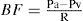It is beyond dispute that elevated intraocular pressure (IOP) is a major risk factor for the development of primary open angle glaucoma (POAG). Nonetheless, only a minority of patients with elevated IOP generate the disease. This leads to the notion that POAG is a progressive optic neuropathy caused by added factors that may work in concert with elevated IOP. Corneal viscoelastic resistance and vascular autoregulation have been associated to the effects of elevated IOP. This article discusses investigational facts suggesting that altered corneal viscoelastic/elastic properties and vascular dysregulation may contribute to the development of POAG in patients with elevated IOP.
Es indiscutible que la presión intraocular (PIO) elevada es un factor de riesgo importante para el desarrollo de glaucoma primario de ángulo abierto (GPAA). Sin embargo, la enfermedad aparece solamente en una minoría de pacientes con PIO elevada, de lo que se deduce que el GPAA es una neuropatía óptica progresiva causada por factores añadidos que posiblemente actúan junto con la PIO elevada. La resistencia viscoelástica de la córnea y la autorregulación vascular se han asociado con los efectos de la PIO elevada. En este artículo se abordan hechos en investigación que indican que la alteración de las propiedades viscoelásticas/elásticas de la córnea y la desregulación vascular pueden contribuir al desarrollo de GPAA en pacientes con PIO elevada.
Glaucoma is a leading cause of preventable blindness in the world. The complete understanding of the pathogenesis of the disease is still very much in debate. Nonetheless, as studies have evolved, elevated intraocular pressure (IOP) has been looked at differently in regards to its contribution to the development of primary open-angle glaucoma (POAG). It is now considered by some researchers as a significant risk factor that may be influenced by viscoelastic and elastic properties of the cornea.1 Furthermore, corneal viscoelastic resistance and vascular autoregulation have been associated to the effects of elevated IOP.2,3 Others with a different perspective suggest that the effect of elevated IOP on the optic nerve head may be at the expense of autoregulatory mechanisms in the oculo-vascular system.4 Conceivably, the possible association of these two factors to elevated IOP may help clarify the reason(s) for clinical differences between patients with POAG and patients with ocular hypertension (OHT).
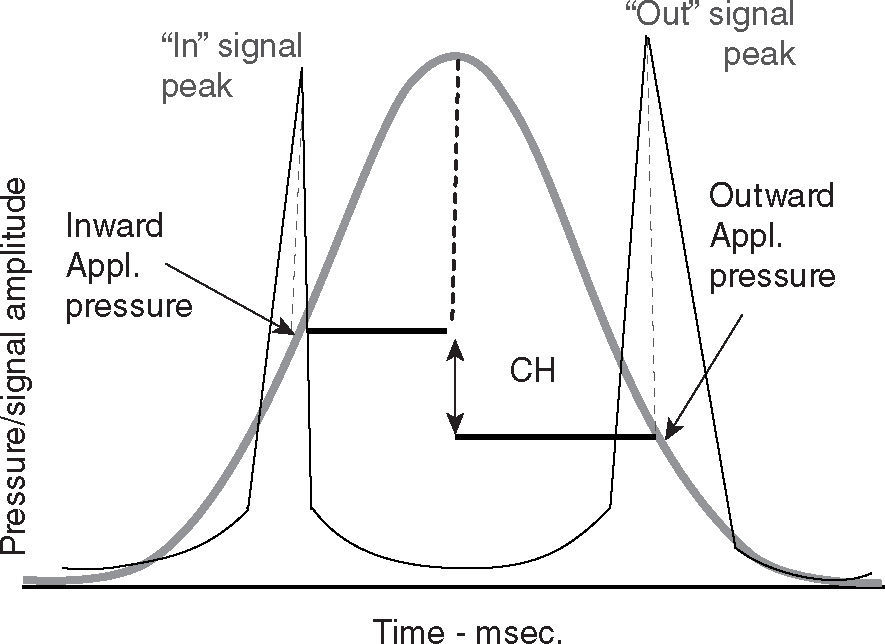
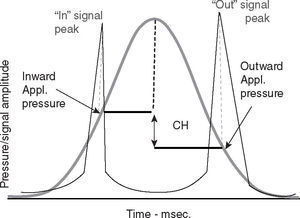
Ocular rigidity has been characterized in part by viscoelastic and elastic properties of the cornea. Measurement of these properties is obtained with the ocular response analyzer (ORA). This instrument is a sophisticated non-contact pneumo-tonometer (Reichert Ophthalmic Instruments, Buffalo NY, USA) that records two applanation pressure measurements from the air pulse delivered to the cornea. One at the applanation point as the cornea is moved inward and another at the applanation point as it moves outward to its original contour. The difference between these two measurements is termed corneal hysteresis (CH) (Figure 1). CH defines the viscoelastic behavior of the cornea in response to air applanation force.5 This viscoelastic response reflects the capacity of the cornea to dissipate air pulse energy delivered by the pneumo-tonometer,6 and appears to be a dynamic resistance element to the outward force exerted by IOP. CH also provides the basis for another ORA parameter called corneal resistance factor (CRF). CRF is a measurement that may better reflect the elastic properties of the cornea.7 Corneal elasticity relates to the resistance encountered by the air pulse of the pneumo-tonometer while deforming the corneal surface6 and seems to be a marker that relates more specifically to the overall ocular structural resistance.
