Visual evoked potentials (VEPs) provide important diagnostic information related to the functional integrity of the visual pathways. The aim of this study was to establish normative values of different components of pattern reversal VEPs on Iranian normal adult subjects.
MethodsMonocular and binocular pattern reversal VEPs were recorded on 59 healthy participants (22.55±3.79 years old) using the Roland RETI system for two check sizes of 15 and 60min of arc. The measured VEP components were the latencies of N75, P100, N135 and amplitude of N75–P100.
ResultsRepeated measures ANOVA showed that viewing eye condition has a significant impact on the amplitude of N75–P100 (P<0.001, F=13.89). Also, the effect of check size on the latencies of N75, P100, N135, amplitude of N75–P100 (P≤0.010), as well as the intraocular difference of P100 latency and amplitude N75–P100 (P=0.007) was significant. More specifically, the amplitude of N75–P100 in both check sizes significantly differed between gender groups (P<0.023).
ConclusionAccording to the results of this study, VEPs components are affected by the stimulus size, monocular and binocular recording conditions and gender. Therefore, it is necessary to determine the normative values of VEPs in each population, so that the results could be used in clinical studies.
Los potenciales evocados visuales (PEV) aportan información diagnóstica importante relacionada con la integridad funcional de las vías visuales. El objetivo de este estudio fue establecer los valores normativos de los diferentes componentes de la reversión del patrón de PEV en adultos normales iraníes.
MétodosSe registraron los valores de reversión monocular y binocular del patrón de PEV en 59 participantes sanos (22,55±3,79 años), utilizando el sistema Roland RETI para dos tamaños de comprobación de 15 y 60min de arco. Los componentes medidos de PEV fueron las latencias de N75, P100, N135 y la amplitud de N75-P100.
ResultadosLa repetición de las medidas ANOVA reflejó que la situación del ojo de visión tiene un impacto significativo sobre la amplitud de N75-P100 (P<0,001, F=13,89). De igual modo, el efecto del tamaño de la comprobación de las latencias de N75, P100, N 135, la amplitud de N75-P100 (P ≤ 0,010), así como la diferencia intraocular de la latencia de P100 y la amplitud de N75-P100 (P=0,007) fue significativo. Más específicamente, la amplitud de N75-P100 en ambos tamaños de comprobación difirió considerablemente entre los grupos de sexos (P<0,023).
ConclusiónCon arreglo a los resultados de este estudio, los componentes de los PEV se ven afectados por el tamaño del estímulo, las situaciones del registro monocular y binocular, y el sexo. Por tanto, es necesario determinar los valores normativos de los PEV en cada población, para poder utilizar los resultados en estudios clínicos.
Visual evoked potentials (VEPs) are electrophysiological potentials of the primary visual cortex in response to visual stimuli, which can be amplified and averaged from the signals of electro-encephalic activity.1 VEPs provide crucial diagnostic information related to the functional integrity of the visual system from the retina to the occipital cortex2–7 and they are sensitive to subclinical optic nerve and macular disorders.6 VEPs can be used as objective and noninvasive methods of evaluating the visual system in children and non-cooperative patients.2
There are two principal stimulus protocols for recording VEPs including flash and pattern VEPs.1 Pattern VEPs are classified to onset–offset pattern VEPs and pattern reversal VEPs.1,8 Because pattern reversal VEPs have low waveform variability between and within subjects, they are widely used for clinical and research purposes.1,9 The components of pattern reversal VEPs waveform are the negative N75 wave, large positive P100 wave and negative N135 wave.1 The P100 latency has very low variability between individuals and is very sensitive to the integrity of neural pathways as a small defect can prolong the time of peak of P100 wave.10–13
In addition to visual pathway defects that can influence the waveform, physiological factors including age,14,15 gender,16 degree of attention and cooperation,17,18 and stimulus parameters1,3,14 such as size and contrast may affect the waveform of VEPs. Longer latency of P100 occurs in the elderly and in males than females.14,16 Numerous studies have assessed the maturation of visual system using VEPs and have observed earlier maturation for larger check sizes than smaller ones.19–22 The latency values of VEPs for large checks and small checks reached adult levels respectively at 1 and 5 year of age20 and the amplitudes of VEPs were not affected by age after 1 years.22,23 Moreover, most studies have reported that the maturation of the visual pathway occurs around 4 years of age.20,22,24
Besides considering physiological factors and stimulus parameters, the results of VEPs should be compared with normal data so as to interpret the normality/abnormality of VEPs. The International Society for Clinical Electrophysiology of Vision (ISCEV) recommends that in each VEPs laboratory normative values be determined using its own stimuli and recording parameters.1 Several studies have determined the normative values of VEPs parameters at their regional level and have evaluated factors affecting VEPs waveforms.16,25–27 Given the importance of establishing the normative values of VEPs in each VEP laboratory, the aim of this research was to identify normative values of pattern reversal VEPs parameters, using the Roland RETI system, on normal adult subjects – living in northeastern Iran.
