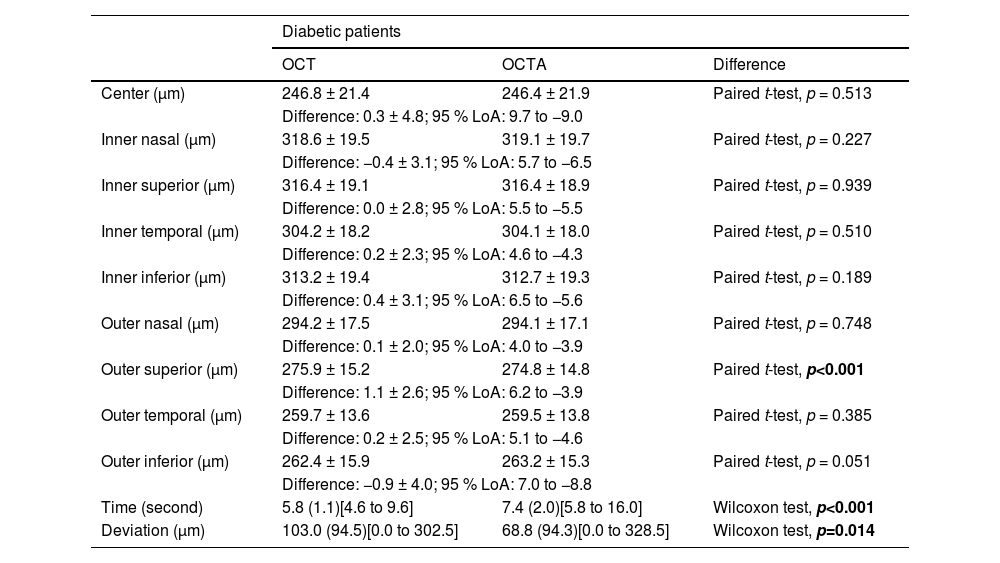
Table 2 shows the OCT and OCTA results. Overall, the OCTA scans required more time to complete than the OCT scans (median of 7.4 s versus median of 5.8 s, p < 0.001). However, no significant difference was identified in macular thickness measurements between the two modes in most ETDRS sectors (95 % limits of agreement within 9.7μm to −9.0μm). A slightly thinner macula was detected at the outer superior quadrant when OCTA acquisition was used (274.8 ± 14.8μm versus 275.9 ± 15.2 μm in OCT, p < 0.001). The difference of 1.1 μm is smaller than the axial and transverse resolution (5 μm and 15 μm respectively) of the Cirrus HD-OCT 5000.1819 This difference can be considered clinically insignificant.
Macular thickness of diabetic patients obtained using optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA), difference and 95 % limits of agreements (LoA) between the two acquisitions. Results are presented in mean ± standard deviation, or mean (interquartile range) with [range].
Significant difference is bold.
Overall, they had a smaller fixation deviation during OCTA (median 68.8μm) than during OCT (median 103.0μm) acquisitions (Wilcoxon test, p = 0.014), because of the higher scanning speed in the OCTA mode resulting in fewer artefacts and higher reliability.
DiscussionBecause OCTA is an advancement over OCT, deriving retinal thickness by using a proprietary OCT algorithm during OCTA acquisition should be feasible. Most practitioners use the OCT and OCTA modes separately to measure retinal thickness and evaluate retinal vasculature. The Cirrus HD-OCT 5000 measures macular thickness during OCTA acquisition. In this study, we compared the macular thickness results obtained when using both modes, and we observed no significant difference in the ETDRS grid. We also observed favorable 95 % limits of agreement for both modes. Compared with the OCT mode, smaller fixation deviation was noted in the OCTA mode, macular thickness results may be more reliable by using the OCTA mode. To sum up, practitioners may not need use a separate OCT mode for acquiring macular thickness after OCTA acquisition. Simultaneous assessment of macular thickness and retinal vasculature in diabetic patients can be achieved by utilizing the OCTA mode provided by the Cirrus HD-OCT 5000. This approach provides significant advantages by reducing patient chair time, enhancing patient comfort and increasing the overall efficiency of the clinical workflow.
The Macular Cube mode yields results other than just macular thickness, such as the ganglion cell-inner plexiform layer (GC-IPL) thickness. In their study, Channa et al.20 reported that GC-IPL was 1.41μm thinner in patients with diabetes with no or mild DR than in non-diabetic participants, indicating neuroretinal changes may occur before vascular changes of DR. They also reported that patients with diabetes with DR above the mild stage had a 1.24μm thinner GC-IPL than that of patients with no or mild DR. Ezhilvendhan et al.21 and Ambiya et al.22 conducted studies investigating the relationship between GC-IPL thickness in both control and diabetic groups, with and without DR. Their findings align with those of Channa et al., indicating a significant reduction in GC-IPL thickness in individuals with diabetes, even prior to the onset of DR. The most prominent changes in GC-IPL thickness were observed following the development of proliferative DR. Ezhilvendhan et al.21 also found that the macular thickness of diabetic group was thinner than the control group. In another study, Lim et al.23 used a Cirrus HD-OCT 5000 to study the relationship between GC-IPL thickness and retinal vasculature. They used a separate 512×128 Macular Cube to obtain the macular GC-IPL thickness after OCTA acquisition. Similarly, Kim et al.3 studied macular thickness, GC-IPL thickness, and vessel density in patients with retinal vein occlusion and performed OCT and OCTA acquisitions separately using a Cirrus HD-OCT 5000.
