Dry eye disease (DED) is often managed with over-the-counter eye drops. This study evaluated the diurnal effects of a single drop of two ocular lubricants (nanoemulsion vs. non-emollient) on tear film lipid layer thickness (LLT) and symptoms of ocular dryness. Subjects were also assessed after 1 month of nanoemulsion eye drop use.
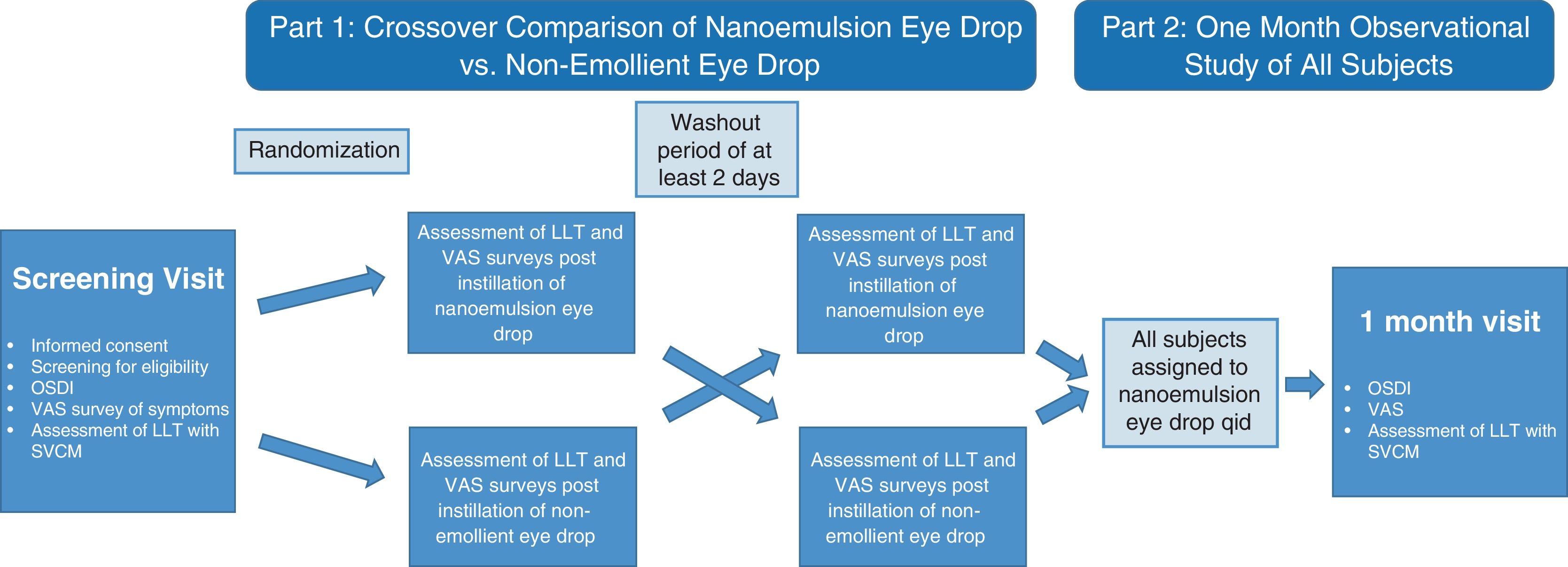
MethodsPart 1 was a cross-over comparison of a nanoemulsion and a non-emollient eye drop. LLT and dry eye symptoms were measured at baseline and at 15min, 1h, 2h, 4h and 6h after instillation of each drop. Part 2 was a 1-month observational study assessing LLT and symptoms after 30-day use of the nanoemulsion drop four times daily (qid).
ResultsTotal of 20 subjects completed the study (mean age=45.6±7.9, 15 female). Part 1 found a significant increase in average LLT 15min after nanoemulsion drop instillation in the overall and inferior third of the tear film for subjects with baseline LLT values <50nm. Average LLT values did not increase after use of the non-emollient. Symptoms of dryness improved up to 6h following instillation of both drops. Part 2 results found that using the nanoemulsion eye drop for 1 month improved symptoms reported on symptom surveys.
ConclusionNanoemulsion eye drop use increased average LLT in subjects with low baseline levels. Statistically and clinically significant improvement in symptoms were found on symptom surveys after qid-use (four times a day) of the nanoemulsion drop. Results suggest that a nanoemulsion eye drop can benefit subjects with dry eye symptoms.
El síndrome del ojo seco (SOS) se trata a menudo con gotas oftálmicas sin prescripción. Este estudio evaluó los efectos diurnos de la aplicación de una única gota de dos lubricantes oculares (nanoemulsión vs. no emoliente) en el grosor de la capa lipídica lagrimal (LLT) y los síntomas de sequedad ocular. También se valoró a los sujetos transcurrido un mes del uso de gotas oftálmicas de nanoemulsión.
MétodosLa Parte 1 consistió en la comparación cruzada de gotas oftálmicas de nanoemulsión y no emolientes. Se midieron LLT y síntomas de ojo seco basales y a los 15 minutos, 1 hora, 2 horas, 4 horas y 6 horas tras la instilación de cada gota. La Parte 2 consistió en un estudio observacional de 1 mes que valoró LLT y síntomas tras el uso de 30 días de la gota de nanoemulsión cuatro veces al día (qid).
ResultadosUn total de 20 sujetos completaron el estudio (edad media=45,6±7,9, 15 mujeres). Los resultados de la Parte 1 reflejaron un incremento significativo en LLT medio a los 15 minutos de la instilación de la gota de nanoemulsión globalmente y en el tercio inferior de la película lagrimal para los sujetos con valores basales de LLT<50nm. Los valores medios de LLT no se incrementaron tras el uso de la gota no emoliente. Los síntomas de sequedad mejoraron hasta 6 horas tras la instilación de ambas gotas. Los resultados de la Parte 2 reflejaron que el uso de gotas oftálmicas de nanoemulsión durante 1 mes mejoraron los síntomas reportados en las encuestas.
