Glaucoma consists of a group of progressive optic neuropathies that are characterized by degeneration of the optic nerve and irreversible visual filed loss. Elevated intraocular pressure is the only proven treatable risk factor and commercial products used for glaucoma treatment are focused in lowering intraocular pressure. These drugs can have various undesirable side effects and this invites to look for new strategies. The purpose of this work is to study the use of a siRNA (small interfering RNA) to selectively silence beta2 adrenergic receptors and to see whether it reduces IOP (intraocular pressure).
Material and methodsTopical instillation of beta2 adrenergic receptors small-interfering RNA (siRNA, 25–250μg) was applied and IOP was measured with a Tonopen XL up to 9 consecutive days. The effect of such siRNA was compared to commercial compounds such as Timoftlol, Trusopt and Xalatan, and it was also analyzed if some anatomical changes occurred by microscopy.
ResultssiRNA designed for beta2 adrenergic receptor induced a reduction of intraocular pressure (IOP) of 30±5%, compared to a control (scrambled siRNA). The results in terms of IOP decrease were similar to that found with commercial compounds but a long-lasting hypotensive action was shown by beta2 adrenergic receptor siRNA treatment as compared to commercial drugs. No apparent side effects were observed in the ocular structures.
ConclusionThe use of siRNA against the beta2 adrenergic receptors could provide an interesting therapeutic strategy for glaucoma treatment.
El glaucoma consiste en un grupo de neuropatías ópticas progresivas caracterizadas por degeneración del nervio óptico y pérdida irreversible del campo visual. La elevación de la presión intraocular es el único factor de riesgo tratable probado, centrándose los productos comerciales para el tratamiento del glaucoma en la reducción de la presión intraocular. Estos fármacos pueden tener diversos efectos secundarios indeseados, lo cual invita a buscar nuevas estrategias. El objetivo de este trabajo es estudiar el uso de un ARNip (ARN pequeño de interferencia) para silenciar selectivamente los receptores adrenérgicos beta2, y comprobar si reduce la PIO (presión intraocular).
Material y métodosSe realizó instilación tópica de ARN pequeño de interferencia para los receptores adrenérgicos beta2 (ARNip, 25-250μg), midiéndose la PIO con Tonopen XL hasta nueve días consecutivos. Se comparó el efecto de dicho ARNip con componentes comerciales tales como Timoftlol, Trusopt y Xalatan, analizándose asimismo mediante microscopio si se producía cualquier cambio anatómico.
ResultadosARNip diseñado para el receptor adrenérgico beta2 indujo una reducción de la presión intraocular (PIO) de 30±5%, en comparación al control (ARNip de secuencia aleatoria). Los resultados en términos de reducción de la PIO fueron similares a los encontrados utilizando componentes comerciales, aunque el tratamiento con ARNip para el receptor adrenérgico beta2 reflejó una acción hipotensora de larga duración en comparación con los fármacos comerciales. No se observaron efectos secundarios aparentes en las estructuras oculares.
ConclusiónEl uso de ARNip contra los receptores adrenérgicos beta2 podría aportar una estrategia terapéutica interesante para el tratamiento del glaucoma.
