To determine whether - and which - higher-order corneal aberrations, up to the sixth order, are induced by photorefractive keratectomy (PRK).
Methods197 eyes of 197 patients have been examined with a corneal aberrometer for a 3.5 and a 6.0mm pupil simulation, both before and 1, 3, 6 months after myopic PRK treatment ranging from −15.25 D to -0.5 D (mean −5.31±2.95 D). The statistical evaluation was performed using a paired Student's T-test.
ResultsAfter PRK there is a clear-cut increase in almost all the higher-order corneal aberrations for both a 3.5 and a 6.0mm pupil simulation. These aberrations tend to normalize after 3 and 6 months mainly for a 3.5mm simulation, whereas such normalization is not present for a 6.0mm simulation.
ConclusionsPRK induces significant aberrations both for 3.5 and 6mm pupils, 1 month after PRK, but a trend towards normalization is evident at the 6 month follow-up for the smaller pupil size.
Determinar en qué medida la queratectomía fotorrefractiva (en inglés, PRK) genera aberraciones corneales de alto orden (hasta sexto orden).
MétodosSe midieron las aberraciones corneales de 197 ojos pertenecientes a 197 pacientes utilizando un aberrómetro corneal y simulando tamaños de pupila de 3,5mm y de 6,0mm. Estas medidas se realizaron tanto antes como transcurridos 1, 3 y 6 meses de una intervención de cirugía refractiva con PRK para corregir la miopía (errores refractivos tratados comprendidos entre -15,25 D y -0,5 D; media: -5,31±2,95 D). Para el análisis estadístico de los datos se utilizó una prueba T de Student para muestras relacionadas.
ResultadosTras la PRK se observa un claro aumento de prácticamente todas las aberraciones corneales de alto orden, tanto para la pupila simulada de 3,5mm como para la de 6,0mm. Para la pupila de 3,5mm, estas aberraciones tienden a normalizarse transcurridos 3 y 6 meses de la operación, mientras que para una pupila simulada de 6,0mm no se aprecia dicha normalización.
ConclusionesLa PRK genera una cantidad significativa de aberraciones, tanto para 3,5 como para 6mm de pupila, según lo observado 1 mes desde la operación de PRK. Sin embargo, a medida que aumenta el tiempo transcurrido desde la operación (hasta la revisión a los 6 meses), se aprecia una tendencia a la normalización para pequeños tamaños de pupila.
Currently laser in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK) are widely accepted techniques to correct myopia, hyperopia and astigmatism.
Although LASIK and PRK may result in a good objective outcome measured by correction of the sphero-cylindrical refractive error, some patients complain of a decrease in visual performance with dissatisfaction with postoperative vision.1-3 For example, a reduction in the natural negative asphericity of the cornea may contribute to a less than optimal visual outcome with possible subjective visual sequelae of glare or halo.4
Some clinical studies suggest that this is due to the fact that the cornea becomes oblate after treatment.2,5,6 Another explanation could be the use of a small optical zone that gives optical zone edge effects and lack of correction of the untreated peripheral cornea, rather than a change in spherical aberration per se. More recent studies blame the induced high-order aberration in the patients complains.
The shape of the anterior corneal surface largely determines the quality of the retinal image, because this surface determines the shape of the tear-air interface, the most powerful image-forming interface in the eye.7 Focusing errors of the corneal surface, such as regular corneal astigmatism, degrade the retinal image. An error in corneal spherical power or astigmatism is easily correctable with spectacles. Optical aberrations of the cornea, such as coma and distortion, are not correctable with spectacles.7 Such aberrations are common in keratoconus and after penetrating keratoplasty, and can develop after radial keratotomy and other refractive corneal surgical procedures.
For this reason we decided to check whether and which corneal aberrations were induced after PRK and if they were related to the kind and amount of treatment.