Autoregulation is a process in which normal blood flow is maintained in response to changes in perfusion pressure. At the eye, perfusion pressure (PP) may be generally expressed as the difference between the blood pressure (BP) and the IOP.8 So, in essence any significant elevation in IOP will reduce perfusion pressure to the eye.8,9 This effect in turn may result in a reduction in blood flow to the optic nerve head (ONH). Nonetheless, only about 20 percent of patients that have persistent elevated IOP develop glaucomatous ONH damage.10 An apparent reason for this is that autoregulation impedes the process. As it occurs in other organs of the human body, ocular autoregulation maintains normal blood flow to the eye in response to changes in perfusion pressure by altering mechanical or chemical physiologic processes that influence the tone of blood vessel walls; thereby modifying vascular resistance. As the equation below shows, blood flow (BF) equals PP (PP, Pa — Pv), over vascular resistance (R).11
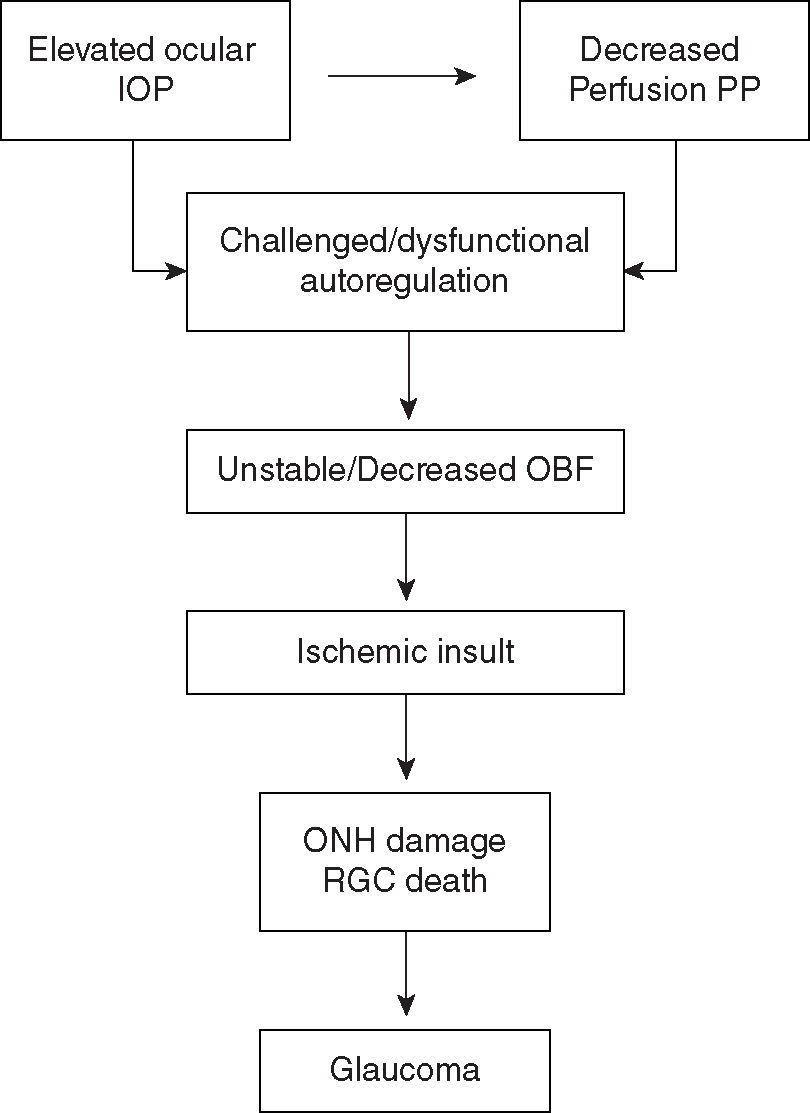
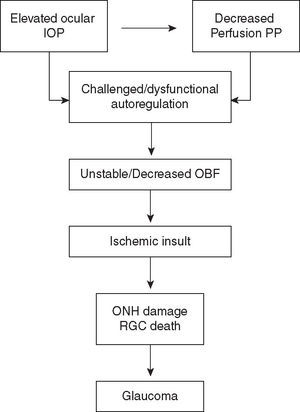
PP at the eye may also be expressed as the difference between the perfusion pressure in arteries entering the eye and perfusion pressure of veins exciting the eye. PP at the venous system exiting the eye needs to be slightly higher or equal to the IOP; otherwise a venous collapse may occur.10 Thus, an increment in venous perfusion pressure provoked by elevated IOP may result in a decrease in ocular PP. Significant elevated IOP and a resultant decrease in ocular PP may challenge or adversely affect ocular vascular regulatory function.12 Furthermore, instability in ocular blood flow (OBF) may ensue when autoregulation dysfunction is present.12 This may lead to ischemic glaucomatous damage to the ONH and retinal ganglion cell (RGC) death, which in turn, may partially lead to further regulatory dysfunction12 (Figure 2).