ConclusiónEl uso de gotas oftálmicas de nanoemulsión incrementó el parámetro LLT medio en los sujetos con bajos niveles basales. Se encontraron mejoras estadística y clínicamente significativas en las encuestas sobre síntomas tras el uso 4 veces al dia de gotas oftálmicas de nanoemulsión. Los resultados sugieren que las gotas oftálmicas de nanoemulsión pueden beneficiar a los sujetos con síntomas de ojo seco.
Dry eye disease (DED) is a common and complex ocular disorder which affects many individuals worldwide. In the United States alone, recent studies have reported that approximately 7% of individuals over the age of 18 have diagnosed DED.1,2 The prevalence of DED is estimated to be even higher in other countries, with some estimates reaching up to 25% in Canada and 33% in Japan.2
In DED, an insufficient tear film causes a variety of symptoms including ocular discomfort, visual disturbances, and physical disruption of the ocular surface.3 Tear film insufficiency may be the result of inadequate aqueous tear production by the main and accessory lacrimal glands, excessive evaporation of the tears from the ocular surface, or a combination of both.3 Evaporative forms of DED are in part due to a lack of tear film stability. This stability may be disrupted for a variety of reasons including inadequate tear lipid.3,4 The tear film lipid layer (TFLL) and the biochemical properties of the TFLL on tear stability and evaporation of the tear film are not yet completely understood.4–8 However, it is generally accepted that maintaining an adequate tear lipid layer is important in managing DED, particularly for those patients with lipid deficiency, as occurs in meibomian gland dysfunction (MGD). It has been hypothesized that emollient-containing eye drops may be superior to their non-emollient counterparts in individuals suffering from evaporative DED, as they may help to replenish a lipid-deficient tear film.9–11 Because the physiologic tear lipid layer plays a vital role in tear film stabilization and reduction in tear film evaporation,3,5,12 it would be reasonable to hypothesize that lipid supplementation of the tear film may subsequently improve the signs and symptoms of those patients with tear film lipid deficiency. Patients with symptoms of DED which are due to a deficiency of aqueous tears may find relief of symptoms with an aqueous eye drop. However, it can sometimes be challenging to assess whether the underlying cause of the disease is due primarily to an aqueous or lipid deficiency, as patients in a pre-clinical disease state may manifest symptoms associated with DED, but not signs.13
Studying the tear lipid layer in conjunction with symptoms of dry eye may provide better insight into how the tear film is altered in DED and help to identify the clinical implications to treating DED patients. Previous studies have been conducted on patients with MGD to determine if adding emollient eye drops can increase the tear lipid layer thickness (LLT).14,15 Using interferometric methods, imaging of the tear lipid layer may be used to evaluate tear film lipid layer thickness and fluidity.4,16 By assessing the LLT before and after the use of an eye drop, changes in the thickness can be assessed.
Treatment of DED may involve one or more interventions depending upon the etiology and symptoms. Advances in tear film science has led to the production of many over-the-counter (OTC) tear substitutes, which target the differential inadequacies of the tear film. As the use of OTC eye drops is often the first line of therapy for DED patients,10,17 it is of vital importance that eye care providers recommend tear supplements that have been shown to be effective in treating the signs and symptoms of DED. Several studies have sought to identify the optimal eye drop to manage the symptoms of DED,17 with emollient eye drops being favored for patients with MGD. While some emollient eye drops increase tear LLT, the viscous nature of these drops may temporarily cause blurred vision and reduced contrast sensitivity following instillation when compared to aqueous eye drops.18 Although these differences are mild, they may be undesirable in individuals suffering from the disease. Those patients with DED due to aqueous insufficiency, rather than lipid insufficiency, or those with very mild symptoms due to lipid layer instability may benefit from a nanoemulsion eye drop formulation. A nanoemulsion is composed of particles that are generally 10–1000nm in size and packed with both oil and aqueous components.19 An eye drop formulated as a nanoemulsion, therefore, allows for the components of the drop to be dispersed onto the surface of the eye similarly to that of an aqueous eye drop, theoretically adding oil to the tear film without the temporary effects that accompany highly viscous eye drops. As OTC eye drops are a regular DED treatment recommended by eye doctors, and can be used by patients to self-treat, such a formulation may benefit both MGD patients and those with aqueous tear-deficient DED.
Studies of tear lipid layer are reliant upon instrumentation based upon the principles of interferometry. Interferometric imaging devices allow for visualization of the tear lipid layer and have been used in previous studies to assess the tear LLT and how it relates to DED.14,15 Previously, a stroboscopic video color microscope (SVCM) developed by King-Smith, has used to quantify tear LLT.16 Understanding how tear LLT is affected by the use of OTC tear substitutes may aid in improved prescribing practices and allow for more targeted development of new dry eye treatments.
The purpose of this study was to compare the effects of a nanoemulsion eye drop (Systane Complete®) versus a non-emollient (Systane Ultra®) eye drop on tear LLT and symptoms of ocular dryness following single-dose drop instillation in individuals suffering from symptoms of dry eye disease. Secondly, this study sought to evaluate symptoms of ocular dryness after regular use of this nanoemulsion eye drop for 1 month in the same study group.
MethodsThis study (ClinicalTrials.gov identifier: NCT03706443) was completed under the approval of the Institutional Review Board at The Ohio State University, located in Columbus, Ohio, USA. Informed consent was obtained from 24 subjects who responded to an advertisement for a dry eye study. Twenty subjects met all inclusion and exclusion criteria and completed the study. Subjects first participated in a double-masked cross-over study to examine the short-term effects of single-drop instillation of the two tested ocular lubricants. Part 2 of the study was a 1-month observational study in which the effects of sustained nanoemulsion eye drop use were evaluated for all subjects. Refer to Fig. 1 for study design.
