Central areolar choroidal dystrophy (CACD) is a rare, inherited disease that can lead to profound visual disturbance. It is characterized by atrophic changes, particularly in the macula. Specific genotypic mutations are responsible for the autosomal dominant form of this disease. However, there is a distinct retinal dystrophy that combines central areolar choroidal dystrophy with autosomal dominantly inherited drusen. It has been proposed that patients with a specific mutation in the peripherin/RDS gene may manifest a combined presentation.
Case reportHere we describe a case of a patient who reported with significantly decreased best-corrected visual acuity of fifteen years of duration in the right eye more than the left. Dilated fundus examination found macular changes that were consistent with central areolar choroidal dystrophy. In addition, there was evidence of surrounding congenital drusen throughout the arcades of both eyes. Electrodiagnostics, optical coherence tomography, and fundus fluorescein angiography were used to confirm the diagnosis of CACD.
ConclusionCentral areolar choroidal dystrophy normally presents without drusen. However, in patients manifesting a specific mutation, central areolar choridal dystrophy may present in conjunction with drusen. It appears that the Arg142Trp mutation is one of the factors predisposing to drusen formation.
La distrofia coroidea areolar central (DCAC) es una enfermedad rara y hereditaria, que puede originar una gran alteración visual. Se caracteriza por cambios atróficos, particularmente en la mácula. Las mutaciones genotípicas específicas son responsables de la forma dominante autosómica de esta enfermedad. Sin embargo existe una distrofia retiniana diferente, que combina la distrofia coroidea areolar central con las drusas autosómicas dominantes hereditarias. Según se ha propuesto, los pacientes con una mutación específica del gen periferina-RDS pueden manifestar una presentación combinada.
Informe de un casoDescribimos aquí el caso de un paciente que se presentó con una disminución considerable de la agudeza visual mejor corregida, desde hacía quince años, más acusada en el ojo derecho que en el izquierdo. El examen del fondo de ojo bajo dilatación mostró unos cambios maculares en consistencia con una distrofia coroidea areolar central. Además, existía evidencia de drusas congénitas circundantes en las arcadas de ambos ojos. Se utilizaron electrodiagnóstico, tomografía de coherencia óptica y angiografía fluoresceínica del fondo de ojo para confirmar el diagnóstico de DCAC.
ConclusiónLa distrofia coroidea areolar central se presenta normalmente sin drusas. Sin embargo, en pacientes que manifiestan una mutación específica, la distrofia coroidea areolar central puede presentarse acompañada de drusas. Parece que la mutación Arg142Trp es uno de los factores que influyen en la formación de drusas.
Central areolar choroidal dystrophy (CACD) is a hereditary retinal disorder that primarily affects the macula. In the early stages, there is subtle, mottled depigmentation in the posterior pole. Ultimately, the depigmentation enlarges into in a well-circumscribed round or oval area of atrophy of the retinal pigment epithelium (RPE) and choriocapillaris in the center of the macula.1,2 Patients usually become symptomatic in the 3rd to 4th decade when a fine, hardly detectable, mottling of the RPE leads to the development of absolute central visual scotomas. Later, between the 4th and 7th decade of life, progressive macular atrophy leads to a dramatic decline in central visual acuity and severe visual disability.3,4 CACD may be autosomal dominant or recessive; however, autosomal-recessive cases are rare.1 Although CACD has a genetic basis, sporadic cases have been reported.5
Autosomal-dominant CACD is heterogeneous, but mutations in the peripherin/RDS (peripherin-2 or PRPH2) gene seem to be the most common cause.1,6 Seven different mutations in the peripherin/RDS have been described in autosomal-dominant CACD.1,5 The p.Arg142Trp mutation in the peripherin/retinal degeneration slow (RDS) gene has been implicated in both autosomal-dominant CACD and dominant drusen.2,7 However, no flecks or drusen are typically seen in CACD.7,8 Therefore, the expression of this particular mutation is not common.