Material and methodsParticipantsA total 59 students of Mashhad University of Medical Sciences (32 females and 27 males) participated in this study. Since the morphologic and metabolic maturation of the visual cortex completes at the age of 18 and VEPs components are not affected after this age,19,20 we selected participants who were 18 years and older (22.55±3.79 years old). All participants had normal general and ocular health and their best-corrected visual acuity was 6/6 or better in each eye at 6m. The procedures of this study were approved by the local Human Research Ethics Committee and were in conformity with the Helsinki Declaration. Also, the written consent form was obtained from the participants.
ProceduresPattern reversal visual evoked potentials (PVEPs) were recorded using the Roland RETI system (Roland, Germany) according to the protocol proposed by the ISCEV.1 Stimuli were black and white checks in two size of 15 and 60min of arc at high contrast (99%) and with reversal rate of 1.535Hz. Considering that the resolution of various regions of the retina is distinct, it is recommended to use two sizes of 15 and 60min of arc for stimulating the central and peripheral regions of the retina.1 The stimuli were presented on a 22-inch CRT monitor with a resolution of 1800×1440 pixels at a repetition frequency of 75Hz (iiyama/vision master pro 513, Taiwan) and at the mean luminance of 52cd/m2, which was measured by the TES 137 luminance meter (ES Electrical Electronic Corp., Taiwan). The VEPs were recorded monocular (right and left eyes) and binocularly with the best optical correction of each participant. The sequence of eye viewing condition was selected randomly within participants. According to the International 10-20 System of electroencephalograph electrode placement28 and using the EEG golden cup electrodes, the active, reference and ground electrodes were placed respectively on the occipital area (Oz), the frontal (Fpz) and the vertex (Cz). To keep the impedance below 5kΩ, the scalp (at the electrode location) was cleaned with an abrasive gel, and then the electrodes were attached to the skull with Ten20 conductive paste.
To reduce noise, the examination room was kept dark and without any distracters. Patients were asked to look at the red cross fixation at the center of checkerboard stimuli at a distance of 1 meter. In each recording, 64 responses were averaged and pattern VEPs components including latencies of N75, P100 and N135 (as microsecond (ms)) and amplitude N75–P100 (as microvolt (μV)) were determined.
Statistical analysisStatistical analysis was performed using SPSS version 15. Repeated measures ANOVA and independent T-test were employed to investigate any effect cause by the viewing eye condition, size of stimulus and gender on VEPs components. P-values less than 0.05 were considered significant. The descriptive results presented in the tables and for pair-wise comparisons are expressed as mean and standard deviation (SD). Eventually, a 95% confidence interval was considered for the difference of latencies of N75, P100, N135 and amplitude of N75–P100.