Glaucoma consists of a group of eye diseases showing a broad spectrum of clinical presentation and unknown aetiologies, that lead to a permanent loss of visual function due to the death of retinal ganglion cells and damage of the optical nerve.1 It is well accepted that a critical risk factor in the pathogenesis of glaucoma is the elevation in intraocular pressure (IOP) and it is the only proven treatable risk factor.2
IOP is generated and maintained by the aqueous humor circulation system in the anterior eye.3 Elevated IOP occurs from an imbalance between production and outflow of aqueous humor. The aqueous humor is a transparent nutritional fluid that provides the nutrients to the inner avascular structures of the eye.4 The control of the aqueous humor production occurs in the ciliary body and it is regulated by the sympathetic nervous system.5 Noradrenaline released from the sympathetic terminals stimulate adrenergic receptors that facilitate the production of the aqueous humor. This fact has been taken by the pharmaceutical companies to develop a series of antagonists of mainly β2-adrenoceptors to reduce aqueous humor production, reducing IOP in glaucoma.6 Other drugs currently used to decrease the rate of aqueous humor inflow are carbonic anhydrase inhibitors and α1-adrenoceptor agonists.6 Additionally, there are pharmacological agents such as cholinoceptor agonists and prostaglandin analogs that lower IOP by increasing the rate of aqueous humor outflow.7,8
Thus, pharmacological reduction of IOP by decreasing the rate of aqueous humor inflow or increasing the rate of aqueous outflow is the current therapeutic approach for preserving visual function in glaucoma patients. However, all the pharmacological agents mentioned can have various undesirable effects. For instance, β2-adrenoceptors blockers can cause substantial cardiovascular and respiratory side effects,8 carbonic anhydrase inhibitors use must be restricted in subjects with renal disease and patients with compromised endothelium,9 prostaglandin analogs can originate conjunctival hyperaemia, lengthening and thickening of eyelashes or changes in eye color.10 Moreover, the efficacy of these drugs is also likely to be reduced after prolonged use.1 Thus, there is the need to discover novel agents from the presently available pharmacological classes. In this context, RNA interference-based therapy for glaucoma can be an attractive alternative approach. RNA interference involves specific gene expression silencing by small molecules of double stranded RNA: small interference RNAs (siRNAs).11
The eye is currently considered a good target for RNAi therapy mainly because it is an accessible and confined compartment. The accessibility of the eye facilitates siRNA delivery, and naked siRNAs have been efficiently applied by topical administration to the anterior segment or by intravitreal injection to the posterior segment.12 In addition, its confined location provides several advantages. Local delivery of compounds to the eye restricts systemic exposure and reduces the amount of compound needed diminishing potential side effects outside the eye. Moreover, the immune system has a limited access to the eye; therefore immune responses to the compound are less likely to occur.13
As the localized delivery of siRNA to the eye is less challenging than for other tissues, there has been significant progress made toward its use as a therapeutic procedure for eye diseases.14 In fact, several siRNA-based therapeutic agents for ocular disorders such as wet age-related macular degeneration and diabetic retinopathy have already reached clinical trials.15–17
In the present work, we analyze the use of siRNA, designed to silence β2-adrenoceptors, in order to reduce IOP in rabbits, studying the characteristics of different concentrations of siRNA on the animal responses and comparing this novel strategy with classical glaucoma treatments.
Materials and methodsAnimals24 male normotensive New Zealand white rabbits, with 6–8 weeks of age, were kept in individual cages with food and water ad libitum and maintained under a controlled 12h/12h light/dark cycles. All the procedures complied with the ARVO Statement for the Use of Animals in Ophthalmology and Vision Research. Animal studies were approved by the Ethical Committee of Complutense University (UCM-CEA) which follows the tenets of the European Communities Council Directive (86/609/EEC).
siRNA and commercial drug applicationAll the siRNA experiments were performed and measured at the same time. A siRNA targeting the beta2 adrenergic receptor (sc-39866) and siRNA scramble (sc-37007) were purchased from Santa Cruz (Hercules, CA, USA).
The siRNAs were topically applied in one eye and the contralateral eye received the same volume of saline solution (0.9% NaCl). The single eye treated with the siRNAs received an amount of 250μg of the siRNA dissolved in 40μL of 0.9% NaCl, daily in a single dose on four consecutive days. For dose–response studies, concentrations of 125μg, and 25μg were dissolved in 40μL of 0.9% NaCl. Experiments were performed using a single blind design: no visible indication was given to the experimenter as to the applied solution (agent or vehicle). IOP was measured for more than 8 days and, afterwards, animals were killed and both eyes were collected.
In the experiments performed with commercially available drugs 40μL of the commercial drugs: Trusopt (dorzolamide) (Merck Sharp & Dohme de España S.A., Madrid, Spain), Xalatan (latanoprost) (Pfizer, Alcobendas, Madrid, Spain) and Timoftol (timolol) (Santen Oy, Tampere, Finlandia) were instilled daily over a period of four consecutive days. IOP levels of rabbits treated with commercial drugs or with siRNAs were measured every hour for a total of 8h, starting 1h after the last administration. In addition, to estimate the mean time effect of these compounds, IOP was also measured for 8 days as described above.
Intraocular pressure (IOP) measurements in rabbitsIOP was measured by means of a Tono-Pen XL contact tonometer (Mentor Massachusetts Inc., Norwell, MA). Topical anesthesia (Colircusi, Laboratorios Cusi, Spain: 0.1mg/ml tetracaine plus 0.4mg/ml oxybuprocaine in 0.9% saline, diluted 1:3 in 0.9% saline) was applied (10μL) to the cornea before each measurement to avoid animal discomfort.