MethodsWe retrospectively investigated the corneal aberrations of 197 eyes of 197 consecutive patients (82 males and 115 females) who had undergone PRK for myopia, myopic astigmatism or compound astigmatism in our Department. The mean age was 32.2±9.1 years (range: from 18 to 59 years). Spherical-equivalent-attempted correction ranged from -15.25 D to -0.5 D (mean: -5.31±2.95 D). Preoperative and follow-up examinations at 1, 3, and 6 months included detailed ophthalmic examination with manifest refraction, and computer-assisted videokeratography (Keratron Scout, Optikon 2000, Rome, Italy) to measure corneal aberrations.
Patients were asked to discontinue wearing contact lenses for at least 1 month before undergoing the last refractive and topographic evaluation, which was performed a few days before the patient underwent PRK. An informed consent was obtained before the surgery. The Ethics Committee decided approval was not required for this study.
Patients with systemic and ocular diseases that could potentially interfere with the healing process of the cornea or with the refractive outcome, such as diabetes, collagenopathies, dry eyes, uveitis, corneal and lens opacities or glaucoma, were excluded from the treatment.
Treatments both as sphere and as cylinder were performed by combining objective and subjective refraction, thereby achieving the best corrected visual acuity. In particular, the cycloplegic refraction was performed during the first visit, whereas the subjective refraction was performed during the last visit before surgery, taking into account the results of the cycloplegic refraction. When a discrepancy between these two methods was found, the final refraction was obtained by means of a duochrome (red-green) test. All treatments were performed under topical anesthesia with oxybuprocaine (Novesina® Novartis Farma, Italy) eye drops. The lids were opened with a speculum, the epithelium was debrided with a mechanical brush epithelial removal, and all treatments were performed with a 193nm excimer laser (Nidek EC 5000) operating in scanning mode.
After treatment, surface smoothing with a PTK treatment was performed by placing 1 drop of a 0.04% hyaluronic acid solution over the cornea, and spreading it out with a 23 gauge cannula. In this way the fluid filled an eventual depression of the cornea, thus protecting the tissue from the laser pulse, and the endpoint of the smoothing was chosen on the basis of microscopy and of clinical evidence.8 A bandage contact lens was applied under sterile conditions on the treated eye immediately following surgery, and was left until complete re-epithelialization. During this period, the operated eyes received the following medications: Diclofenac sodium 0.1% eye drops twice a day for the first 2 days, nethylmicin preservative-free eye drops until reepithelialization and preservative-free artificial tears for 1 month. After re-epithelialization clobetasone eye drops were prescribed to all patients for 1 month in a tapered dose, as follows: one drop four times a day for the first week, one drop three times a day for the second week, one drop twice a day for the third week, and one drop once a day for the last week.
Four topographies were performed on each eye, and the one selected for the study was chosen based on the following criteria: the least eyelid coverage to allow the processing of the greatest area; correct centring; correct focusing with thin, regular, continuous rings; and absence of dry spots or excess pooling of tears along the inferior lid margin.9
The Keratron instrument was correctly calibrated at the start of each examination session.
We did not use topography maps with poor or questionable mire images for analysis, nor did we use maps that had missing data within the zone of analysis.
Using corneal topographic data, the machine calculated the variance between the reference wavefront and the actual wavefront over the pupil size of interest. To determine the positive and negative values of the Zernike terms, a reference surface sphere is determined from the average surface curvature of the corneal topography. Lower order aberrations of prism, sphere, cylinder, and axis are parcelled out and ignored because they can be corrected with traditional spectacle corrections. In this study, we evaluate the higher-order aberrations at different pupil sizes (3.5mm - 6mm), since no standard pupil has been established for reporting ocular aberrations. Analyses were obtained by using Microsoft Excel 7 (Microsoft Corp., Seattle, WA). The statistical evaluation was performed with a paired two-tailed Student's T test.
