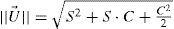To evaluate postoperative clinical outcomes, and corneal High Order Aberrations, among eyes with hyperopia up to +5 D of spherical equivalent, that have undergone LASIK treatments using the SCHWIND AMARIS laser system.
MethodsAt six-month follow-up, 100 eyes with preoperative hyperopia or hyperopic astigmatism up to +5 D of spherical equivalent were retrospectively analysed. Standard examinations, pre- and postoperative wavefront analysis with a corneal-wavefront-analyzer (OPTIKON Scout) were performed. Aberration-Free aspheric treatments were planned with Custom Ablation Manager software and ablations performed using the SCHWIND AMARIS flying-spot excimer laser system (both SCHWIND eye-tech-solutions). LASIK flaps were created using a LDV femtosecond laser (Ziemer Group) in all cases. Clinical outcomes were evaluated in terms of predictability, refractive outcome, safety, and wavefront aberration.
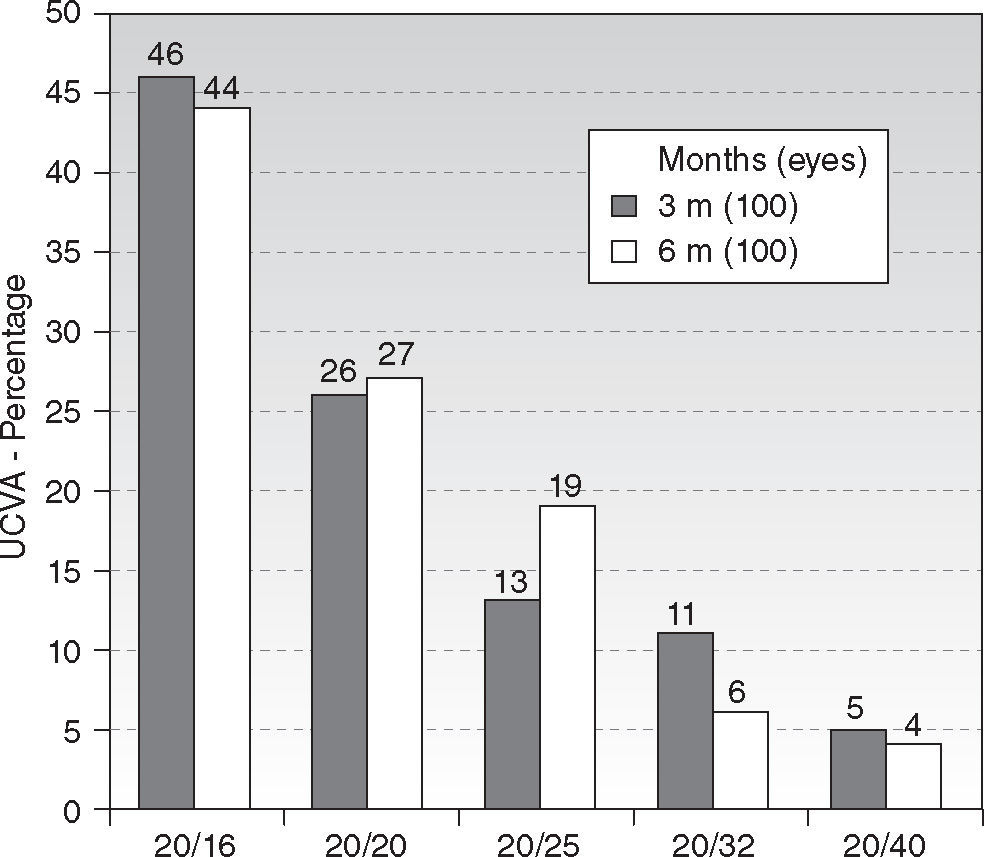
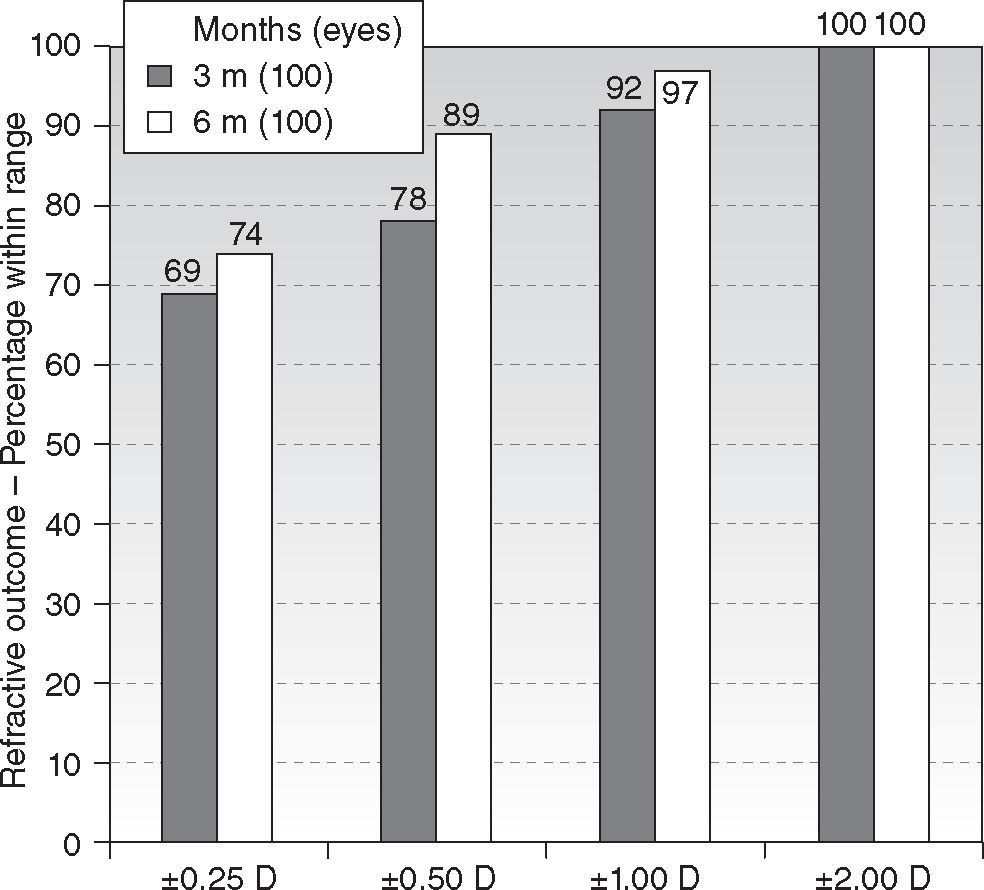
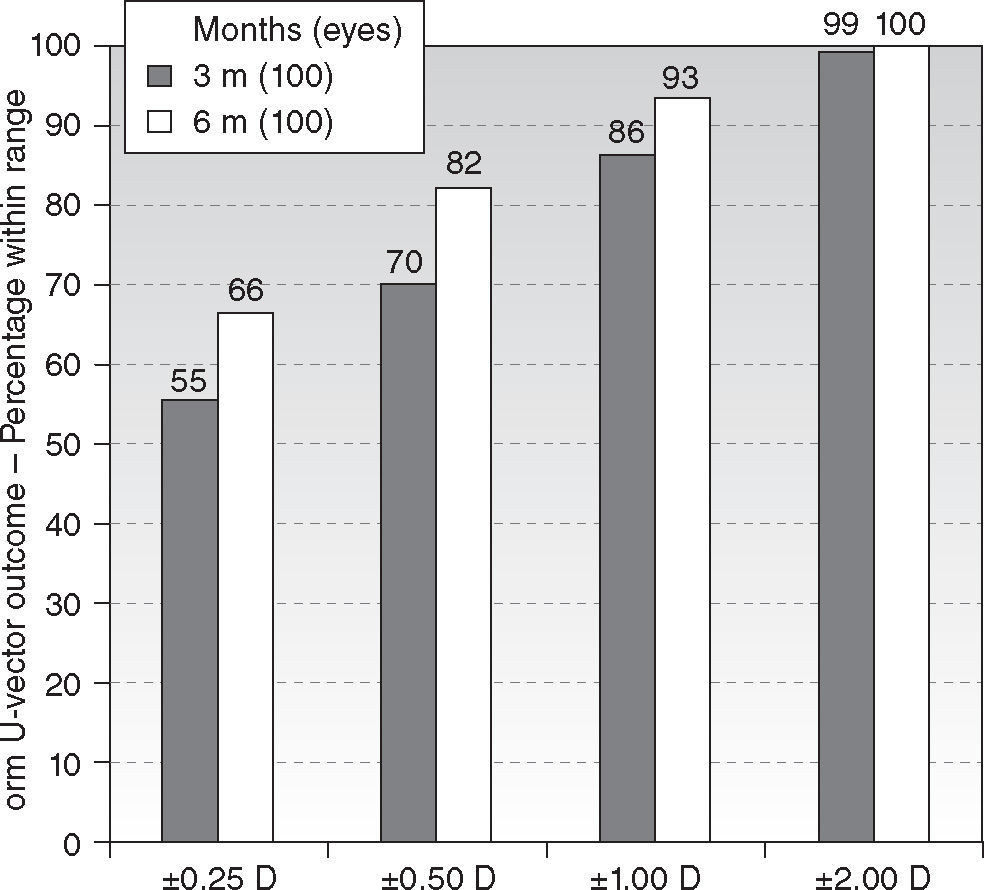
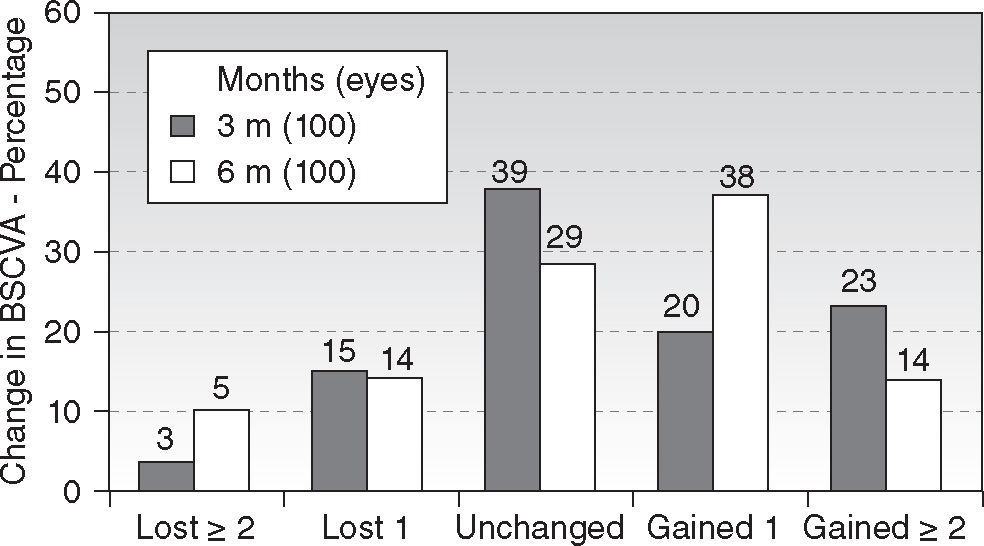
ResultsAt six month, 90 % of eyes achieved ≥ 20/25 UCVA and 44 % achieved ≥ 20/16 UCVA. Seventy-four percent of eyes were within ± 0.25D of spherical equivalent and 89 % within ± 0.50D, with 94 % within 0.50D of astigmatism. Mean spherical equivalent was −0.12 ± 0.51D and 0.50 ± 0.51D for the astigmatism. Fifty-two