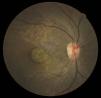
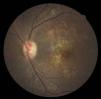
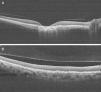
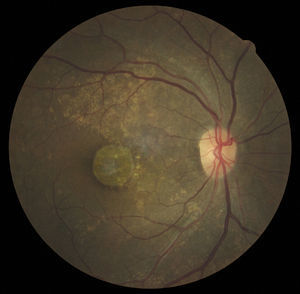
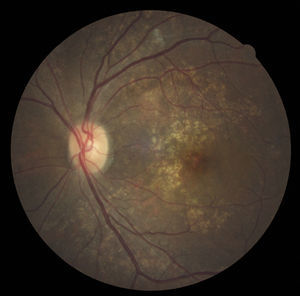
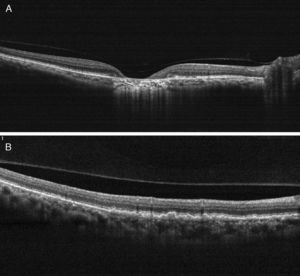
Case reportA 47-year-old black female presented with a chief complaint of decreased vision in her right eye (OD) for the past 15 years. Her ocular history included a prior episode of anterior uveitis in the OD which resolved with conventional treatment. Medical history was remarkable for hypertension and type 2 non-insulin dependent diabetes of 17 years duration. Her systemic medications included glyburide, metformin, and lisinopril. Her family history was remarkable for diabetes, hypertension, and glaucoma in her mother. Examination revealed best corrected visual acuity of OD 20/200 and OS 20/25. Pupillary reactions were normal and there was no restriction of gaze. Visual fields were full to finger counting. The intraocular pressures were 20mmHg in OD and 17mmHg in OS. Slit lamp evaluation exhibited nuclear sclerosis and cortical cataracts in OU with a posterior subcapsular cataract in OS. Fundus examination was significant for a well-circumscribed area of choroidal and retinal pigment epithelial atrophy involving the macula in OD, and overall geographic atrophic changes in OS (Figs. 1 and 2). Drusenoid deposits surrounded both maculae. The optic nerves were pink with distinct margins and the peripheral fundus was unremarkable in OU. Optical coherence tomography was performed in office. On OCT examination in OD, the atrophic macular area correlated with a total absence of the outer retinal layers with shadowing into the choroid. (Fig. 3A) In addition, the OCT revealed “lumpiness” at the level of the retinal pigment epithelium, consistent with the heavy drusenoid deposition. (Fig. 3B) Based on clinical appearance and OCT, a tentative diagnosis of CACD was made and the patient was sent for further evaluation including fluorescein angiography and electrodiagnostic evaluation.
(A) On OCT examination in OD, the atrophic macular area correlated with a total absence of the outer retinal layers with shadowing into the choroid. (B) OCT revealing bumpiness at the level of the retinal pigment epithelium consistent with the drusenoid deposits seen throughout the posterior pole.
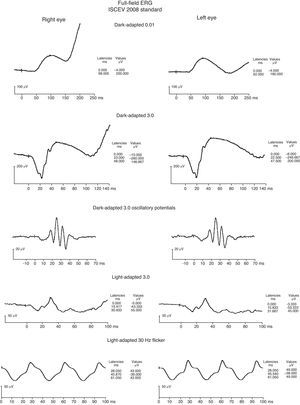
Both a flash electroretinogram (fERG) and multifocal electroretinogram (mfERG) were performed. (Figs. 4–6) The fERG findings showed normal rod responses along with a mild reduction in the photopic response amplitude and minimal delay, with the right eye affected to a greater degree. These findings are consistent with the most common fERG pattern (normal responses overall with slightly impaired photopic responses) seen in patients with central areolar choroidal dystrophy.2,9 The mfERG findings further mapped the amplitude reduction across 50° of the posterior pole. The results indicated a loss of central cone population, consistent with patient's loss of vision in both eyes, and with the foveal area response significantly more depressed in the right eye. It is worth noting that the retinal dysfunction noted with her mfERG is not limited to the demarcated central atrophic retinal areas, but expands beyond centrifugally. This finding has been reported in the literature in patients with central areolar choroidal dystrophy, and is thought to reflect a more diffuse retinal involvement relative to the clinically evident morphological changes.10,11 The mfERG is able to detect abnormalities localized in the macula, and is therefore a very useful instrument in the diagnosis of macular dystrophies such as CACD.11 It is also useful to help mapping areas of visual loss in patients unable to perform reliable visual field testing.
fERG shows normal scotopic responses OU (dark-adapted waveforms above), reduced photopic response amplitudes and minimal delay OU (light-adapted waveforms above), with right eye 30Hz flicker responses slightly reduced compared to left eye. These findings are consistent with the most common fERG pattern seen in patients with central areolar choroidal dystrophy.