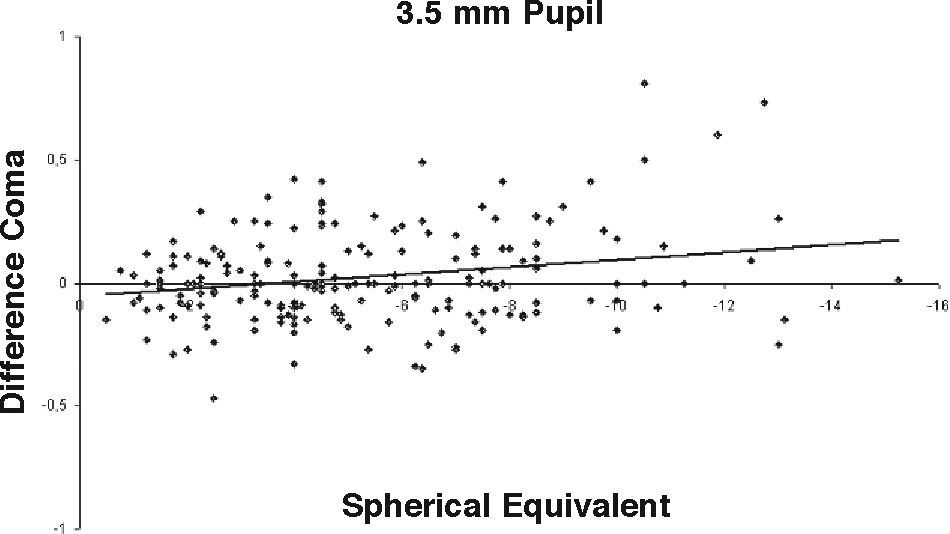
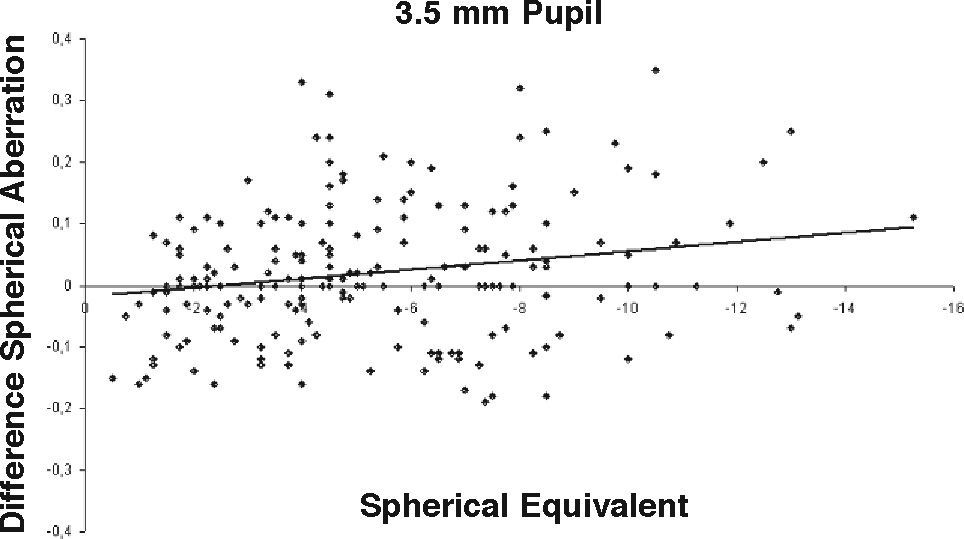
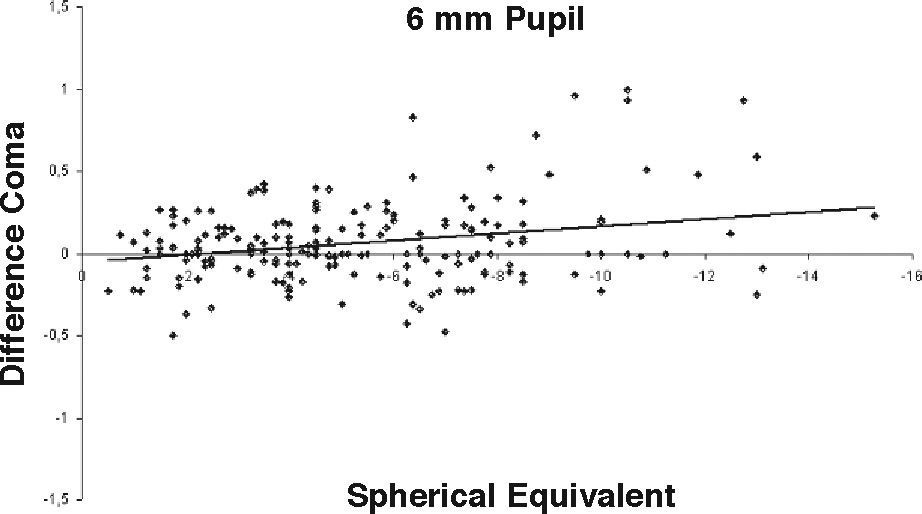
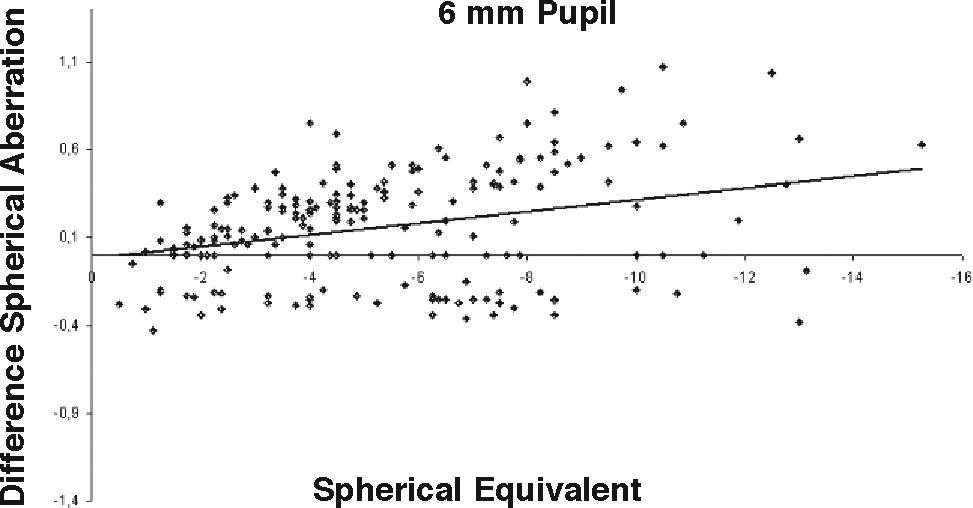
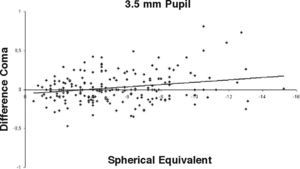
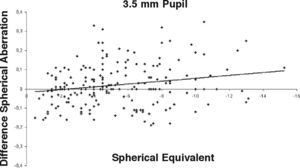
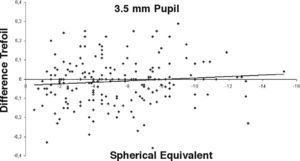
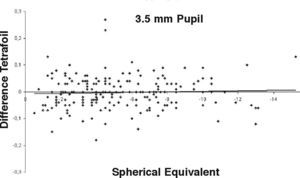
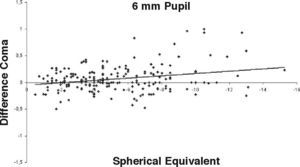
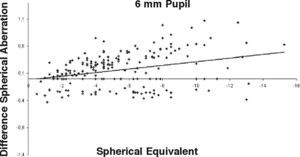
ResultsOur data show that one month after PRK there is a marked increase in almost all the higher-order corneal aberrations, both for a 3.5 and a 6.0mm pupil simulation. These aberrations tend to normalize after 3 and 6 months mainly for a 3.5mm simulation, whereas such normalization is not present for a 6.0mm simulation (Tables 1-3). Moreover, there was a good correlation between the amount of treatment and the increase in spherical aberration and coma, whereas such a correlation was not so evident for the other aberrations (Figures 1-8).