Study design. After baseline assessment, subjects were randomized to the nanoemulsion or non-emollient eye drop for assessment of LLT and symptoms of ocular dryness at various time points after instillation of the eye drop. After a washout period of at least 2 days, the subjects crossed over for assessment with the second eye drop. All subjects then utilized the emollient eye drop four-times daily for 30±2 days and subjects returned for additional assessment.
All subjects suffered from symptoms of self-reported dry eye and were required to have a tear lipid layer thickness <75nm in either eye at baseline examination to be consistent with previous dry eye studies measuring LLT.14,15 No other diagnostic tests were performed to grade the severity or etiology of the dry eye.
Exclusion criteria included: use of any ocular prescription medication, active ocular inflammation, systemic diseases, previous ocular surgery (including refractive surgery within five years or cataract surgery within one year), self-reported pregnancy, or current use of punctal plugs. Individuals who were unable to provide analyzable data due excessive bodily or ocular movements during the measurement periods were also excluded. Subjects were instructed to refrain from using eye makeup or eye drops on study visit days, and were additionally instructed to not rub his or her eyes for the duration of each visit. Subjects were permitted to use eye makeup during the 1-month observational portion of the study, but were instructed to refrain from use at the 1-month follow-up visit.
Equipment and surveysTear film lipid layer thickness was measured using a stroboscopic video color microscope (SVCM), developed by King-Smith, which has been used for various tear film imaging studies.14,16 This instrument uses interferometric principles to capture the tear film lipid layer and report it as a thickness measurement in nanometers. The SVCM uses a stroboscopic white light source to illuminate a 6mm diameter region of the ocular surface, and records a 900-frame video over 40s (∼22images/s) using a high-resolution camera (1400×1000 pixels).15 Custom software was used to convert the video into LLT points throughout the field at every frame. These thickness values were reported in nanometers, and were further evaluated by dividing the overall field into equal thirds which assessed the upper, middle and lower segments of the test area.
Two surveys were used to assess symptoms of subjects: the Ocular Surface Disease Index (OSDI), and a unique visual analog scale (VAS) symptom survey created specifically for the study. Both the OSDI and VAS dry eye symptom surveys similar to the one used here have been shown to be valid and reliable for monitoring of dry eye symptoms over time.20,21 The VAS survey was designed to target distinct features of ocular dryness and included five symptoms characteristic of dry eye disease: ocular dryness, light sensitivity, eye fatigue, blurred vision, and discomfort. The severity of symptoms for each eye was reported using a tick-mark on a 100mm line, with 0mm identified as non-symptomatic and 100mm identified as maximally symptomatic.
Test materialsTear LLT measurements were taken after use of two different eye drops. Systane Complete® (Alcon Laboratories, Inc., Fort Worth, TX, USA) was utilized as the emollient containing eye drop and contains propylene glycol 0.6% (lubricant), and inactive ingredients including boric acid, dimyristoyl phosphatidylglycerol, edatate disodium, hydroxypropyl guar (HPG), mineral oil, polyoxl 40 stearate, polyquaternium-1 0.001%, sorbitan tristearate, sorbitol and purified water. This nanoemulsion contains HPG, which allows cross-linking of the eye drop on the ocular surface after instillation, and subsequently improves contact between the drop and the eye.22,23 Systane Ultra® (Alcon Laboratories, Inc., Fort Worth, TX, USA) was used as the non-emollient containing eye drop, and contains polyethylene glycol 400 0.4% (lubricant), and propylene glycol 0.3% (lubricant), as well as the inactive ingredients aminomethylpropanol, boric acid, HPG, polyquaternium-1 0.001%, potassium chloride, purified water, sodium chloride, and sorbitol. Both drops contain hydrochloric acid and sodium hydroxide for pH adjustment.
Examination proceduresFollowing the informed consent process, subjects completed OSDI and VAS surveys, and were examined under the biomicroscope to rule out active ocular inflammation or significant eye disease. Baseline tear LLT was obtained using the SVCM on each eye. During each SVCM measurement, subjects were instructed to blink naturally every 4s as indicated by the examiner to approximate a typical blink rate for the duration of the recording (40s). Only individuals who were able to provide analyzable data and had a LLT<75nm in either eye continued after screening examination. Of the 24 subjects screened, 20 were eligible to continue in the study and immediately begin the first of two eye drop exposure visits.
Subjects were randomized to either the nanoemulsion or non-emollient eye drop for their first eye drop exposure visit. One drop was instilled into each eye with minimal lid manipulation as to not stimulate meibomian gland secretion. Subjects completed a VAS symptom survey and LLT was measured at 15min, 1h, 2h, 4h and 6h following eye drop instillation. A second eye drop exposure visit occurred after a two days wash-out period using the drop not given at the first eye drop exposure visit.
After completing both eye drop exposure visits, all subjects were dispensed the nanoemulsion eye drop, and instructed to use the drop in each eye four times daily (qid). Subjects were instructed to use no other eye drops during the 30-day test period. Subjects were seen for follow-up examination in 30 days (±2 days). At the one month follow-up visit, subjects were asked to complete both dry eye symptom surveys, and SVCM measurements were taken for each eye following survey completion. Subjects were instructed to discontinue their drop usage at least 4h prior to clinical assessments at the one month follow-up visit. Examination procedures are summarized in Fig. 1.
Statistical analysisThe sample size for this study was driven by previous emollient eye drop studies, but was largely a pilot study as this particular nanoemulsion eye drop had not been previously tested for LLT interaction.
Paired t-tests were used to determine if significant changes in tear LLT and dry eye symptoms were found at each tested time point, including 1-month follow up testing, when compared to baseline values. For Part 1, each time point was compared to baseline LLT and symptoms for that study visit day. For Part 2, LLT and symptoms were compared to baseline values on the screening visit day.
Analysis of data was performed on the eye with the lower LLT measurement at the screening examination. In total, 10 right eyes were analyzed, and 10 left eyes were analyzed.