Side effectsThe assessment of the ocular tolerance of the RNAi sequences, were followed several aspects and parameters from the tear film, ocular anexa and ocular surface. The analysis were performed and graded before and after the topical application of siRNA with the Efron grading scales. A Topcon SL-8Z slit lamp and a Topcon SP-2000P specular microscope (Topcon Spain, Madrid, Spain), as well as a digital camera and a “de visu” exam were used for every experiment. Slit-lamp images were captured under 10× augments, a diffuser filter and a medium degree of illumination. The images were recorded and analyzed by IMAGEnet 2000 system software (Topcon).
Data analysisAll data are presented as the mean±S.E.M. Significant differences were determined by two-tailed Student's t-tests. The plotting and fitting of all the data was carried out with the computer program Prism GraphPad v.4.0 (Prism, USA).
ResultsEffect and specificity of beta2 adrenergic siRNA on IOPThe effect of a siRNA designed for silencing the beta2 adrenergic receptor was tested together with a scramble siRNA in order to confirm the specificity of the tested siRNA. Measurement of intraocular pressure (IOP) was monitored during more than 8 days applying both siRNA under the conditions described in methods (250μg of the siRNA in 40μL).
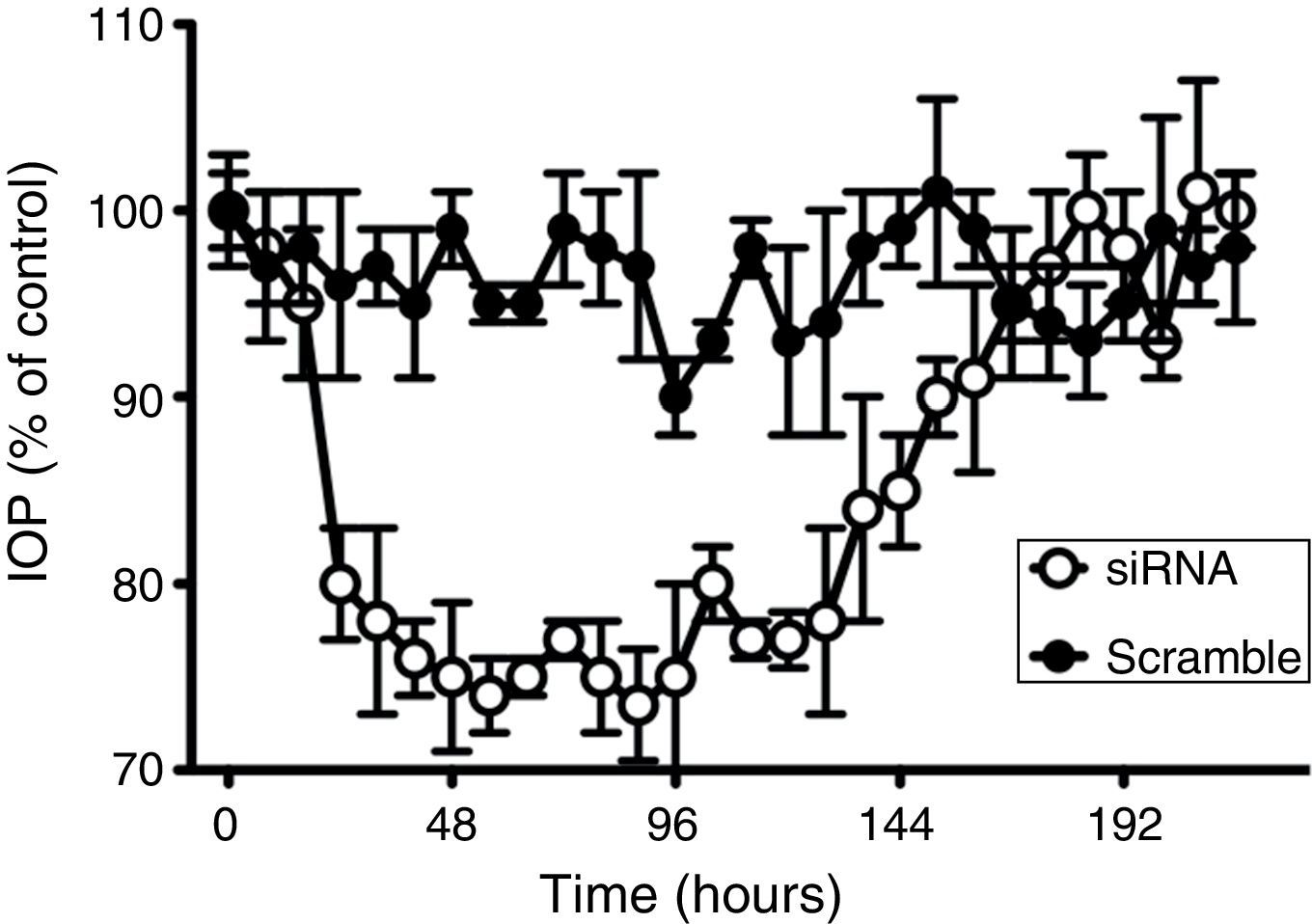
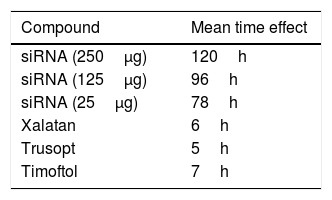
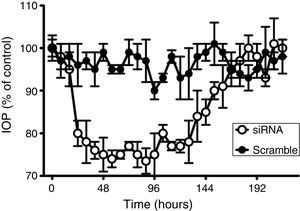