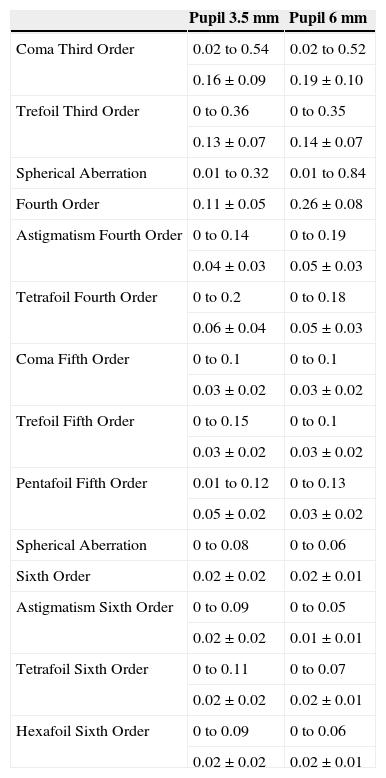
Values of corneal aberrations (range, mean ± standard deviation) in microns before PRK for a 3.5 and 6mm pupils
| Pupil 3.5 mm | Pupil 6 mm | |
| Coma Third Order | 0.02 to 0.54 | 0.02 to 0.52 |
| 0.16±0.09 | 0.19±0.10 | |
| Trefoil Third Order | 0 to 0.36 | 0 to 0.35 |
| 0.13±0.07 | 0.14±0.07 | |
| Spherical Aberration | 0.01 to 0.32 | 0.01 to 0.84 |
| Fourth Order | 0.11±0.05 | 0.26±0.08 |
| Astigmatism Fourth Order | 0 to 0.14 | 0 to 0.19 |
| 0.04±0.03 | 0.05±0.03 | |
| Tetrafoil Fourth Order | 0 to 0.2 | 0 to 0.18 |
| 0.06±0.04 | 0.05±0.03 | |
| Coma Fifth Order | 0 to 0.1 | 0 to 0.1 |
| 0.03±0.02 | 0.03±0.02 | |
| Trefoil Fifth Order | 0 to 0.15 | 0 to 0.1 |
| 0.03±0.02 | 0.03±0.02 | |
| Pentafoil Fifth Order | 0.01 to 0.12 | 0 to 0.13 |
| 0.05±0.02 | 0.03±0.02 | |
| Spherical Aberration | 0 to 0.08 | 0 to 0.06 |
| Sixth Order | 0.02±0.02 | 0.02±0.01 |
| Astigmatism Sixth Order | 0 to 0.09 | 0 to 0.05 |
| 0.02±0.02 | 0.01±0.01 | |
| Tetrafoil Sixth Order | 0 to 0.11 | 0 to 0.07 |
| 0.02±0.02 | 0.02±0.01 | |
| Hexafoil Sixth Order | 0 to 0.09 | 0 to 0.06 |
| 0.02±0.02 | 0.02±0.01 |
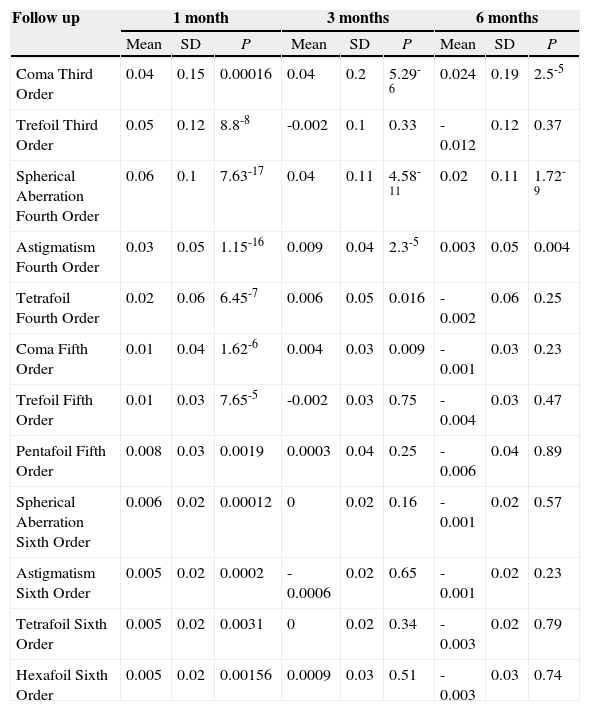
Differences (mean ± standard deviation and t test) in microns between corneal aberrations (preoperative aberrations have been subtracted from the postoperative ones) at 1, 3 and 6 months after PRK for a 3.5mm pupil. SD means standard deviation
| Follow up | 1 month | 3 months | 6 months | ||||||
| Mean | SD | P | Mean | SD | P | Mean | SD | P | |