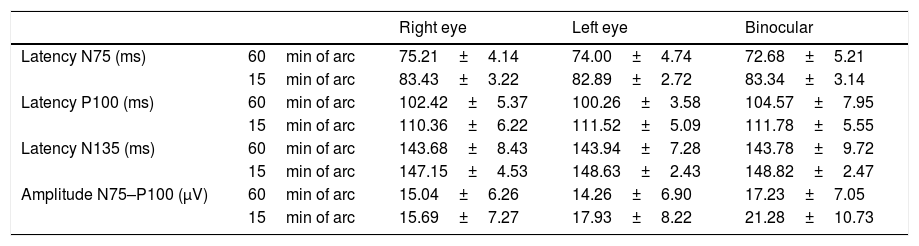
ResultsThe Kolomogrov–Smirnov analysis exhibited a normal distribution for the data (P>0.05). The mean and standard deviation of latencies of N75, P100 and N135 and amplitude of N75–P100 in two different checker board size are illustrated in Table 1 for each viewing eye condition (right, left eye and binocular). Paired T-test demonstrated that there was no significant difference in components of VEPs between right and left eyes (P>0.05).
The mean and SD of VEP components in two checker board size and under 3 eye viewing conditions.
| Right eye | Left eye | Binocular | ||
|---|---|---|---|---|
| Latency N75 (ms) | 60min of arc | 75.21±4.14 | 74.00±4.74 | 72.68±5.21 |
| 15min of arc | 83.43±3.22 | 82.89±2.72 | 83.34±3.14 | |
| Latency P100 (ms) | 60min of arc | 102.42±5.37 | 100.26±3.58 | 104.57±7.95 |
| 15min of arc | 110.36±6.22 | 111.52±5.09 | 111.78±5.55 | |
| Latency N135 (ms) | 60min of arc | 143.68±8.43 | 143.94±7.28 | 143.78±9.72 |
| 15min of arc | 147.15±4.53 | 148.63±2.43 | 148.82±2.47 | |
| Amplitude N75–P100 (μV) | 60min of arc | 15.04±6.26 | 14.26±6.90 | 17.23±7.05 |
| 15min of arc | 15.69±7.27 | 17.93±8.22 | 21.28±10.73 |
(ms): microsecond; (μV): micro volt.
Repeated measures ANOVA revealed that the effect of viewing eye condition is significant only on the amplitude of N75–P100 (P<0.001, F=13.89). Paired-wise comparison using Bonferroni correction showed that the amplitude of N75–P100 was larger in binocular viewing than monocular viewing (for binocular when compared with the right eye: 5.91; 95% CI: 3.50, 8.32; P<0.001 and compared with the left eye: 5.33; 95% CI: 3.39, 7.27; P<0.001).