Although both the OCT and OCTA modes scan the same 6 × 6mm² square, the Macular Cube mode offers a higher sampling density along the horizontal than along the vertical meridian. In their study, Kim et al.24 reported that the GC-IPL thickness was not substantially affected when the deviation was within 59μm horizontally or 47μm vertically. This is equivalent to 5 pixels of horizontal deviation and 1 pixel of vertical deviation. The 6 × 6 mm² OCTA square has a transverse resolution of 17.2 µm along both the horizontal and vertical meridians. Transverse resolution can be further improved to 12.2 µm by using a smaller 3 × 3 mm² OCTA scan area. However, a smaller scan area cannot cover the ETDRS outer ring. To obtain a high-resolution retinal vasculature image, the scan area should be small. Vujosevic et al.25 used swept-source OCT to determine macular thickness by using a 6-mm scan (transverse resolution: 20μm), and they scanned the retinal vasculature with a 3 × 3 mm² pattern (transverse resolution: 9.4μm).
In patients with diabetes, OCTA acquisition required more time to complete than did OCT acquisition. However, fixation was superior when using OCTA (68.8μm deviation) than when using OCT (103.0μm deviation) acquisition. Lam et al.26 suggested that patients with high myopia should be corrected with contact lenses rather than using the built-in corrective lenses. This is because contact lens correction may reduce fixation deviation and enhance fixation stability. Therefore, we speculate that the high scanning speed and transverse resolution of OCTA may reduce fixation deviation.
OCT has gained increasing significance in the diagnosis, monitoring, and identification of prognostic biomarkers for managing diabetic cases such as DR and diabetic macular edema (DME). In determining the need for interventions like repeat anti-VEGF injections, switching therapeutic agents (e.g., intraocular corticosteroids), initiating laser treatment, or considering vitrectomy surgery, OCT findings play a crucial role. This imaging modality allows practitioners to assess various morphological features including focal or diffuse DME, hyperreflective dots, disorganization of retinal inner layers, hard exudates, intraretinal or subretinal fluid, center-involving or center-sparing DME, and tractional retinal detachment.
OCT has now become the gold standard for diagnosing and monitoring DME, with central retinal thickness being utilized as an enrollment criterion in many clinical trials. In modern clinical trials investigating the efficacy of anti-VEGF therapy for DME, macular thickness is often used as a secondary anatomical endpoint, with improvements in BCVA being reported alongside reductions in OCT-measured macular thickness.
In this study, we compared the macular thickness results by using the ETDRS grid rather than by referring to just one location (e.g. subfoveal thickness). The macular thickness of the ETDRS central 1-mm subfield provides a more accurate diagnosis of diabetic macular edema and clinically significant macular edema than that provided by fundus photographs.27 To decrease the false-positive rate resulting from the use of fundus photographs, Wong et al.28 suggested a macular thickness of 300μm at the ETDRS central 1-mm circle as the cut-off for defining diabetic maculopathy. Generally, DR is common among patients with diabetes.29 Therefore, more macular thickness and retinal vasculature evaluations should be performed.
OCTA has become popular in clinical practice. It can detect non-exudative choroidal neovascularization (CNV) in wet AMD.5 Bailey et al.30 reported that the presence of non-exudative CNV may substantially increase (18-fold) the risk of exudative CNV. However, although OCTA is superior to other invasive angiography modalities, Told et al.4 found that it is less effective than indocyanine green angiography for detecting CNV. Nevertheless, recent studies have indicated that the use of fluorescein angiography has declined since the introduction of OCTA.3132 This shift in the employment of imaging tools may be explained by the improvements in image quality and the non-invasive nature of OCTA. Hence, OCTA can be used as a supplementary tool for indocyanine green angiography, especially in the wide-field mode.33 The limitations of OCTA, however, are that although retinal thickness information may be measured, image export is manual and no comparison with the built-in normative macular thickness database is available.
This study has some limitations. First, almost all patients with diabetes in this study had normal retina with good BCVA. More severe cases of DR may affect the segmentation of different retinal layers. Second, the OCTA device used in this study had two scanning speeds for both OCT and OCTA acquisitions. The latest model, however, has the same scanning speed (100 kHz) for both modes of acquisition. Although only one OCTA device was used in this study, the Cirrus model is considered to have better performance than other OCTA devices, as well as fewer artefacts and higher reliability.34
In conclusion, OCTA acquisition can be used for macular thickness measurement. In addition, the thickness results obtained with the OCT and OCTA modes by using the Cirrus HD-OCT device were comparable. Finally, a higher scanning speed with a higher transverse resolution may improve fixation during OCTA acquisition. The Cirrus HD-OCT 5000 offers the OCTA mode, which allows for the simultaneous assessment of macular thickness and retinal vasculature in diabetic patients, a condition that frequently affects the elderly population. It seems unnecessary to acquire macular thickness measurements using a separate optical coherence tomography mode. This approach offers notable benefits in terms of reducing patient chair time and optimizing the efficiency of the clinical workflow. However, further research is required to ascertain if similar findings apply to other concurrent health conditions.
Funding supportWe would like to thank the funding support from UGC Matching Grant Scheme ZEZK.
Author contributionsWHY. Conceptualization, data curation, formal analysis, investigation, methodology, project administration, Writing – original draft, Writing – review & editing
AR. Conceptualization, methodology, project administration
ZCYB. Conceptualization, methodology
LCWS. Conceptualization, methodology, project administration
CLYG. Conceptualization, methodology
LAKC. Conceptualization, formal analysis, methodology, Writing – original draft, Writing – review & editing
Statement of ethicsWritten informed consent was obtained before any ophthalmic examination.
We thank the Areas of Excellence Committee, The Hong Kong Polytechnic University for providing funding to buy the high-resolution optical coherence tomography angiography system. Part of the materials was presented as poster at the 2021 American Academy of Optometry annual meeting.