| Coma Third Order | 0.04 | 0.15 | 0.00016 | 0.04 | 0.2 | 5.29-6 | 0.024 | 0.19 | 2.5-5 |
| Trefoil Third Order | 0.05 | 0.12 | 8.8-8 | -0.002 | 0.1 | 0.33 | -0.012 | 0.12 | 0.37 |
| Spherical Aberration Fourth Order | 0.06 | 0.1 | 7.63-17 | 0.04 | 0.11 | 4.58-11 | 0.02 | 0.11 | 1.72-9 |
| Astigmatism Fourth Order | 0.03 | 0.05 | 1.15-16 | 0.009 | 0.04 | 2.3-5 | 0.003 | 0.05 | 0.004 |
| Tetrafoil Fourth Order | 0.02 | 0.06 | 6.45-7 | 0.006 | 0.05 | 0.016 | -0.002 | 0.06 | 0.25 |
| Coma Fifth Order | 0.01 | 0.04 | 1.62-6 | 0.004 | 0.03 | 0.009 | -0.001 | 0.03 | 0.23 |
| Trefoil Fifth Order | 0.01 | 0.03 | 7.65-5 | -0.002 | 0.03 | 0.75 | -0.004 | 0.03 | 0.47 |
| Pentafoil Fifth Order | 0.008 | 0.03 | 0.0019 | 0.0003 | 0.04 | 0.25 | -0.006 | 0.04 | 0.89 |
| Spherical Aberration Sixth Order | 0.006 | 0.02 | 0.00012 | 0 | 0.02 | 0.16 | -0.001 | 0.02 | 0.57 |
| Astigmatism Sixth Order | 0.005 | 0.02 | 0.0002 | -0.0006 | 0.02 | 0.65 | -0.001 | 0.02 | 0.23 |
| Tetrafoil Sixth Order | 0.005 | 0.02 | 0.0031 | 0 | 0.02 | 0.34 | -0.003 | 0.02 | 0.79 |
| Hexafoil Sixth Order | 0.005 | 0.02 | 0.00156 | 0.0009 | 0.03 | 0.51 | -0.003 | 0.03 | 0.74 |
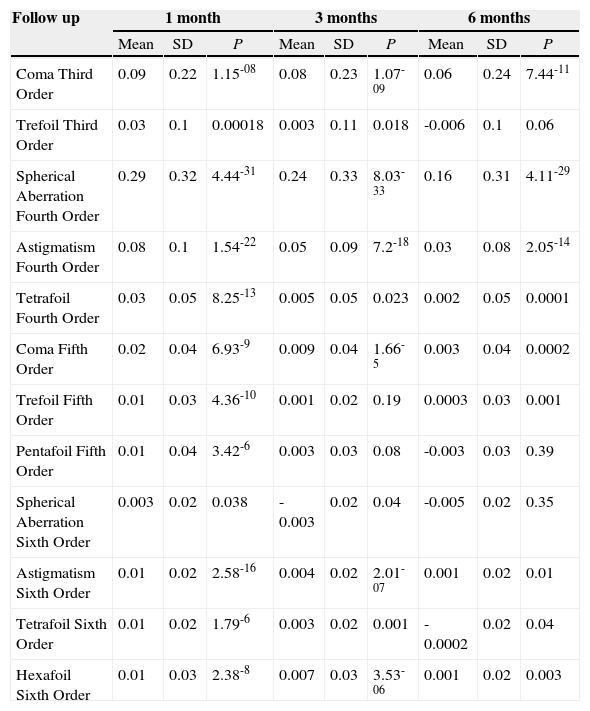
Differences (mean ± standard deviation and t test) in microns between corneal aberrations (preoperative aberrations have been subtracted from the postoperative ones) at 1, 3 and 6 months after PRK for a 6mm pupil. SD means standard deviation
| Follow up | 1 month | 3 months | 6 months | ||||||
| Mean | SD | P | Mean | SD | P | Mean | SD | P | |
| Coma Third Order | 0.09 | 0.22 | 1.15-08 | 0.08 | 0.23 | 1.07-09 | 0.06 | 0.24 | 7.44-11 |