DiscussionCorneal biomechanicsStatistical analysis exposing the relationship between CH and central corneal thickness (CCT), and CRF and CCT in OHT and POAG eyes revealed to be proportional.3 In other words, the higher the CCT, the higher the CH and CRF. Although the analysis proved to be statistically significant, the correlation coefficient was not very strong. This may imply that CH and CRF are related to CCT but are different corneal biomechanical values to assess ocular rigidity. Besides this relationship showing a positive effect, data also demonstrates that CH and CRF values tend to be greater in OHT patients. Shah et al.3 demonstrated that in a group of 216 eyes with POAG and 199 with OHT, the latter group had statistically significant higher CH and CRF values. Sullivan-Mee et al.13 also revealed in their study that OHT subjects had higher mean CH and CFR values than the POAG group.
In addition, a relationship of CH and CRF with the development and progression of glaucoma is seen. Luce and Taylor6 state that the Ocular Hypertension Treatment Study (OHTS), and other studies on the subject, point out the significance of CCT in diagnosing and managing the progression of glaucoma. Data of these studies rightfully imply that low CCT may be a risk factor for the development and the progression of glaucoma. Likewise, studies assessing CH and CRF values obtained with the ORA insinuate this type of relationship. Anand et al.14 found in their study that asymmetric POAG was associated with asymmetry in ORA measurements. Hence, lower CH values were linked to more advanced forms of POAG and had a positive correlation with advanced visual field loss. Moreover, Luce and Taylor6 found that compared to normal patients POAG patients had lower than average CH and CRF values. However, it is worth noting that in this study POAG eyes showed a negative correlation between CH and CRF values related to sustained elevated IOP. The eyes with severely elevated pressures had much lower than average CH values and higher CRF values. Alternatively, this may imply that a sustained elevated IOP in POAG results in a decreased dynamic resistance and stiffer elastic behavior of the cornea. Understandably, increased corneal stiffness can be expected at significantly elevated IOP6 since the cornea forms part of the overall structural rigidity of the eye. Utilizing Friedenwald equation for ocular rigidity in their study, Hommer et al.15 found that the corneal stiffness was significantly higher in POAG patients than in normal patients. It should be noted, however, that the structural rigidity of the eye cannot be attributed to corneal properties alone. Biomechanical properties of the sclera, choroid, Bruch's membrane, and retina also play an imperative role in the overall rigidity of the eye.15
AutoregulationAlbeit the precise mechanism is not fully understood, it is generally accepted that vascular autoregulation ensures adequate irrigation to the ONH.16 Research literature and clinical studies on OBF suggest that ocular PP decreases in response to elevated IOP and ischemic insult to the ONH may arise in the presence of autoregulatory dysfunction.16,17 Consequently, this may lead to glaucomatous ONH damage and RGC death.17–20 Research data suggest that abnormalities in lipid metabolism, primary vasospastic syndrome, and vascular endothelial dysfunction may be responsible for ocular circulatory differences and ONH susceptibility in response to elevated IOP.12,17,21
AtherosclerosisAtherosclerosis is a chronic, progressive vascular disease that may reduce the autoregulatory capacity of the optic disc.22 The occurrence of atherothrombosis in this disease may lead to impediment of BF. In a study performed on monkeys, Faraci et al.23 found that atherosclerosis increases the response of vascular endothelial cells for the activation of leukocytes and platelets. Extracellular transduction signaling provoking abnormalities in endothelial cell function appears to be the triggering mechanism for thrombus formation.24 Moreover, altered serotonin released by endothelial cells seems to play an active role in the impediment of BF. Hayreh et al.22 demonstrated in their study on atherosclerotic monkeys that the presence of serotonin during atherothrombotic pathogenesis induced vasospasm of the central retinal artery and posterior ciliary artery, consequently impairing BF. However, a cause-effect relationship between atherosclerosis and POAG has not been proven. In fact, the prospective, population-based Rotterdam Eye Study found atherosclerosis not to be a significant risk factor for POAG.25