percent of eyes improved BSCVA vs. only 19 % losing lines of BSCVA. Predictability slope for refraction was 1.03 and intercept +0.01 D. On average, negative corneal spherical aberrations were significantly increased by the treatments, no other aberration terms changed from pre- to postoperative values.
ConclusionsLASIK for hyperopia and hyperopic astigmatism with SCHWIND AMARIS yields very satisfactory visual outcomes. Preoperative refractions were postoperatively reduced to subclinical values with no clinically relevant induction of corneal HOA.
Evaluar los resultados clínicos posoperatorios, aberraciones corneales de orden superior, entre ojos con una hipermetropía de hasta 5 dioptrías de equivalente esférico, previamente sometidos a tratamientos LASIK con el sistema de láser SCHWIND AMARIS.
MétodosTras 6 meses de seguimiento, se analizaron de manera retrospectiva 100 ojos con hipermetropía o astigmatismo hipermetrópico preoperatorio de hasta 5 dioptrías de equivalente esférico. Se llevaron a cabo exploraciones estándar, análisis de frente de onda preoperatorio y posoperatorio con un analizador de frente de onda corneal (OPTIKON Scout). Se diseñaron tratamientos asféricos sin aberraciones con el software Custom Ablation Manager y se realizaron ablaciones utilizando el sistema de láser excímero de punto flotante SCHWIND AMARIS (ambas tecnologías son de SCHWIND Eye-tech Solutions). En todos los casos, los colgajos de LASIK se crearon utilizando un láser de femtosegundo LDV (Ziemer Group). Los resultados clínicos se evaluaron a nivel de previsibilidad, resultado de refracción, seguridad y aberración de frente de onda.