OD mfERG findings show marked 1st order response amplitude reduction across the central retina with a normal to trace delay in implicit time. Note the marked central response amplitude depression in the foveal zone, however the retinal dysfunction noted in the mfERG is not limited to the demarcated central atrophic area as can be seen in the retinal photo (Figs. 1 and 2). This finding of retinal response depression on mfERG testing extending beyond the borders of the ophthalmoscopic lesions has been reported in patients with CACD, and is thought to reflect a more diffuse retinal involvement relative to the clinically evident morphological changes.
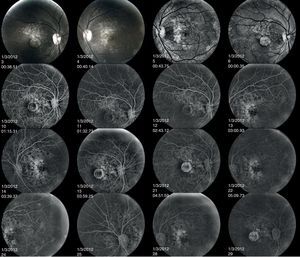
Fluorescein angiography revealed small areas of hyperfluorescence corresponding to the RPE atrophy in the early stages. In the later frames, there is loss of choriocapillaris in the area of RPE atrophy. The hard drusen can be distinguished as small, well-defined, hyperfluorescent lesions (Fig. 7).
Several clinical entities resemble the fundoscopic appearance described and the differential diagnoses included central areolar choroidal dystrophy, cone dystrophy, macular degeneration, and Stargardt's Disease. In our patient, the diagnosis of central areolar choroidal dystrophy was made by clinical evaluation, ancillary testing and by exclusion.
DiscussionCentral Areolar Choroidal Dystrophy was initially called Central Senile Areolar Choroidal Atrophy by Nettleship in 1884.12 In 1939, Sorsby renamed the condition Choroidal Angio-Sclerosis. The current name of CACD was designated after the inheritance pattern was deemed primarily dominant. Since CACD is predominantly an autosomal dominant disorder with complete or almost complete penetrance, almost all people carrying the peripherin/RDS (Arg142Trp) mutation can be considered potential CACD patients.5,7
Central areolar choroidal dystrophy is a heterogeneous progressive macular dystrophy that primarily affects the choriocapillaris. Clinically, it presents as a regional, geographic area of atrophy involving the choroid and RPE. Fine symmetric mottling of the RPE in the macula can resemble that of a bulls-eye.13 Patients are initially asymptomatic with normal visual acuity. Between the 3rd and 4th decades, the area may begin to mirror that of a macular coloboma.14 Over time, retinal blood vessels begin to course over the lesion and sclera becomes visible. Visual acuity may drop to the 20/50 level at this stage.13 As the patient gets older (5–6th decade), the lesion may begin to spread more peripherally leading to further visual disturbance.
Four clinical stages of the disease have been described.5 Stage 1 is characterized by subtle focal parafoveal pigmentary RPE changes that can be observed ophthalmoscopically. Stage 2 would appear as an oval-to-round, mildly atrophic, hypopigmented area. Stage 3 is characterized by one or more patches of well-demarcated RPE atrophy outside the fovea. In stage 4, the atrophic area involves the fovea which results in a profound decrease in vision.
The retinal findings seen in CACD vary and range from minimal RPE changes without drusen to CACD associated with dominant drusen. Literature on similar clinical presentations is limited. Klevering et al.7 report on an entity called Zermatt macular dystrophy which affects patients in their late teens and twenties and presents with drusen-like deposits and central pigmentary changes. Focal atrophic defects with eventual geographic atrophy and severe visual loss results in these patients. Interestingly, this macular dystrophy is linked to a mutation in the Arg172Trp mutation of the RDS/peripherin gene which is located alongside the Arg142Trp mutation in the large intradiscal loop D2 of the RDS/peripherin protein.7,15 It is possible that drusen and CACD are variable expressions of the same disease, as these are allelic disorders of the RDS/peripherin gene.4,7 Mutations in the peripherin/RDS gene have also been linked to retinitis pigmentosa, progressive macular degeneration, cone-rod dystrophy, and pattern dystrophy.7,16
Differential diagnosisBecause of the type of fundus changes seen in CACD, differential diagnosis can be quite challenging. Several of the macular dystrophies can present with symmetric RPE mottling, bull's eye maculopathy, and central geographic atrophy mimicking those changes seen in early CACD. Cone dystrophy presents with a bull's eye appearance in the macula appearing as a central reddish circular area (pigment clumping), which is surrounded by depigmented areas. It can be autosomal dominant or recessive and usually presents in the first to second decade with moderate to severe vision loss. Fluorescein angiography will show window defects late if there is chorioretinal atrophy or breaks. Electrodiagnostic testing typically demonstrates a moderate reduction and delay of the b-wave on fERG testing and a normal EOG. CACD will have a normal to slightly subnormal fERG and EOG. Additionally, with mfERG testing the implicit time in CACD is normal (as in our patient) or minimally delayed, this is in contrast to a more moderate delay in cone dystrophy patients. The differential diagnosis can also be established by looking for the presence of large choroidal vessels that characterize CACD and are rarely encountered in cone dystrophy.4,7