Following data collection, subjects were stratified into groups with higher (≥50nm, n=5) and lower (<50nm, n=15) baseline tear LLT for additional statistical analysis. The stratification of groups into <50nm and ≥50nm was based on two studies in which the average LLT value was reported to be 42nm in one study and 53nm in the other study.6,24
Results20 subjects completed the study: subjects ranged from 33 to 60 years of age (mean=45.6; standard deviation=7.9) and included five males and 15 females. The mean LLT for all subjects at baseline was 45.15±12.42nm.
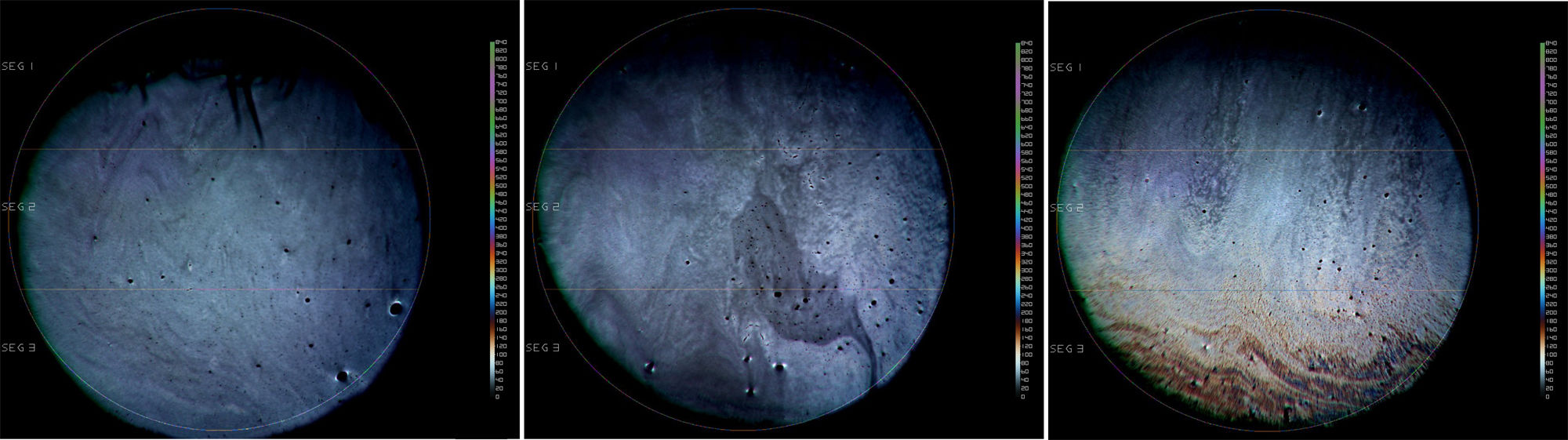
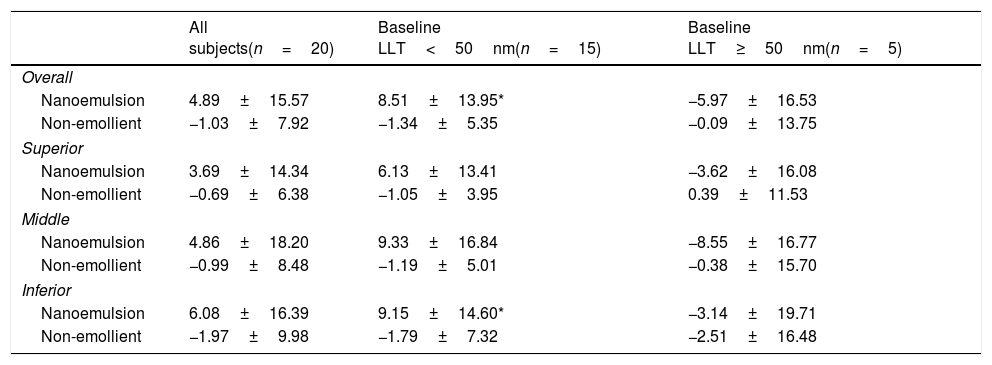
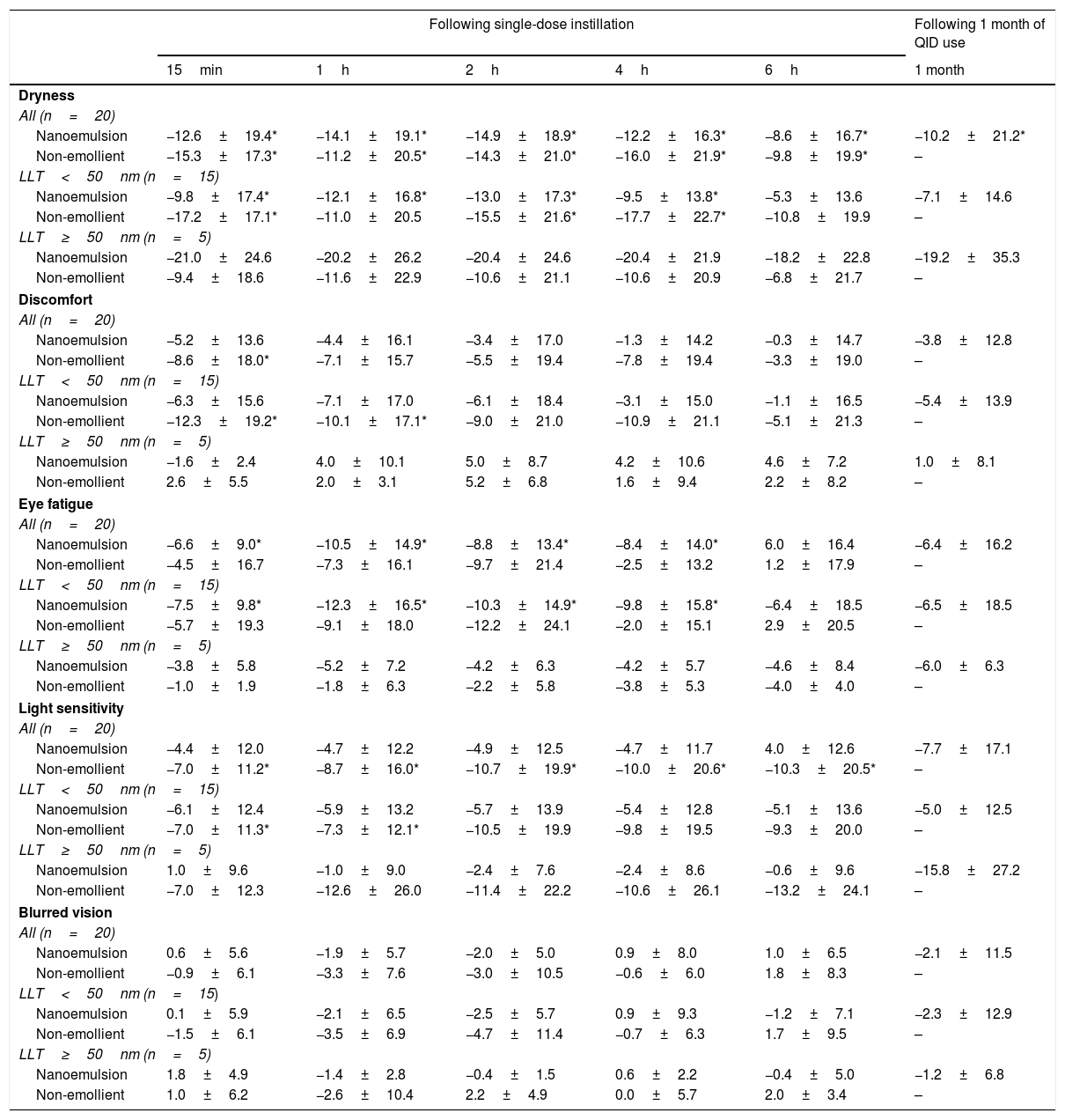
Part 1: Cross-over eye drop visitsAnalysis of the entire study cohort (baseline LLT<75nm, n=20) showed no significant increases in LLT at any time point following single-drop instillation of either the non-emollient or emollient-containing nanoemulsion eye drops. A post hoc stratification was conducted to determine if subjects with lower or higher initial LLT measurements at baseline had different effects. In the cohort of subjects with a baseline LLT<50nm (n=15), there was a significant increase in the mean LLT 15min after the nanoemulsion eye drop instillation in both the overall (8.51±13.95, p=0.03) and inferior third (9.15±14.60, p=0.03) of the measured area. For the same cohort, the mean change in LLT 15min after instillation of the non-emollient eye drop in the overall (−1.34±5.35, p=0.4) and inferior third (−1.79±7.32, p=0.4) were not statistically significant. In the cohort of subjects with a baseline LLT ≥50nm (n=5), no significant changes in mean LLT occurred with use of the nanoemulsion or non-emollient eye drop at any time point following single-drop instillation. Table 1 summarizes the changes in LLT 15min following single-drop instillation of both the nanoemulsion and non-emollient eye drops, as well as the significance at this time point for each cohort. No significant increase in LLT occurred for either drop at any time point beyond 15min for any cohort examined, and therefore, LLT measurements made beyond 15min are not presented in Table 1. Fig. 2 contains images of the lipid layer collected from a single subject at baseline and 15min after instillation each of the study eye drops.