ResultadosAl cabo de 6 meses, el 90 % de los ojos alcanzaron agudeza visual sin corrección (AVSC) > 20/25 AVSC (UCVA) y el 44 % alcanzaron > 20/16 AVSC. El 74 % de los ojos se encontraban en +0,25 dioptrías de equivalente esférico y el 89 % en ±0,50 dioptrías, con el 94 % en 0,50 dioptrías de astigmatismo. La media del equivalente esférico fue de −0,12 ± 0,51 dioptrías y de 0,50 ± 0,51 dioptrías para astigmatismo. El 52 % de los ojos vieron mejorada la mejor agudeza visual con gafa (MAVCG) (BSCVA) frente a solamente un 19 % que perdieron líneas de MAVCG. La pendiente de previsibilidad de refracción fue de 1,03 y la ordenada en el origen, de +0,01 dioptrías. Como promedio, la aberración esférica negativa de la córnea fue significativamente incrementada por los tratamientos y ningún otro término de aberración cambió entre los valores preoperatorios y posoperatorios.
ConclusionesUtilizar LASIK para la hipermetropía y el astigmatismo hipermetrópico con SCHWIND AMARIS produce unos resultados visuales muy satisfactorios. Las refracciones previas a la operación se vieron reducidas a valores subclínicos después de la operación, sin inducción clínicamente relevante de aberraciones de orden superior de la córnea.