Nevertheless, as a systemic process, atherosclerosis has recognized association with vascular conditions that have a possible correlation with POAG. For example, atherosclerosis is the most common pathologic process affecting the carotid system that leads to cerebrovascular accidents.26 It also shows a trend of high prevalence among patients with aortic valve stenosis that have experienced episodes of myocardial infarction.27 Both of these conditions have been demonstrated to have a relatively strong association with POAG.20,28,29
In brief, atherosclerosis appears to have some influence on OBF. It also appears to play a role in autoregulatory dysfunction of vascular conditions that have potential association with POAG. However, further investigation is necessary to comprehend how endothelial pathophysiology in atherosclerosis relates to ocular autoregulatory dysfunction in POAG.
VasospasmVasospasm is a reversible, exaggerated constriction or closure of blood vessels that leads to excessive reduction in BF.30,31 This excessive vasoconstriction usually results in insufficient oxygen supply to the surrounding tissue.31 Many vasospastic episodes arise secondary to underlying diseases such as prinzmetal's angina and multiple sclerosis.32 However, a primary vasospastic syndrome has also been identified in patients having such a predisposition when exposed to cold stimuli and emotional stress.33 These patients often suffer episodes of ocular vasopasm as well as other systemic vasospasmic manifestations such as cold extremities, migraine and low BP.33–35 Ocular vasospastic episodes have been related to glaucoma.33,34 Disturbances in BF to the ONH seem to occur in some glaucoma patients as a results of episodic local vasospasms.36 Moreover, systemic vasospasmic manifestations have been associated to glaucomatous deterioration.37–40 Nicolela et al.37 concluded in their study that glaucoma patients with evidence of vasospasmic effects in their extremities were more likely to show visual field deterioration after cooling stimulus than glaucoma patients without acral vasospasm. Hence, suggesting that vasospastic responses may be involved in glaucomatous progression.
Vasospasm of the brain vessels is linked to the pathogenesis of migraine.38 Migraine, a condition more commonly encountered in females, has a well documented association with glaucoma progression.39,40 In their respective studies, McKendrick et al. and the Collaborative Normal-Tension Glaucoma Study Treatment Group found that migraine is an independent risk factor for the progression of glaucomatous visual field loss.39,41 Furthermore, Drance et al.42 concluded from their visual field data collection analysis of 160 patients with normal tension glaucoma (NTG) that the occurrence of migraine in conjunction with NTG was more common in women. However, the study also concluded that gender and presence of migraine contribute separately to the risk of progression of visual field loss.42 Low BP has also been associated with vasospasm and considered a risk factor in glaucoma.33,35 However, low systemic pressure related to glaucoma may or may not be secondary to vasospasm. Pache et al.43 concluded in their study that vasospasm and low BP may be separate risk factors for glaucomatous damage. Nonetheless, it is a general agreement that non-physiologic nocturnal systemic pressure dipping causing marked circadian fluctuations in ocular PP is linked to vasospasm and glaucoma progression.44–46
This evidence suggests that vasospasm may represent a particular way by which glaucomatous damage can occur. Seemingly, an environment of dysregulated blood flow may result from these episodes, thus putting the ONH at a greater risk of ischemic damage.34 Furthermore, vasospasm may play a more significant role in POAG ONH damage than atherosclerosis. Atherosclerotic patients tend to overcome with more proficient autoregulation function the challenges of ocular perfusion.18 OBF fluctuates more dramatically in vasospastic patients due their susceptible hyper-responsiveness to cold stimuli and emotional stress, which may as well lead to reperfusion damage to the ONH.18,33,47
Vascular endothelial dysfunctionEndothelial vascular dysfunction is also implicated in the autoregulatory pathophysiology associated to POAG.47 Endothelial biochemical alterations initiated by oxidative stress are known to alter the vascular tone and destabilize vascular regulation function.48 Nitric oxide (NO) and endothelin-1 (ET-1) are the endothelial vasoactive mediators that sustain the physiological balance between vasodilator and vasoconstrictor pathways, respectively.49,50 NO is made available via endothelial cell synthesis and endothelin-B receptor (ETB) stimulation.12 ETB receptors activity is triggered through interaction with ET-1 that is synthesized and released by endothelial cells. ET-1 interacts primarily with endothelin-A receptor to mediate physiological vasoconstriction. A decrease in the biosynthesis and/or bioavailability of NO and an excess of ET-1 causes altered vasoreactivity (increased vasoconstriction), which leads to an imbalance in basal vascular tone.47,51