Summary of changes to average LLT (±standard deviation) 15min after instillation of the two study eye drops for each cohort (all subject, subjects with baseline LLT<50nm, and subjects with baseline LLT ≥50nm). A 6mm diameter of the tear film was analyzed and the average change in LLT is reported for the overall area measured. The measured area was also split into equal thirds for analysis of the superior, middle, and inferior portions of the tear film.
| All subjects(n=20) | Baseline LLT<50nm(n=15) | Baseline LLT≥50nm(n=5) | |
|---|---|---|---|
| Overall | |||
| Nanoemulsion | 4.89±15.57 | 8.51±13.95* | −5.97±16.53 |
| Non-emollient | −1.03±7.92 | −1.34±5.35 | −0.09±13.75 |
| Superior | |||
| Nanoemulsion | 3.69±14.34 | 6.13±13.41 | −3.62±16.08 |
| Non-emollient | −0.69±6.38 | −1.05±3.95 | 0.39±11.53 |
| Middle | |||
| Nanoemulsion | 4.86±18.20 | 9.33±16.84 | −8.55±16.77 |
| Non-emollient | −0.99±8.48 | −1.19±5.01 | −0.38±15.70 |
| Inferior | |||
| Nanoemulsion | 6.08±16.39 | 9.15±14.60* | −3.14±19.71 |
| Non-emollient | −1.97±9.98 | −1.79±7.32 | −2.51±16.48 |
Stroboscopic video color microscope (SVCM) images taken from an individual subject who best represented average LLT at baseline and 15min post-instillation of the emollient and non-emollient eye drops in the lower LLT (<50nm) cohort. (A) Baseline image taken prior to drop instillation. (B) Image taken 15min following instillation of the non-emollient eye drop. (C) Image taken 15min following instillation of the nanoemulsion eye drop. The brighter colors correspond to a thicker lipid layer as depicted on the key to the right of each image.
Analysis of VAS surveys for all subjects (n=20) showed significant improvements in many symptoms following instillation of both the nanoemulsion and non-emollient eye drop. The change in VAS scores revealed significant improvement in average dryness up to 6h following instillation of both the nanoemulsion (8.6±16.7mm, p=0.03) and non-emollient (9.8±19.9, p=0.02) eye drops. Analysis of these surveys also showed an improvement in average eye fatigue up to 4h following instillation only for the nanoemulsion drop (8.4±14.0mm, p=0.01), and significant improvement in average light sensitivity up to 6h (10.3±20.5, p=0.02), and average ocular discomfort up to 15min (8.6±18.0, p=0.045) following drop instillation only for the non-emollient eye drop. These VAS scores were further evaluated by cohort as presented in Table 2.