The introduction of the excimer laser for refractive surgery has opened up the possibility to change the corneal curvature to compensate for refractive errors of the eye. In the case of small spot hyperopic laser in situ keratomileusis (H–LASIK), excimer laser systems produce a steepness of the cornea by ablating mainly at the periphery of the cornea.
H-LASIK treatments induce aberrations.1 Studies 2,3 attempted to determine the changes in corneal asphericity after H-LASIK, and found a extreme corneal prolateness, indicating large amounts of induced negative spherical aberrations. This is predominantly caused by the loss of efficiency4–7 at the periphery and the biomechanics of the cornea, and has been described previously by other authors.8,9
The pitfalls of H–LASIK are historically important and should be mentioned, as the main problems in decentrations, decreases in best corrected visual acuity, very high frequency of retreatments, frequent residual refractive error, induction of astigmatism and induction of high levels of corneal aberrations, specifically, and spherical aberration.
Later studies on hyperopic treatments with excimer lasers also suggested an increase in negative spherical aberration.10,11
By comparison of the intended and achieved topographical changes after H-LASIK surgery, de Ortueta et al.12 obtained information as to whether the corneal power was changed as calculated, thus providing help in optimizing the ablation profiles (either in terms of nomogram adjustments or more sophisticated optimization algorithms like multifocal algorithms). Additionally, actual under- or overcorrection can be differentiated from under- or overcorrection due to wrong intended correction in this way.
A study by O’Brart et al.13 analyzing hyperopic LASEK using a Munnerlyn based classical profile and a 7 mm optical zone with a total treated zone of 9 mm demonstrated that the induced aberrations were lower than with the Munnerlyn profile in LASIK. A recent study by de Ortueta et al.,14 using aspheric aberration neutral profiles showed that induced aberrations are less than in previous publications. This study was undertaken to evaluate postoperative clinical outcomes and High Order Aberrations (HOA), among eyes with hyperopia up to +5 D of spherical equivalent, that underwent LASIK treatments using the SCHWIND AMARIS laser system.15
MethodsStudy Design and Patient Eligibility CriteriaThis is a retrospective analysis of consecutively operated eyes by a single surgeon (MCA). The first consecutive 100 eyes (50 patients) with preoperative manifest hyperopia or hyperopic astigmatism treated using the AMARIS “aberration neutral” (Aberration-Free™) aspheric ablation profiles were retrospectively analysed. In fact, there was no nomogram check before commencing the study, and there were no eyes excluded because lost to follow-up or because they required re-treatments.
This is the first series of eyes operated with this particular treatment algorithm. Due to its retrospective nature, no investigational review board or other regulatory oversight was required. The level of statistical significance was taken as P < .05.
Inclusion criteria for review were preoperative hyperopia or hyperopic astigmatism targeted for emmetropia, best spectacle corrected visual acuity (BSCVA) ≥ 20/25 (logMAR ≤ +0.1), < 0.75 μm root mean square (RMS) of the HOA of the corneal wave aberration for 6-mm diameter, and successful completion of 6-month follow-up. All 50 patients (100 eyes) fulfilled the criteria for being taken in the retrospective analysis, and so no one was excluded.
Laser DescriptionAll ablations were non-customised based on “aberration neutral” profiles and calculated using the ORK-CAM software module. Aspheric aberration neutral16 (Aberration-FreeTM17) profiles are not based on the Munnerlyn proposed profiles,18 and go beyond that by adding some aspheric characteristics to balance the induction of spherical aberration,9,19 (prolateness optimization20,21).
The aberration neutral (Aberration-FreeTM) profile is aspherical-based,22–24 including a multidynamic aspherical transition zone,23 aberration and focus shift compensation due to tissue removal,17 pseudo-matrix based spot positioning,23 enhanced compensation for the loss of efficiency7,25, and intelligent thermal effect control;26–28 all based on theoretical equations validated with ablation models and clinical evaluations.
A 6.7 mm central corrected ablation zone was used in all eyes with a variable transition size automatically provided by the laser related to the planned refractive correction (6.9 mm to 9.2 mm). The ablation was performed using the AMARIS29 excimer laser (SCHWIND eye-tech-solutions, Kleinostheim, Germany) which is a flying-spot laser using ablative spot voxels locally considered through a self-constructing algorithm. In addition, there are a randomized flying-spot ablation pattern and controls for the local repetition rates to minimize the thermal load of the treatment.30
Ablations were centred on the corneal vertex (CV) using the pupillary offset, i.e., the distance between the pupil centre and the normal CV measured by videokeratoscopy (Keratron Scout topographer, Optikon 2000 s.p.a., Rome, Italy). The measurement was performed under photopic conditions of 1500 lux, similar to the conditions under the operating microscope. This method was suggested and described by de Ortueta and Arba Mosquera31 and comparatively tested by Arbelaez et al.32 The excimer laser allows for modification of the ablation centration from the pupillary centre with an offset by entering either X and Y Cartesian values or R and u polar values in a regular treatment. The measurement of the pupillary offset was translated into the treatment planning as polar coordinates to be manually entered into the excimer laser computer.