As observed in Fig. 1, whereas beta2 adrenergic receptor siRNA application clearly induced a hypotensive effect reducing IOP 74.0±2.0% (n=8), after the treatment with the scramble siRNA, rabbit IOP did not significantly change during the time of the experiment, confirming both the effect and the specific action of beta2 adrenergic receptor siRNA. Concerning the mean time effect (duration of the effect), the application of the aforementioned siRNA produced a maximal effect that lasted for about 120h (Table 1).
Comparative time-course of changes in intraocular pressure in response to beta2 adrenergic receptor siRNA and scramble siRNA. Effects of scramble siRNA (filled circles) and beta2 adrenergic receptor siRNA (open circles) on intraocular pressure were followed for 216h. 100% represents the intraocular pressure before the application of siRNAs which corresponded to a IOP of 15.2±1.8mm Hg. Values represent the mean±S.E.M. (n=8).
Mean time effect of the tested compounds.
| Compound | Mean time effect |
|---|---|
| siRNA (250μg) | 120h |
| siRNA (125μg) | 96h |
| siRNA (25μg) | 78h |
| Xalatan | 6h |
| Trusopt | 5h |
| Timoftol | 7h |
The mean time effect of each dose of beta2 adrenergic receptor siRNA as well as the commercial drugs Xalatan, Trusopt and Timoftol was calculated as the difference between the half maximal time-point of recovery and the time at which the half maximal effect on IOP was achieved.
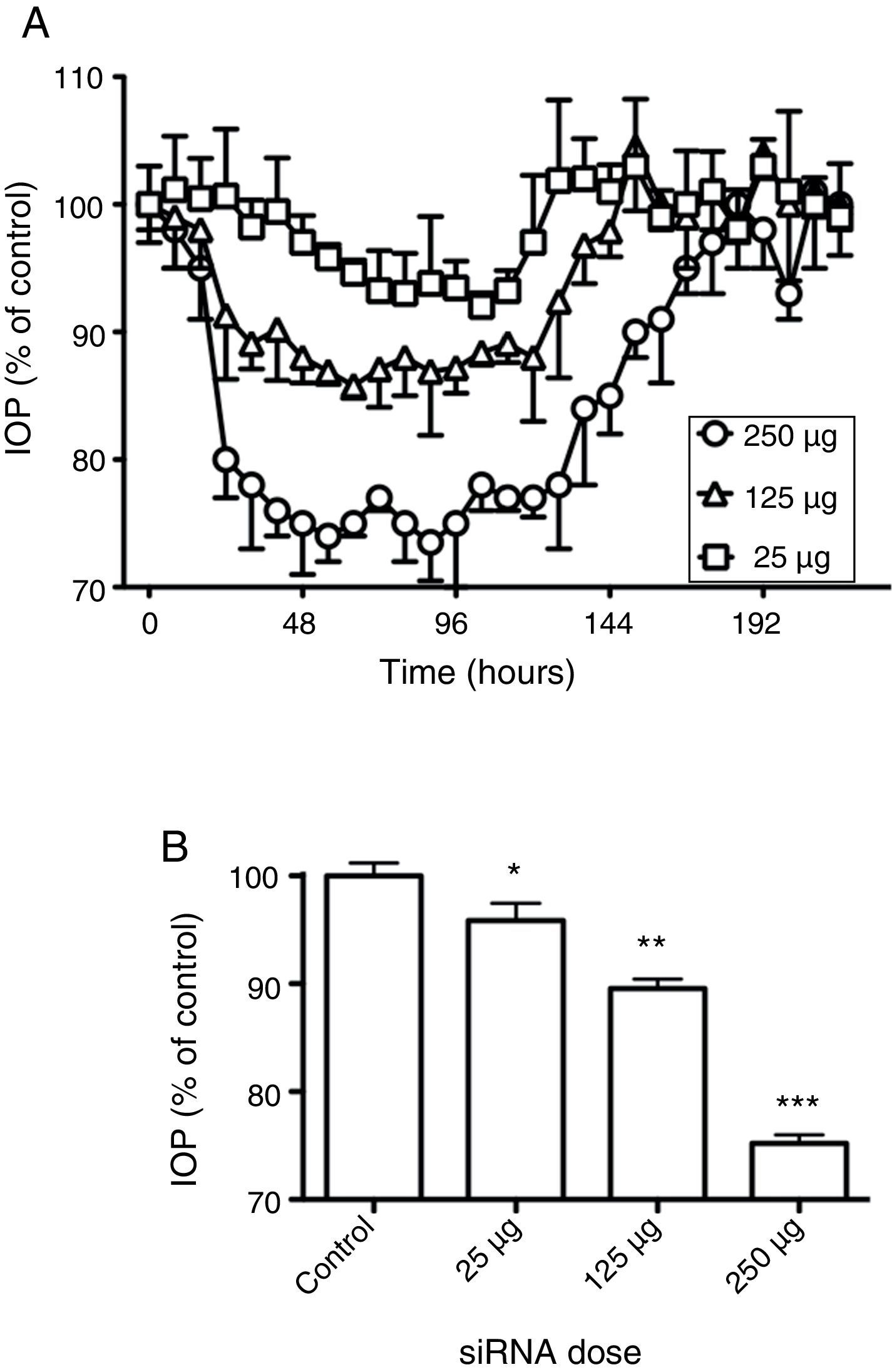
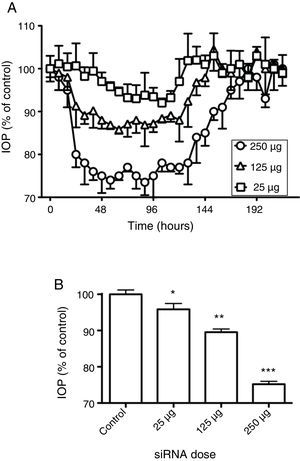
To see whether the silencing of beta2 adrenergic receptors produced changes in the IOP when different doses were applied, the rabbits were treated with the siRNAs against the beta2 adrenergic receptor and monitored in their IOP for more than 8 days. Three different concentrations of the beta2 adrenergic receptor siRNA were tested: 250μg, 125μg and 