Summary of changes to VAS symptom scores (±standard deviation) from baseline at each measured time point following instillation of the nanoemulsion and non-emollient eye drops (Part 1) and at 1 month follow-up (Part 2). Negative values indicate an improvement in symptoms.
| Following single-dose instillation | Following 1 month of QID use | |||||
|---|---|---|---|---|---|---|
| 15min | 1h | 2h | 4h | 6h | 1 month | |
| Dryness | ||||||
| All (n=20) | ||||||
| Nanoemulsion | −12.6±19.4* | −14.1±19.1* | −14.9±18.9* | −12.2±16.3* | −8.6±16.7* | −10.2±21.2* |
| Non-emollient | −15.3±17.3* | −11.2±20.5* | −14.3±21.0* | −16.0±21.9* | −9.8±19.9* | – |
| LLT<50nm (n=15) | ||||||
| Nanoemulsion | −9.8±17.4* | −12.1±16.8* | −13.0±17.3* | −9.5±13.8* | −5.3±13.6 | −7.1±14.6 |
| Non-emollient | −17.2±17.1* | −11.0±20.5 | −15.5±21.6* | −17.7±22.7* | −10.8±19.9 | – |
| LLT≥50nm (n=5) | ||||||
| Nanoemulsion | −21.0±24.6 | −20.2±26.2 | −20.4±24.6 | −20.4±21.9 | −18.2±22.8 | −19.2±35.3 |
| Non-emollient | −9.4±18.6 | −11.6±22.9 | −10.6±21.1 | −10.6±20.9 | −6.8±21.7 | – |
| Discomfort | ||||||
| All (n=20) | ||||||
| Nanoemulsion | −5.2±13.6 | −4.4±16.1 | −3.4±17.0 | −1.3±14.2 | −0.3±14.7 | −3.8±12.8 |
| Non-emollient | −8.6±18.0* | −7.1±15.7 | −5.5±19.4 | −7.8±19.4 | −3.3±19.0 | – |
| LLT<50nm (n=15) | ||||||
| Nanoemulsion | −6.3±15.6 | −7.1±17.0 | −6.1±18.4 | −3.1±15.0 | −1.1±16.5 | −5.4±13.9 |
| Non-emollient | −12.3±19.2* | −10.1±17.1* | −9.0±21.0 | −10.9±21.1 | −5.1±21.3 | – |
| LLT≥50nm (n=5) | ||||||
| Nanoemulsion | −1.6±2.4 | 4.0±10.1 | 5.0±8.7 | 4.2±10.6 | 4.6±7.2 | 1.0±8.1 |
| Non-emollient | 2.6±5.5 | 2.0±3.1 | 5.2±6.8 | 1.6±9.4 | 2.2±8.2 | – |
| Eye fatigue | ||||||
| All (n=20) | ||||||
| Nanoemulsion | −6.6±9.0* | −10.5±14.9* | −8.8±13.4* | −8.4±14.0* | 6.0±16.4 | −6.4±16.2 |
| Non-emollient | −4.5±16.7 | −7.3±16.1 | −9.7±21.4 | −2.5±13.2 | 1.2±17.9 | – |
| LLT<50nm (n=15) | ||||||
| Nanoemulsion | −7.5±9.8* | −12.3±16.5* | −10.3±14.9* | −9.8±15.8* | −6.4±18.5 | −6.5±18.5 |
| Non-emollient | −5.7±19.3 | −9.1±18.0 | −12.2±24.1 | −2.0±15.1 | 2.9±20.5 | – |
| LLT≥50nm (n=5) | ||||||
| Nanoemulsion | −3.8±5.8 | −5.2±7.2 | −4.2±6.3 | −4.2±5.7 | −4.6±8.4 | −6.0±6.3 |
| Non-emollient | −1.0±1.9 | −1.8±6.3 | −2.2±5.8 | −3.8±5.3 | −4.0±4.0 | – |
| Light sensitivity | ||||||
| All (n=20) | ||||||
| Nanoemulsion | −4.4±12.0 | −4.7±12.2 | −4.9±12.5 | −4.7±11.7 | 4.0±12.6 | −7.7±17.1 |
| Non-emollient | −7.0±11.2* | −8.7±16.0* | −10.7±19.9* | −10.0±20.6* | −10.3±20.5* | – |