Late stage CACD shares similar clinical characteristics with atrophic age-related macular degeneration (AMD), such as geographic atrophy and drusen.17,18 Several features may aid in distinguishing between the two. In the early stages of CACD, subtle parafoveal pigmentary RPE changes with or without the presence of an oval, mildly atrophic hypopigmented area in the center of the fovea can be seen. In AMD, there is usually hard/soft drusen concentrated in the macula, or hyperpigmentation. The geographic atrophy of AMD usually arises from these drusen or hyperpigmentation.18,19 In all but the initial stage of CACD, a speckled fundus autofluorescence (FAF) pattern that is sharply demarcated from the surrounding retina, regularly oval shaped and confined to the central macula is observed. Although speckled FAF patters have been described in AMD, the alterations were less regularly shaped, less well demarcated, and often extended beyond the macula.20,21 Drusen associated with CACD show intense fundus autofluorescence, whereas those drusen seen in AMD do not.7,18,22 In the speckled FAF areas in CACD, OCT will show a corresponding disorganization of the photoreceptors and a loss of photoreceptor outer segments, seen as a disruption of the inner-outer photoreceptor junction and thinning of the Outer Nuclear Layer.23 OCT of AMD may also exhibit sub-RPE deposits, whereas these are extremely rare in CACD. The atrophic zones in geographic atrophy were are highly irregular and show residual material on Bruch's membrane. In contrast, in CACD the atrophic areas are smooth and homogeneous. The higher occurrence of sub-RPE deposits and irregular structure of retinal layers within the atrophic lesion in AMD patients indicates different pathogenic mechanisms for CACD and AMD. Choroidal neovascularization is also rare in CACD due to the absence of a functional choriocapillaris or choroidal vascular system.22 Besides the circumscribed atrophic lesions that characterize the late stages in both disorders, drusen-like deposits have been described in a limited number of patients with CACD.
The prevalence of end-stage AMD has been estimated at 3% in people aged over 65 years, and climbs to 11% in those over 85 years old. Therefore, young patients with atrophic lesions should be suspected of having entities other than AMD until proven otherwise.24
Two angiographic patterns of wet AMD have been described, classic and occult choroidal neovascular membrane (CNV). Classic CNV shows up in the early phase as a bright, well delineated area of hyperfluorescence which increases in intensity and size through the mid and late frames. This is due to progressive leakage of the dye that obscures the boundaries of the CNV. With occult CNV, the fluorescein pattern varies from speckled hyperfluorescence initially with persistence of leakage in later frames to late leakage of dye at the level of the RPE in the late phases of the angiogram (not corresponding to the area of the CNV).25
Stargardt's disease or fundus flavimaculatus can present with an array of fundus changes including a beaten-bronze appearance to the macula and yellow, fish-tail shaped flecks in the retina. The fERG is usually normal in these patients, while the mfERG usually reveals markedly reduced focal foveal and central retinal responses, even in the presence of normal acuity.26 The dominance pattern is the same as CACD. In Stargardt's/fundus-flavimaculatus, the macula is mottled; whereby with CACD, the macula is excavated and atrophic. Additionally, the fish-tail flecks seen outside the macula in Stargardt's are not seen in CACD. Early on, the two entities are difficult to differentiate. However, in Stargardt's fundus fluorescein angiography will exhibit continual macular hyperfluorescence and mid-peripheral involvement with a silent choroid.25
Central areolar dystrophy (CAD) of the RPE must also be differentiated from CACD. CAD of the RPE almost exclusively affects white females.22 Visual acuity begins to be compromised in the late 5th to early 6th decade of life. Fundus findings are similar, however CAD of the RPE is usually unilateral and the atrophic changes affect the RPE and the choroid.18,25
ManagementDue to the hereditary nature of CACD, it is crucial to educate the patient and refer the patient for genetic counseling. Low vision rehabilitation can be especially useful in affected patients. Visual acuity prognosis is poor; however, peripheral vision is preserved so mobility can be fairly maintained. Even though management options are scarce, proper diagnosis is essential in order for these patients to be properly counseled.
Conflicts of interestThe authors have no conflicts of interest to declare.