Nevertheless, statistical analysis illustrated that the effect of check size on latencies of N75 (P≤0.001, F=132.54), P100 (P≤0.001, F=62.32) and N135 (P=0.010, F=8.42) and amplitude of N75–P100 (P≤0.001, F=24.55) is significant. The mean and standard deviation of intraocular latencies and amplitude differences are given in Table 2. Furthermore, the intraocular difference of P100 latency and amplitude of N75–P100 significantly differed in both sizes of checker board (P=0.007).
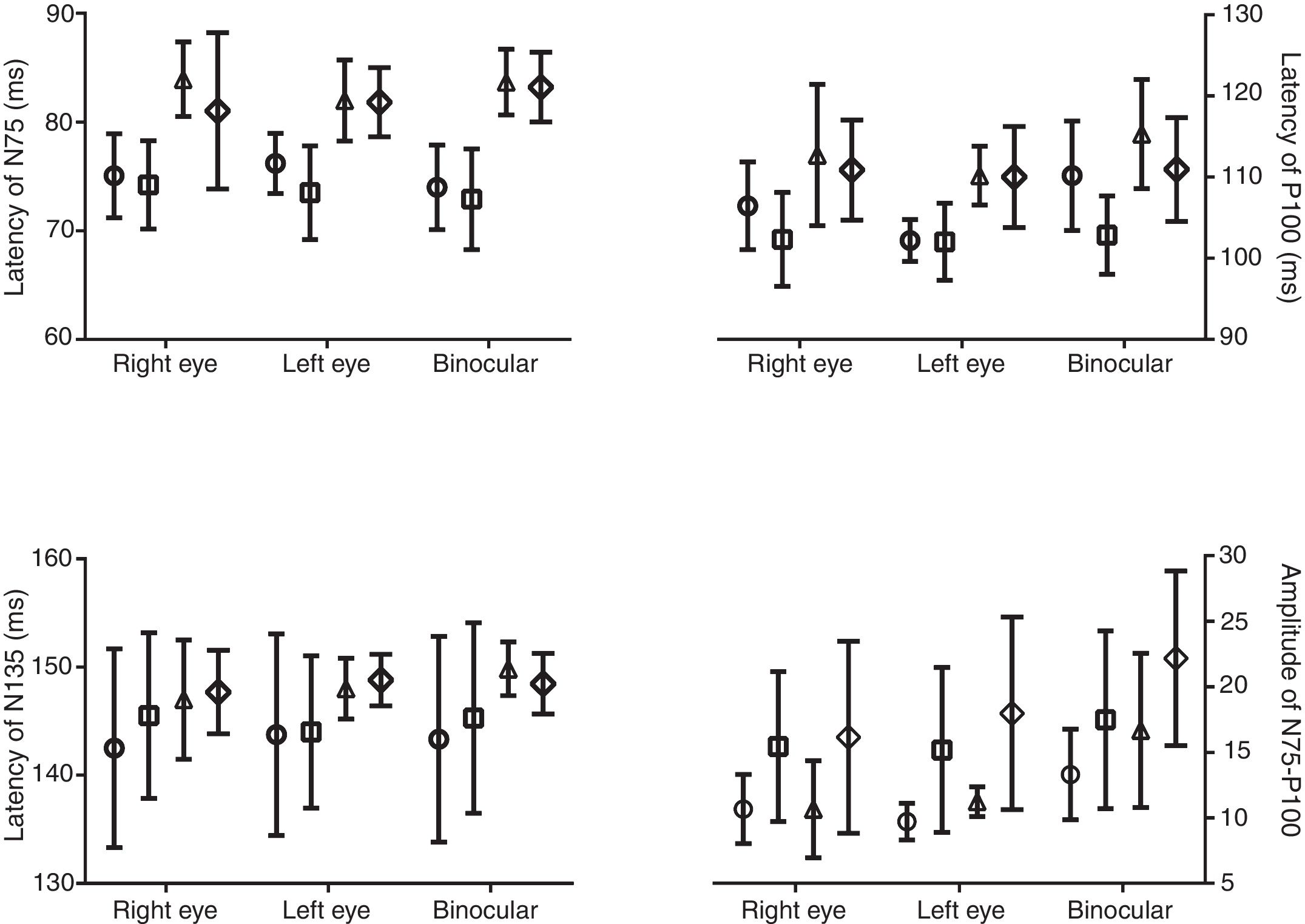
Independent T-test showed that the amplitude of N75–P100 in both check sizes and in each eye viewing condition has a significantly difference between gender groups (P<0.023) (Fig. 1). Other components of VEPs, however, did not significantly differ between males and females (P>0.05).
DiscussionThe high sensitivity of VEPs to lesions on the visual pathways from the retina to the occipital cortex has led to this method being used as a quantitative and objective tool for assessing the integrity of the visual system.4,6,12,29 Different components of pattern reversal VEPs are dependent on stimulus parameters1 and physiological factors.30,31 According to the results of this study, all the components of pattern reversal VEPs depend on stimulus size, such that longer latencies are associated with smaller check sizes. This finding is supported by study of Kurita-Tashima et al.32 which observed an inverse linear relationship between the latency of VEPs and stimulus check size. Moreover, given that the P100 latency has high sensitivity for detecting subclinical lesions of optic nerve and demyelinating disorders,12,13,33 the results should be interpreted based on the size of stimuli.
In this study, it was seen that the amplitude of N75–P100 is larger under binocular VEPs recording conditions. This enhancement of amplitude in binocular VEPs rather than its monocular has also been confirmed in previous studies.2,34 Di Summa et al.,34 observed a binocular summation on the amplitudes of N70 and P100, while binocular viewing did not affect the latencies of transient VEPs.