| Trefoil Third Order | 0.03 | 0.1 | 0.00018 | 0.003 | 0.11 | 0.018 | -0.006 | 0.1 | 0.06 |
| Spherical Aberration Fourth Order | 0.29 | 0.32 | 4.44-31 | 0.24 | 0.33 | 8.03-33 | 0.16 | 0.31 | 4.11-29 |
| Astigmatism Fourth Order | 0.08 | 0.1 | 1.54-22 | 0.05 | 0.09 | 7.2-18 | 0.03 | 0.08 | 2.05-14 |
| Tetrafoil Fourth Order | 0.03 | 0.05 | 8.25-13 | 0.005 | 0.05 | 0.023 | 0.002 | 0.05 | 0.0001 |
| Coma Fifth Order | 0.02 | 0.04 | 6.93-9 | 0.009 | 0.04 | 1.66-5 | 0.003 | 0.04 | 0.0002 |
| Trefoil Fifth Order | 0.01 | 0.03 | 4.36-10 | 0.001 | 0.02 | 0.19 | 0.0003 | 0.03 | 0.001 |
| Pentafoil Fifth Order | 0.01 | 0.04 | 3.42-6 | 0.003 | 0.03 | 0.08 | -0.003 | 0.03 | 0.39 |
| Spherical Aberration Sixth Order | 0.003 | 0.02 | 0.038 | -0.003 | 0.02 | 0.04 | -0.005 | 0.02 | 0.35 |
| Astigmatism Sixth Order | 0.01 | 0.02 | 2.58-16 | 0.004 | 0.02 | 2.01-07 | 0.001 | 0.02 | 0.01 |
| Tetrafoil Sixth Order | 0.01 | 0.02 | 1.79-6 | 0.003 | 0.02 | 0.001 | -0.0002 | 0.02 | 0.04 |
| Hexafoil Sixth Order | 0.01 | 0.03 | 2.38-8 | 0.007 | 0.03 | 3.53-06 | 0.001 | 0.02 | 0.003 |
In normal eyes, retinal image quality is related to optical aberrations. Optical aberrations can be classified in two categories.10
- 1.
Second-order aberrations (spherocylindrical errors that include defocus and astigmatism) have the largest impact on visual acuity, and can be corrected with spectacles, contact lenses, and conventional laser surgery.
- 2.
Higher-order aberrations (i.e. third-order and above), include trefoil, coma, spherical aberrations and others.