25μg, dissolved in 40μL of 0.9% NaCl (Fig. 2). As it shown in Fig. 2A the lowest dose of siRNA tested only induced a slight modification in IOP with a reduction of 8% 102h after application. The dose of 125μg evoked an IOP decrease of around 10% at 24h, with the highest effect (14% of reduction) observable 60h after application and sustained in the next 24h.
Effect of siRNAs on intraocular pressure. (A) Time-course of changes in intraocular pressure in response to siRNAs instillation. Effects of siRNAs at different concentrations on intraocular pressure were followed for 216h. (B) Comparison between the maximal effects obtained for the siRNAs. 100% represents the intraocular pressure before application of siRNAs. Values represent the mean±S.E.M. (n=8, *p<0.05, **p<0.01, ***p<0.001 vs. control).
The highest dose of beta2 adrenergic receptor siRNA (250μg), induced a robust reduction in IOP which started to be measurable 24h after the instillation of the siRNA and which was statistically significant for 5 days. The maximal reduction obtained after silencing the beta2 receptor was 30±5% compared to control. This and similar values were maintained for 1–2 days, with the IOP remaining low for three more days (with a mean of 30% of reduction) and then returning slowly toward control values (Fig. 2A). The maximal reduction in IOP obtained for each given dose is presented in Fig. 2B, where it can be seen that the reduction of IOP is dependent of the doses assayed. Interestingly, it was possible to observe that the higher the dose of beta2 adrenergic receptor siRNA was applied the longer mean-time effect was obtained (Table 1).