| LLT<50nm (n=15) | ||||||
| Nanoemulsion | −6.1±12.4 | −5.9±13.2 | −5.7±13.9 | −5.4±12.8 | −5.1±13.6 | −5.0±12.5 |
| Non-emollient | −7.0±11.3* | −7.3±12.1* | −10.5±19.9 | −9.8±19.5 | −9.3±20.0 | – |
| LLT≥50nm (n=5) | ||||||
| Nanoemulsion | 1.0±9.6 | −1.0±9.0 | −2.4±7.6 | −2.4±8.6 | −0.6±9.6 | −15.8±27.2 |
| Non-emollient | −7.0±12.3 | −12.6±26.0 | −11.4±22.2 | −10.6±26.1 | −13.2±24.1 | – |
| Blurred vision | ||||||
| All (n=20) | ||||||
| Nanoemulsion | 0.6±5.6 | −1.9±5.7 | −2.0±5.0 | 0.9±8.0 | 1.0±6.5 | −2.1±11.5 |
| Non-emollient | −0.9±6.1 | −3.3±7.6 | −3.0±10.5 | −0.6±6.0 | 1.8±8.3 | – |
| LLT<50nm (n=15) | ||||||
| Nanoemulsion | 0.1±5.9 | −2.1±6.5 | −2.5±5.7 | 0.9±9.3 | −1.2±7.1 | −2.3±12.9 |
| Non-emollient | −1.5±6.1 | −3.5±6.9 | −4.7±11.4 | −0.7±6.3 | 1.7±9.5 | – |
| LLT≥50nm (n=5) | ||||||
| Nanoemulsion | 1.8±4.9 | −1.4±2.8 | −0.4±1.5 | 0.6±2.2 | −0.4±5.0 | −1.2±6.8 |
| Non-emollient | 1.0±6.2 | −2.6±10.4 | 2.2±4.9 | 0.0±5.7 | 2.0±3.4 | – |
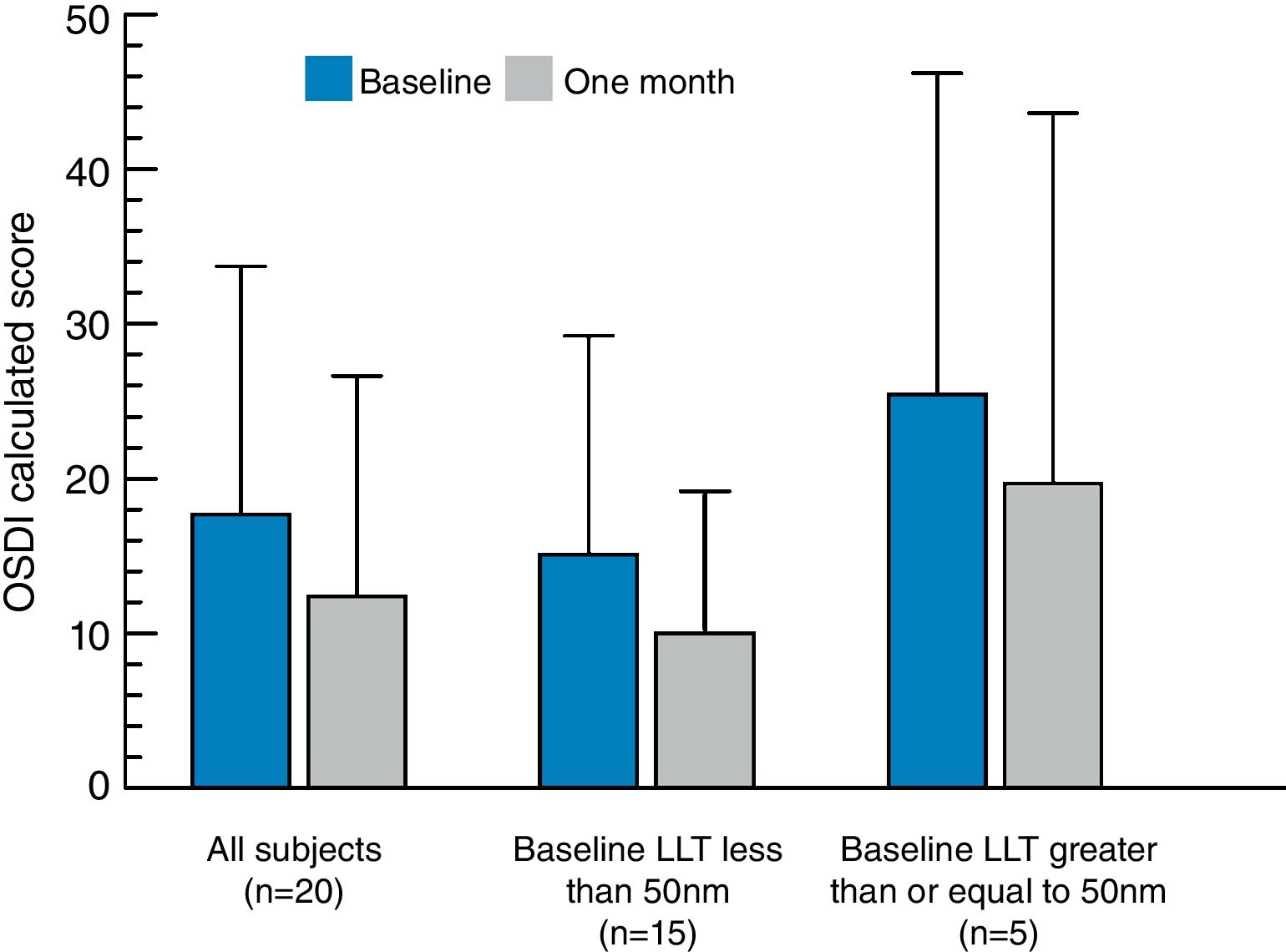
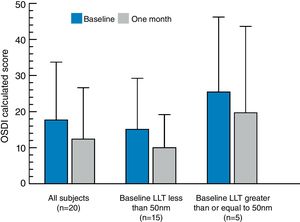
There was no significant difference when comparing the initial LLT measured at baseline to the LLT measurement taken after one month of qid use of Systane® Complete. The average OSDI calculated score at the baseline visit was 17.7 (±16.0) and the average OSDI score after using the eye drops qid for 1 month was 12.4 (±14.2). The change in OSDI calculated score at baseline versus the 1 month follow-up visit is presented in Fig. 3. The decrease of 5.3 in the average OSDI score from baseline to 1 month post eye drop use was statistically significant (p=0.03). The average VAS dryness score decreased by 10.2±21.2 (p=0.045) from baseline (26.3) to 1 month (16.1). Comparison of the average baseline and 1 month VAS scores for blurred vision, eye fatigue, light sensitivity, and discomfort showed a numerical reduction in symptoms at 1 month, but these differences were not statistically significant. Table 2 summarizes the changes in symptom scores and significance for each cohort.
Calculated OSDI scores (±standard deviation) for each study cohort (all subjects, subjects with baseline LLT<50nm, and subjects with baseline LLT ≥50nm) at baseline and at 1-month follow-up after qid use of the nanoemulsion eye drop. The observed change in OSDI for all subjects (n=20) exhibited statistical significance (p=0.03). No significant differences in OSDI score were found for either the low (<50nm LLT at baseline) or high (≥50nm LLT at baseline) cohorts.