Interocular asymmetries of VEPs are very important in the diagnosis of unilateral lesions of the visual pathways and macula diseases.33,35 Previous studies have shown interocular VEPs delays are significantly longer in patients with maculopathy compared with normal individuals.4,6,35 Hence, one of the objectives of this study was to determine the mean of interocular difference of pattern reversal VEPs parameters in normal individuals. The normative values of interocular differences of VEPs components are presented in Table 2. Any interocular difference exceeding the stated values should be considered a caution for possible unilateral defects in the visual pathway and macular lesions and, thus, it requires a more detailed ophthalmic and neurologic evaluation.
The current study showed the amplitude of N75–P100 is larger in females compared to males. The effect of gender on VEPs has been investigated in previous studies.16,30 In spite of non-clearance of the exact cause of gender difference in VEPs components, some studies have associated larger amplitude and shorter latencies in females to their smaller head size and endocrinal differences between genders.16,25,31 As a result, the impact of gender difference on VEPs must be considered when interpreting the results and comparing them with normal values of VEPs.
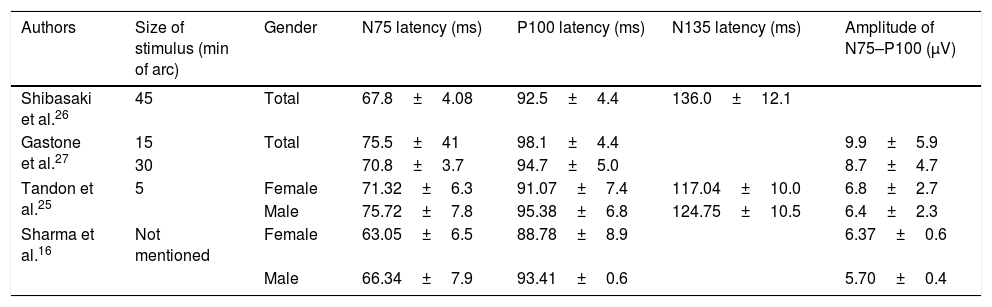
The normative values of VEP components of as observed in the current study and previous studies are given in Tables 1 and 3 respectively. The variation in the values of VEPs may be due to the difference in stimulus parameters, recording instruments, and ethnicity. Racial and ethnic differences in ocular structure and visual function have been reported in healthy eyes.36–38 Because of these dissimilarities, it is important to assess the normative databases of each population. While VEPs are widely used as an objective measure of the visual function in both research and clinical examinations, it is necessary for each electrophysiology laboratory to determine the normative values of VEPs components using its own stimuli and parameters of recording. This is a preliminary step for the application of this test as a diagnostic tool in ocular and neurological disorders.
The normal values of VEP components in previous studies.
| Authors | Size of stimulus (min of arc) | Gender | N75 latency (ms) | P100 latency (ms) | N135 latency (ms) | Amplitude of N75–P100 (μV) |
|---|---|---|---|---|---|---|
| Shibasaki et al.26 | 45 | Total | 67.8±4.08 | 92.5±4.4 | 136.0±12.1 | |
| Gastone et al.27 | 15 | Total | 75.5±41 | 98.1±4.4 | 9.9±5.9 | |
| 30 | 70.8±3.7 | 94.7±5.0 | 8.7±4.7 | |||
| Tandon et al.25 | 5 | Female | 71.32±6.3 | 91.07±7.4 | 117.04±10.0 | 6.8±2.7 |
| Male | 75.72±7.8 | 95.38±6.8 | 124.75±10.5 | 6.4±2.3 | ||
| Sharma et al.16 | Not mentioned | Female | 63.05±6.5 | 88.78±8.9 | 6.37±0.6 | |
| Male | 66.34±7.9 | 93.41±0.6 | 5.70±0.4 |
(ms): microsecond; (μV): micro volt.
This project was supported by Mashhad University of Medical Sciences.
Conflicts of interestThe authors declare that they have no conflict of interest.
This research was financially supported by the research deputy of Mashhad University of Medical Sciences (MUMS). Pattern reversal VEPs were recorded at the Electrophysiology Laboratory in the Optometry Department of Mashhad University of Medical Sciences.