The AMARIS laser system works at a true repetition rate of 500 Hz and produces a beam size of 0.54 mm Full-Widthat-Half-Maximum (FWHM) with a superGaussian ablative spot profile.33,34 High-speed eye-tracking (pupil and limbus tracker with cyclotorsional tracking35) with a 1050-Hz acquisition rate is accomplished with a 3-ms latency time.36
AMARIS technology is treating the astigmatism not by superimposing the spherical component with positive or negative cylindrical patterns, nor is it treating either positive or negative cylindrical patterns depending on the sign of the spherical component. Rather, the system analytically creates an aspherotoric volume, which is then discretised into laser pulses sorted spatially and temporally in a pseudo-random fashion. In that way, there is no sequentialization of the sphere and astigmatic components (nor the other way round), but both components are progressively and simultaneously corrected.
Further the AMARIS system has demonstrated its abilities for sparing corneal tissue37 as well as a very even and gently ablation preserving preoperative bilateral symmetry.38
Preoperative TestingSix-months follow-up was available in 100 of these eyes (100 %), and their preoperative data were as follows: mean manifest spherical equivalent refraction +3.02 ± 2.06 D (range, +0.13 to +5.00 D); mean manifest astigmatism magnitude 1.36 ± 1.61 D (range, 0.00 to 5.00 D). In all eyes, we measured corneal topography39 and derived corneal wavefront40,41 analyses (Keratron-Scout, OPTIKON2000, Rome, Italy), manifest refraction, and uncorrected and best spectacle-corrected Snellen visual acuity42 (UCVA and BSCVA respectively). Results are reported for the measurements performed preoperatively and at three and six months after surgery.
We acquire, under non pharmacologically dilated pupils, non-cycloplegic conditions, and natural dim light conditions (to avoid pharmacologically-induced pupil shifts43–45), 3 aberrometries (Ocular Wavefront Analyzer, SCHWIND eye-tech-solutions GmbH & Co.KG, based on Irx3, Imagine Eyes, Orsay, France) and objective refractions for each eye of the patient.46 In order to minimize the potential accommodative response of the patients, we ask them to “see-through-thetarget” instead of “looking at the target.” In this way, patients do not try to get a sharp image from the +1.5 D fogged target, since they were instructed to see-through-the-target. From those aberrometries, we calculate the mean, and select the most representative one (the aberrometry map with the highest similarity to the mean).
We assessed subjective refraction based upon non-pharmacologic and non-cycloplegic conditions, under natural photopic illumination. We use the objective refraction provided by the aberrometer analyzed for a sub-pupil of 4 mm diameter, as starting refraction for this step. This is particularly useful for determining the magnitude and orientation of the astigmatism.47,48 We measure manifest refraction, UCVA and BSCVA42. Further rules that we impose for accurately determining the manifest subjective refractions among equal levels of BSCVA are: taking the measurement with the most positive spherical equivalent (unmasking latent hyperopia), if several of them are equal in terms of spherical equivalent, we choose the measurement with the least amount of astigmatism (reducing the risk of postoperative shifts in the axis of astigmatism).
Surgical Technique and Postoperative TreatmentFor corneal and conjunctival anaesthesia, two drops of proparacaine HCl 0.5 % (Aurocaine®, Aurolab, Madurai, India) were instilled three times before shifting the patient to the Operation Theatre. All flaps were created using a LDV femtosecond laser (Ziemer Group) with superior hinges, 110 μm nominal flap thickness, and 9.0 mm or 9.5 mm nominal flap diameter. A 9 mm marker was used to ensure centration and to be able to objectively measure the amount of applanation. Online pachymetry49 was performed before and after lifting flap (stromal bed thickness) with the integrated optical coherence pachymeter (Heidelberg Engineering, Heidelberg, Germany). After lifting the flap, ablation was performed preserving flap edges, hinge, and inner face of the flap disk from being ablated. Contact lens was applied at the end of surgery (Biomedics 55 evolution, Ocular Sciences, Cooper Vision, Hamble, UK) in eyes with ‘achieved’ flap thickness less than 110 microns to avoid flap displacements, dislocations or striae.
One eye drop Tobradex (Alcon Inc, USA) 3 times a day was used for 1 week along with Oasis soft plugs extended duration (6404 Glendora CA) and preservative free artificial tear drops during the first three months.
Outcome MeasuresSafety and effectiveness metricsEfficacy: We analysed the number of eyes with postoperative UCVA in scale from 20/16 to 20/40.
Refractive Outcome: Every dioptric power can be represented by means of a point in Euclidean three-dimensional space. The relationship between visual acuity and refractive power can be represented by closed surfaces of constant visual acuity in symmetric dioptric power space. The power of these three component lenses may be interpreted as (x,y,z) coordinates of a vector representation of the power profile (the U-vector).