Odd-order or coma-like aberrations are asymmetric aberrations, i.e., the optical properties of the cornea are not alike on opposite sides of the corneal surface. Even-order or spherical-like aberrations are symmetric aberrations, i.e., the optical properties of the cornea are alike on opposite sides of the corneal surface.11 Spherical aberration smears out features of the image in a radially symmetric fashion, resulting in a ghosting halo around letters in a chart and is often accompanied by night vision complaints while coma is associated with streakiness of images. They normally degrade retinal image quality only by a small amount, contribute to limiting the resolution of the human eye and can represent up to 10% to 15% of the total aberration error.
In the normal eye, aberrations are relatively low and are dominated by odd-order (coma-like) aberrations.12 They increase with pupil size: the larger the pupil, the more significant the effect on vision quality, they can change with accommodation and can fluctuate slightly from moment to moment with tear film changes. These aberrations occur naturally but can also be induced by excimer laser refractive correction, where they may become more visually problematic, as they can be associated with complex visual complaints, such as glare, halos, and night driving difficulty.
Refractive surgery, including radial keratotomy (RK),13-15 PRK,16-18 and LASIK,19 has been shown to increase the magnitude of the corneal first surface aberrations by over a log unit and their distribution is dominated by fourth-order aberrations.13,16
In our study we decided to check only the corneal aberrations as the refractive surgery induces changes only at the corneal level, whereas other internal structures are not expected to change. Moreover, the corneal front surface in the normal eye contributes approximately to half the total aberrations of the eye. The contributions are age-dependent, and increase substantially with surgery and disease.20
Videokeratography provides a detailed description of the corneal surface with much more information about corneal shape, or topography.20 The color dioptric map introduced by Maguire, Singer, and Klyce21 is an important advance, because it allows visual inspection of optical zone size,22 centration, 23 and regularity,24 but the qualitative interpretation of the pattern of colours limits their usefulness. Moreover, the retinal image is a composite of light refracted by all points on the cornea that lie over the entrance pupil, whereas the map provides information only about individual points on the cornea.25 For this reason it is difficult to look at a pattern of colours in a topographic map and determine whether the corneal shape explains a particular patient's loss in visual acuity or astigmatism.
To enable the quantification of corneal first surface aberrations17 and their effect on visual performance, new videokeratographers that utilize the Zernike polynomial fitting have been designed.13,14
In describing ocular aberrations, Zernike polynomials have the advantage that each Zernike term has a coefficient with a magnitude and sign that indicates the relative strength and direction of the aberration contributed by that term and the value of each mode's coefficient represents the root mean square (RMS) wavefront error attributable to that mode.26 These modes can be mathematically described independently of one another, and higher-value coefficients identify the modes having the greatest impact on the total RMS wavefront error of the eye. Unfortunately this mathematical independence does not mean that their impact on visual performance is independent.
It has not yet been established how many Zernike terms are sufficient to describe a given surface, and whether the accuracy of the decomposition process is adequate.27-29
Even if it has been shown that Zernike polynomial fitting fails to represent all the visually significant corneal aberrations, 30 it is an accepted measurement in research and is becoming accepted in leading clinical practices to describe the wavefront error of the eye.
Our study has the limitation of a short follow–up, as it has been shown that after PRK wound healing and topographic remodelling often take one year; however, this study proves that several high-order aberrations are induced by PRK treatment 1 month after PRK, both in photopic (3.5mm pupil) and scotopic (6mm pupil) conditions, whereas there is a tendency to return to the preoperative parameters in photopic conditions, but in case of dilated pupils they tend to be worse.
Nowadays this finding could be very important as vision scientists and refractive surgeons have adopted wavefrontsensing technology to assess the optical aberrations of the eye and to guide customized corneal ablations.17,31-34 In fact, if we study the difference between the aberrated wavefront of light reaching the retina and the theoretical, ideally focused wavefront we will examine the total aberrations of the eye. If for any reason a change in the internal eye aberrations should occur (e.g. undetected change in the lens clarity), this could compensate the surgically induced ones, and could affect our results. In our case, examining only the corneal aberrations, we can really understand whether, and which, corneal aberrations are induced by PRK.