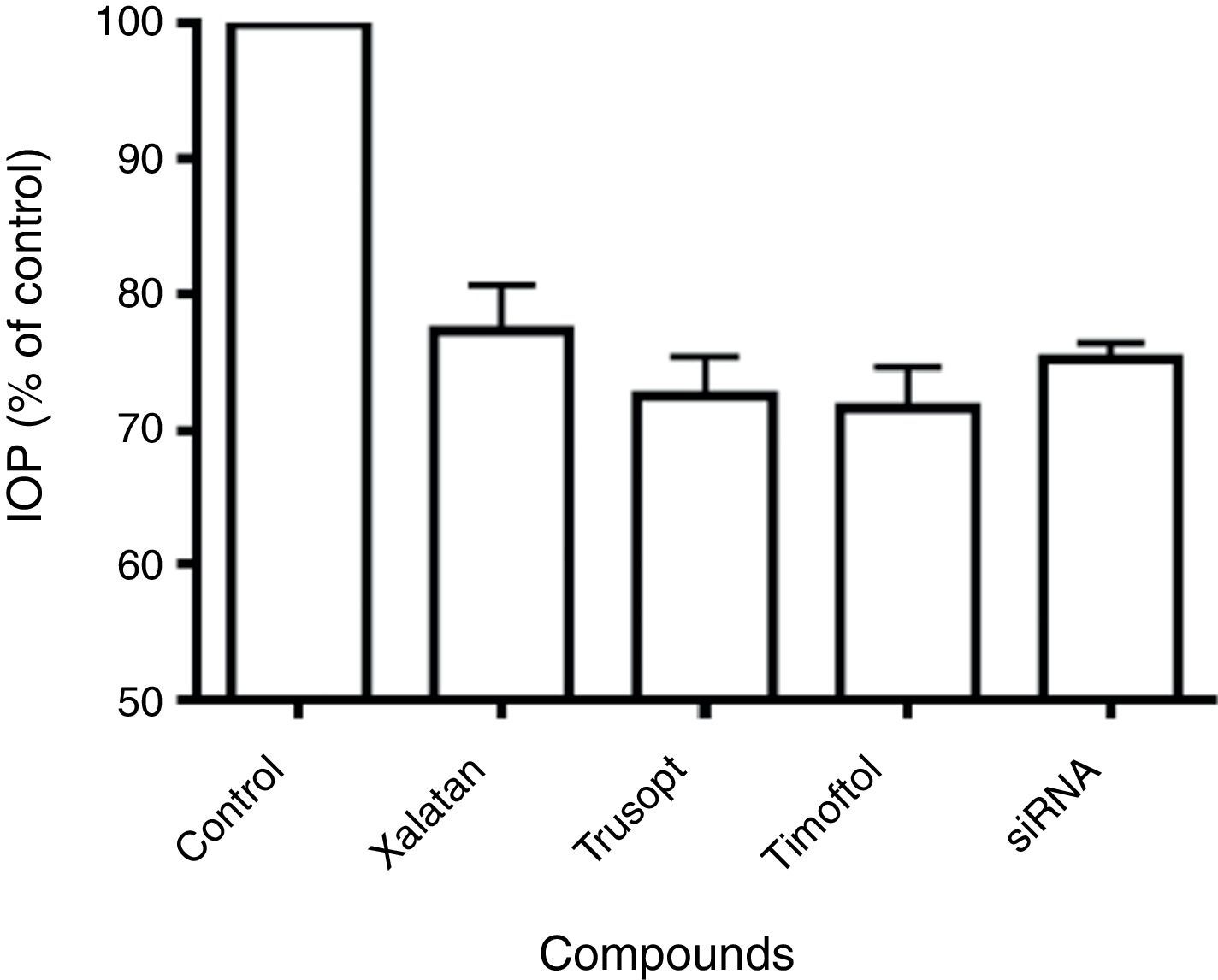
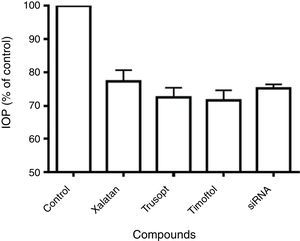
Comparative studies with commercial drugsThe effect of beta2 adrenergic receptor siRNA on IOP of New Zealand White rabbits was compared with that induced by ophthalmic commercial drugs: Trusopt (dorzolamide), Xalatan (latanoprost) and Timoftol (timolol). As observed in Fig. 3, any of those compounds produced a decrease in IOP of between 20 and 35%, this reduction in IOP was similar to that found with beta2 adrenergic receptor siRNA treatment.