The U-vector50 can be represented as a vector in the 3-dimensional double angle astigmatism space.51 The norm of this vector correlates to the dioptric blur and to visual acuity52 and can be formulated as:
We analysed the mean values of spherical equivalent and astigmatism, and the number of treatments with postoperative refraction within 0.25, 0.50, 1.00 and 2.00 D, as well as, the number of treatments with norm of postoperative U-vector within 0.25, 0.50, 1.00 and 2.00 D. We assessed the statistical significance of the postoperative status compared to the preoperative baseline using paired Student’s T-tests.
Safety: We analysed differences in BSCVA postoperative compared to the preoperative baseline for each eye. We assessed the statistical significance of the postoperative status compared to the preoperative baseline using paired Student's T-tests.
Predictability: We plotted scattergrams for the achieved spherical equivalent and astigmatism corrections versus the attempted ones (both at the corneal plane, where the ablation procedure occurs). We analysed slope and intercept of the correlations. We assessed the statistical significance of the correlations using Student's T-tests, the Coefficient of Determination (r2) was used, and the significance of the correlations has been evaluated considering a metric distributed approximately as t with N—2 degrees of freedom where N is the size of the sample.
Changes in corneal wavefront aberrationOptical errors centred on the line-of-sight, representing the Wavefront Aberration, are described by Zernike polynomials53 and coefficients in OSA standard,54 and analysed for a standardised diameter of 6 mm for corneal wavefront.
We analysed mean values and differences, in each of the 30 HOA terms of the Zernike expansion to the seventh order, postoperative compared to the preoperative baseline for each eye. We assessed the statistical significance of the postoperative status compared to the preoperative baseline using paired Student's T-tests.
ResultsPatient Demographics and Adverse eventsThe mean age at the time of the surgical intervention was 37 years (from 21 to 59). Forty-six percent of the patients were males and 54 % females.
Neither adverse events nor complications were observed intra- or postoperatively. In the 6 months follow-up no retreatment was performed.
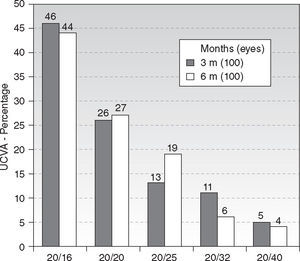
Efficacy and Refractive OutcomeAt six-month, UCVA was 20/16 or better in 44 % of the treatments (44 eyes), and 20/25 or better in 90 % (90 eyes) (Figure 1). The efficacy index scored 0.89. There were no statistically significant differences between the 3 and 6 months follow-ups.
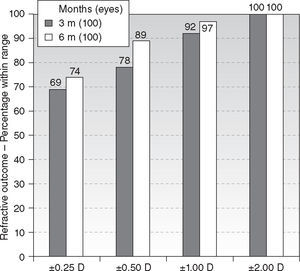
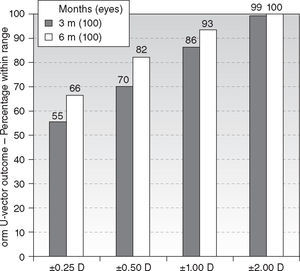
Six-month postoperatively, mean residual spherical equivalent was −0.12 ± 0.51D (range, −0.75 to + 1.75D) (P < .0001) and mean residual astigmatism 0.50 ± 0.51D (range, 0.00 to 1.75D) (P < .0001). Eighty-nine percent eyes (89) were within ± 0.50D of attempted spherical equivalent correction (Figure 2), with ninety-four percent eyes (94) within ± 0.50D of attempted astigmatic correction, ninety-seven percent eyes (97) within 1.00D, and ninety-three percent eyes (93) within 1.00D of the norm of the residual U-vector (Figure 3). There were no statistically significant differences between the 3 and 6 months follow-ups.
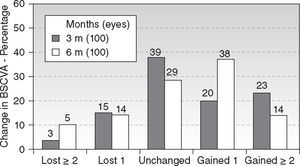
Regarding safety, 14 % of eyes (14 eyes) gained two lines of BSCVA (Figure 4) (P < .01). The safety index scored 1.10. There were no statistically significant differences between the 3 and 6 months follow-ups.
The achieved refractive change, defined as the vectorial difference in the astigmatism space of postoperative and preoperative refractions (incorporating spherical equivalent and astigmatism) at the corneal plane, was significantly correlated with the intended correction (r2 = .90, P < .0001). Regression slope was 1.03, very close to the ideal correction. The achieved changes in astigmatism was significantly correlated with the intended correction (r2 = .88, P < .0001). The regression slope of 0.91 indicates slight undercorrections. There were no statistically significant differences between the 3 and 6 months follow-ups.