A number of vascular diseases that potentially contribute to the development and progression of POAG are associated with endothelium-dependent altered vasoreactivity.12 Dyslipidemia for example, has been associated with inhibition of endothelial NO signaling in many organs including the eye. Kawakami et al.52 demonstrated in a study performed in mice that apolipoprotein CIII in hyperlipidemia impairs insulin uptake by endothelial cells, necessary for the production of NO. A similar study demonstrated that mice lacking nitric oxide synthase (NOS), the enzyme present in endothelial cells that mediates the conversion of the amino acid L-argenine to NO, had hyperlipidemia.53 Likewise, faulty ET-1 activity provoking pathological vasoconstriction has been identified in conditions such as hypertension and diabetes.54,55
A series of studies have investigated the role of endothelial mediators in POAG. It has been demonstrated that intravenous administration of ET-1 reduces significantly retinal blood flow.56 From their study based on IOP increments in living isolated rat retinas, Rigosi et al.57 suggest that prolonged or recurrent IOP elevation may induce ET-1-mediated retinal capillary dysregulation contributing to neuronal damage overtime.
In a study evaluating glaucomatous field progression in 31 patients with POAG, specific radioimmunoessay revealed that 16 of these patients had increased ET-1 plasma levels.58 Another study observing the effect of dual endothelin receptor blockage on OBF reveals that oral administration of bosetan, a dual endothelin receptor antagonist, increases ocular blood flow in patients with POAG.59
Polak et al.60 demonstrated in their study on NOS inhibition of the ocular vasculature that patients with POAG yielded significantly less percent reduction of blood flow to the ONH than healthy controls. Furthermore, the gene expression of NOS may be of significance in some glaucoma patients. Logan et al.61 found in their study a statistical significant difference in the distribution of allele frequencies of a specific NOS gene isoform, between subjects with glaucoma that had a history of migraine and control subjects. Other studies on the NOS-mediated L-arginine-NO pathway in eyes of patients with POAG suggest that the pathway is genetically defective.31,62
Altered endothelial vasoreactivity may be a determinant in the development and progression of POAG. However, the specific involvement of endothelial mediators such as ET-1, and NOS in the autoregulatory pathophysiology of POAG has yet to be completely elucidated. Nonetheless, it appears endothelial dysfunction, either primary or secondary to vascular diseases, contributes to POAG pathology.
ConclusionClearly, POAG is a progressive optic neuropathy that has a multi-factorial etiology, in which elevated IOP is the most important risk factor. Yet, research evidence and clinical experience clearly indicates that elevated IOP alone is not at work here. Research observations on altered corneal biomechanics and vascular dysregulation in association to elevated IOP suggest their possible contribution to the disease process. Comparing the two factors, however, it appears altered autoregulation function has a more positive correlation with POAG. A relatively strong association exists between decreased ocular PP precipitated by elevated IOP and decrease OBF in patients with vascular regulatory dysfunction.12 However, the exact pathophysiological mechanism of how this provokes glaucomatous damage still remains uncertain.16
Research data on corneal biomechanics appears to be a bit more contending. While results demonstrate consistent lower CH and CRF values in patients with POAG,3,13 further longitudinal studies may be needed to determine if indeed in vivo CH and CRF values are independent predictors of glaucoma susceptibility. Besides, the possibility remains that alterations in corneal biomechanics may be a resultant of POAG rather than a risk factor.6
In conclusion, POAG pathogenesis is a topic that warrants further pathophysiological studies to elucidate what risk factors other than elevated IOP are relevant for the development and progression of the disease. Certainly, vascular dysregulation and alterations in corneal biomechanics are factors to consider in POAG pathogenesis. Hopefully, future glaucoma research will determine more specific variables which may further clarify their correlation with the disease process.
Conflict of interestsThe authors have no conflict of interests.