Previous studies using highly viscous emollient eye drops found significant increases in the LLT in the inferior portion of the tear film 15min after use of the drops.14,15 No previous studies have determined the amount or concentration of lipid needed to increase the LLT enough to maintain tear stability. The formulation of the nanoemulsion eye drop used in this study is less viscous upon immediate application to the eye and thickens by process of polymer crosslinking when in contact with the pH of the ocular surface.25 This method of delivering an emollient to the ocular surface is different than some previously tested,14,15 in which the emollient is viscous upon instillation and remains unchanged after instillation. As some individuals find less viscous eye drops more visually tolerable,18 it was hypothesized that this formulation would minimize transient visual effects after drop instillation, yet still contain enough emollient to result in a thicker, and therefore, more stable tear LLT. The results of this study found that when evaluating the change in LLT over the inferior ocular surface, as has been the standard in previous studies,14,15 those with baseline LLT values at 50nm or below resulted in a 9.15±14.60nm increase in mean LLT when using the nanoemulsion study eye drop. While this increase is less than those found following the use of typical emollient eye drops,14,15 the results of this study reveal that in subjects with low initial LLT values, a statistically significant increase in LLT is possible with an eye drop that has a less viscous application to the ocular surface. No increase in tear LLT was found after nanoemulsion eye drop instillation in individuals with high baseline LLT levels (n=5). In fact, an average decrease in tear LLT was found at this time point, suggesting that individuals with high LLT may have different ocular surface interactions with various drop formulations. It is also possible that high standard deviations in this cohort influenced average values at this measurement time. Certainly, a limitation of this study is the small sample size, particularly for the higher LLT cohort.
Tear LLT and symptoms of DED have previously been correlated by Blackie et al.,12 however, work performed by King-Smith et al. has suggested that the thickness of the tear lipid layer may only be one of many tear film properties affecting lipid layer integrity.6 Therefore, it was important to evaluate subjective findings in all LLT cohorts with use of both nanoemulsion and non-emollient eye drops. Those subjects in the cohort with symptoms of dry eye but with a tear LLT greater than 50nm at baseline did not have a statistically significant change in LLT, yet the VAS surveys of the entire pool of subjects showed an improvement in ocular dryness with use of the emollient eye drop. The fact that the LLT did not increase in this cohort after instillation of the emollient eye drop but symptoms of ocular dryness improved suggests that improvement in symptoms were likely due to the aqueous component of this eye drop alone. Utilizing an eye drop which can improve symptoms of DED, despite the etiology, makes nanoemulsion eye drops like the one used in this study attractive for the treatment of individuals suffering from symptoms of dryness, but who have perhaps not yet undergone a dry eye workup to determine the underlying cause of the disease.
Symptoms and LLT measurements assessed after the 15min time point showed diverging results. Despite the lack of a statistically significant increase in LLT at the 1h time point or beyond, VAS symptom scores were improved in several categories in both arms of the experiment for up to 6h. A study by Papas et al. determined the just-noticeable difference (JND) in ocular comfort utilizing a VAS is between 7 and 8 units, with any change in VAS score at this level or greater considered clinically significant.26 Using this criteria, the VAS changes after use of the emollient eye drop were both statistically significant and clinically significant for symptoms of dryness at all time points measured including 6h post-instillation. The changes in VAS scores for eye fatigue similarly had statistically significant and clinically significant improvements up to and including the 4h time point. Changes in symptoms when using the non-emollient eye drop showed similar improvements in dryness up to the 6h time point, but improvements in ocular discomfort were statistically and clinically significant only at the 15min post-instillation assessment. The non-emollient eye drop improved symptoms of light sensitivity at all time points measured.
The level of improvement in OSDI and VAS scores after 1 month of daily use (qid) of the nanoemulsion eye drop is relevant on a clinical level. A study by Miller determined the specifications for a minimally clinically important difference (MCID) for the average dry eye severity.27 MCID as it relates to DED specifies what improvement in OSDI symptom score correlates to improved clinical signs in dry eye patients.27 Miller et al. found that MCID values for individuals with mild-moderate DED were 4.5–7.3.27 Therefore, the 5.3 unit decrease in average OSDI for the entire study cohort found in our analysis was both statistically and clinically significant. Additionally, VAS surveys showed a significant improvement in symptoms of ocular dryness after 1 month use of the emollient eye drop qid (10.2±21.2, p=0.045). This decrease was also found to be clinically-significant.26
There were several limitations to this study. Specifically, Part 2 of the study examined sustained use of the nanoemulsion eye drop, but did not employ a cross-over design as Part 1 did. Therefore, it cannot be determined how sustained use of the non-emollient eye drop affected symptoms of dry eye compared to sustained use of the nanoemulsion eye drop. Additionally, no tests were performed during this study to categorize subjects based on underlying etiology or to characterize the severity of subject's dry eye disease. Instead, subjects were stratified into groups based on baseline LLT. Certainly, further studies of tear LLT and how it relates to dry eye disease severity and etiology is needed to fully understand the implications of clinical treatment of the disorder.
Several factors may contribute to the development of DED, including age, increasing screen and device time, and environmental factors.2 With the prevalence of DED seemingly on the rise, it is critical that eye care providers are able to recommend appropriate eye drop formulations and dosing patterns to effectively target both aqueous-deficient and evaporative forms of DED.
In this study population, subjects with low LLT levels had an average increase in LLT after utilizing a nanoemulsion eye drop. Improvements in average VAS symptoms scores for all subjects were noted for the emollient and non-emollient eye drops during the cross-over portion of the study. Use of the nanoemulsion eye drop also improved some symptoms of dry eye after 1 month of regular use. Results suggest that individuals suffering from dry eye symptoms may benefit from both single-dose instillation and sustained use of this type of eye drop regardless of natural lipid layer thickness values.
FundingThis study was sponsored by Alcon, Inc, and conducted by the Innovation in Vision and Eye Care Research Group (iVERG) at The Ohio State University College of Optometry.
Conflicts of interestJS Fogt: Consulting – Alcon.
K Weisenberger: No disclosures.
N Fogt: No disclosures.