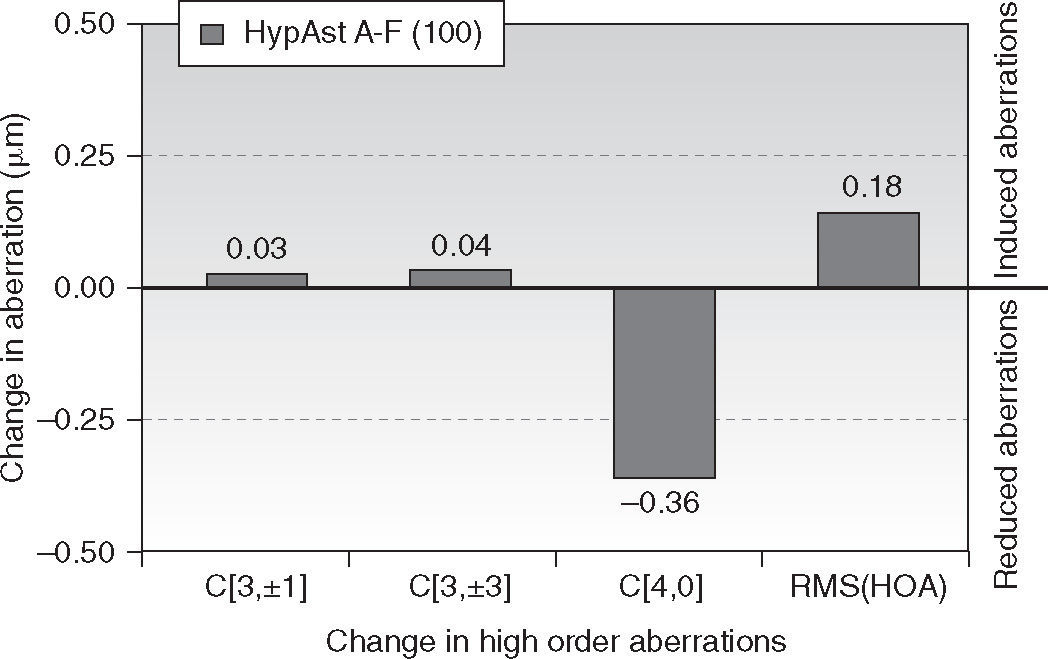
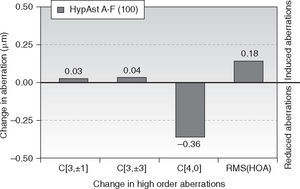
Changes in corneal wavefront aberrationAt 6-month follow-up, corneal trefoil aberration increased by 0.03 μm (P = .1), corneal coma aberration increased by 0.04 μm (P = .1), corneal spherical aberration decreased by −0.36 μm (P < .0001), and corneal RMS of the HOA increased by 0.18 μm (P < .05) (all at 6 mm anaylsis diameter).
DiscussionIn this study, results were good and promising. We can conclude that CAM Aberration-Free Hyperopic treatments
produce safe and predictable ablations on the cornea. From post-op VA, we have got 90 % eyes in UCVA 20/25 or better and more than 50 % eyes improved their pre-op BSCVA, due to the minimum aberrations induction by the ORK-CAM aspherical profile. From the refractive power change (in terms of achieved correction), we can see that both the sphere and cylinder corrections are quite accurate, predictable and stable from the first month follow-up.Advanced H-LASIK treatments with AMARIS system yield excellent outcomes. Refractions were reduced to subclinical values. Mean spherical equivalent was −0.12 ± 0.51D and astigmatism 0.50 ± 0.51D. Seventy-four percent of eyes were within ± 0.25D of emmetropia. Fifty-two percent of eyes gained lines of BSCVA. In Aberration-Free profiles the induction of aberrations fell well below the clinical relevance (< 0.25 D). Only negative spherical aberration was significantly induced, but negative spherical aberration increases depth of focus, and thus it may help when approaching presbyopia.
In these 100 treatments no retreatments were performed in the first 6 months of follow-up, and after this timeline 5 retreatments were performed due to undercorrection or hyperopic regression. Overall, in our 30-month experience with AMARIS, of 348 simple hyperopia or compound hyperopic astigmatism treatments performed with AMARIS with more than 6 months follow up, only 15 retreatments were performed (4 %). Even with today's technology, retreatments are more frequent in hyperopia. The reasons for the unexpectedly low retreatment ratio in our hyperopic series might be associated to several factors:
On the one hand we do not base our corrections on the manifest refraction or on the cycloplegic one. We use the objective refraction provided by the aberrometer analyzed for a sub-pupil of 4 mm diameter, as starting refraction, which is particularly useful for determining the magnitude and orientation of the astigmatism, and then we push the refraction under test to the the most positive spherical equivalent (unmasking latent hyperopia) consistent with the highest BSCVA scored by the patient. This does not mean, that objective refraction based on wavefront measurement is more effective compared to subjective or cicloplegic refraction, rather that it is a systematic starting refraction for the subjective refraction analysis. Further, if several refraction datasets are equal in terms of spherical equivalent, we choose the measurement with the least amount of astigmatism (reducing the risk of postoperative shifts in the axis of astigmatism).
Moreover, we centre our treatments not on the pupil or on the 1st Purkinje image, but on an objective assessment of the corneal vertex as determined by the videokeratoscopy. This offset for the treatment is actually based on the vectorial mean of 4 well acquired topographies per treated eye. The technological improvements of the AMARIS system may also help at that aim, since it incorporates a six-dimensional eye-tracking subsystem which tracks not only pupil movements, but rolling movements of the eye, as well as, torsional movements and movements along the propagation axis of the laser.
Finally, we used the objective refraction provided by the aberrometer analyzed for a sub-pupil of 4 mm diameter, as starting refraction for subjective refraction. This is particularly useful for determining the magnitude and orientation of the astigmatism. Further, we selected the measurement with the most positive spherical equivalent (unmasking latent hyperopia).
The results report an improvement of BSCVA of 2 or more lines in 14 % of eyes. It is hard to understand why after the surgical correction of hyperopia, with less magnification of retinal image, compared to spectacle and without evident improvement in ocular aberration, we found an improvement in the resolving power of the eye. The minimum aberrations induction can justify no reduction of BSCVA, but not such improvement.
Although having measured HOA of the whole eye, we analyzed only the HOA of the cornea. This has several reasons: on the one hand, we used ocular aberrometry only to derive refraction, i.e. low order aberration at 4-mm diameter, so we did not have always large analysis diameters available (6-mm or more) for ocular wavefront (either pre- or postoperatively); on the other hand we were not interested on the magnitude of the aberrations, but rather on the change of aberrations.
Papers in which direct comparison of induced corneal and ocular wavefront aberrations over the same sample was studied, showed that the induction of anterior corneal aberrations was always, at least, as high as the induction of ocular wavefront aberrations for the entire eye. Marcos et al.55 found that ocular and corneal aberrations increased statistically significant after LASIK myopia surgery, by a factor of 1.92 (ocular) and 3.72 (corneal), on average. They found a good correlation (r = 0.97; P < .0001) between the aberrations induced in the entire optical system and those induced in the anterior corneal surface. However, anterior corneal aberrations increased more than ocular aberrations, suggesting also changes in the posterior corneal surface. Lee et al.56 found that after laser refractive surgery, anterior corneal aberration and ocular aberration increased equally and showed statistically significant correlations. They found no statistically significant differences of internal optics aberration values in coma, spherical aberration, and RMS for HOA. Arbelaez et al.16 found that comparing corneal and ocular aberrations, the amount of induced aberrations was very similar for spherical aberration and coma. For the RMS for HOA corneal induced aberrations were moderately higher, despite not statistically significant, than ocular induced aberrations. Arba-Mosquera and de Ortueta57 found that induced corneal and ocular spherical aberrations were correlated in a statistically significant manner, with ocular spherical aberration being induced at a rate of half of the induced corneal spherical aberration in patients preoperative and in the same patients after LASIK for myopic astigmatism.
Hyperopic Aberration-Free Treatments with the SCHWIND AMARIS are safe and very predictable. Results were achieved without applying additional nomograms. 6-month follow-up time shows the good performance of the system. To evaluate the long-term stability further follow up is necessary. However, de Ortueta et al.58 determined good refractive and topographic stability after H-LASIK already from the 3 month of follow-up with little to no regression occurring up to 36 months.
De Ortueta12 found after H-LASIK with the ESIRIS system a good predictability, with 92 % of the eyes (61) having a postoperative refraction within ± 0.50 D of the attempted correction. Moreover, as expected, the achieved refractive change was significantly correlated with intended refractive correction (r2 = 0.91), and was very close to the ideal correction. These values are similar to the ones in this study for 1-year follow-up (95 % within 0.50 D).
The conventionally accepted limits for H-LASIK (about 5D of spherical equivalent) are lower than the ones accepted for myopic LASIK (up to about 10D if the residual stromal bed is thicker than 250–300 μm). One of the causes is that the induction of aberrations per achieved diopter is higher in hyperopic treatments.9 The centration of refractive surgery remains also controversial. The offset between the corneal vertex and pupil centre is higher in hyperopic eyes, with a nasal fixation in most of the cases. This is also a problem to take into account.59 Hyperopic eyes are usually short in axial length, showing higher values for the angles alpha, kappa, and lambda. This also causes an offset between the corneal vertex and the pupil centre of higher magnitude than in myopic eyes, making it difficult to decide where to centre the refractive procedure.
It is possible that the ‘improved’ results are simply due to treatment centration, rather than to the new AMARIS technology. New ablation patterns, which minimize the induction of aberrations, will allow us to perform H-LASIK more predictably and safely and may allow us in the future to treat higher hyperopia. At this aim, the limits to the steepness of the central cornea with regards to quality of vision and tear film stability shall be carefully considered.
Reducing the induction of aberrations after H-LASIK will possibly allow us to treat higher hyperopia, however further studies are necessary to confirm this hypothesis.
Conflict of interestsAuthors Arbelaez and Vidal have no proprietary interest in the materials presented herein.
Arbelaez receives travel expenses from SCHWIND eye-tech-solutions.
Arba-Mosquera is employee of SCHWIND eye-tech-solutions.