Maximal IOP reduction elicited by siRNA against the beta2 adrenergic receptor and anti-glaucomatous drugs. Maximal IOP reduction (% of control) for beta2 adrenergic receptor siRNA tested (250μg) was compared with the hypotensive action induced by Xalatan, Trusopt and Timoftol. Control (100%) represents the intraocular pressure before the application of any drug (15.2±1.8mm Hg). Values represent the mean±S.E.M. (n=8).
In addition, the effect of the beta2 adrenergic receptor siRNA in terms of time effect was also compared with conventional pharmacological agents and the mean time effect of each compound was calculated as the difference between the half maximal time-point of recovery and the time at which the half maximal effect on IOP was achieved (Table 1). Commercial drugs showed mean time effect values between 5 and 7h, whereas the effect of the beta2 adrenergic receptor siRNA was significantly longer exhibiting values between 78 and 120h for the higher doses assayed.
DiscussionGlaucoma is one of the leading causes of blindness in the world. Anti-glaucomatous drugs currently prescribed can have various undesirable effects and their efficacy is also subject to a reduction after prolonged use.1 Moreover, to maintain adequate levels of IOP, frequent instillation is necessary, which can lead to poor compliance of the patient. Therefore, the development of new therapeutic strategies to circumvent the drawbacks associated to the presently available pharmacologic classes is required. In this context, we analyzed siRNA-based therapy for glaucoma.
In particular, we focused our attention on beta2 adrenergic receptors, since their commercial inhibitors are effective in lowering IOP, the primary indication of glaucoma.6 The hypotensive action of a commercial sequence of beta2 adrenergic receptor siRNA was tested in rabbits. The results confirmed the reduction of the IOP in rabbits, in a marked and sustained way. The frequency of application is a factor to be considered to achieve this IOP reduction in a reproducible mode. In this case, beta2 adrenergic receptor siRNA was administered daily in a single dose on four consecutive days in rabbits. This approach, the use of siRNA to reduce IOP has been also observed by other authors, with similar results to the ones here presented.18,19 In a clear difference with those works, the reduction in IOP observed at the highest siRNA concentration showed a decreased in IOP around 30% while in the work by Moreno-Montañes and co-workers it only reduced around 20%.19 Also, the concentrations used by these authors, up to 900μg/day are far from the doses here studied, which were 250μg/day. The present study was performed in rabbits while the one of 900μg/day was carried out in humans. Although the aqueous humors in rabbits and humans are not so different, it would be interesting to carry out experiments in humans to confirm the potential higher efficacy of the siRNA used in our work.
The value of IOP reduction obtained using siRNA was similar to that found with drugs currently prescribed for glaucoma treatment such as Xalatan, Trusopt or Timoftol. Although the recommended dosage for Trusopt or Timoftol in some patients is one drop in the affected eye twice a day, we administrated these drugs following similar experimental conditions than those used for siRNA application (a daily single dose on four consecutive days). Clearly, differences in application frequency of therapies can have influence in the results. In order to compare the hypotensive effect induced by siRNA treatment with the effect produced by drugs we used the same dosage regimen.
The effect of beta2 adrenergic siRNA treatment was significantly longer in terms of time-effect as compared to conventional pharmacological agents. While the commercial compounds reduced IOP for a few hours, the siRNA effect lasted for several days. In a previous study,18 a single dose of a siRNA for the beta receptor, provided a mean time effect of 91h, while in our case it was about 120h. Therefore, it seems that comparing commercial compounds as well as other siRNA treatments already published, it seems that the characteristics of the sequence we use is also better producing a longer lasting effect.
Comparing to the commercial drugs, it is a clear that siRNA effect, which uses a different molecular mechanism to those used by commercial medicines, provide a more profound and sustained reduction in IOP. When RNAi therapy is used, upon cessation of treatment the silenced protein has to be re-synthesized in order to recover its activity and consequently it prolong the duration of the treatment as compared to conventional treatments. Thus, siRNA against beta2 adrenergic receptor enables to obtain a significant reduction in IOP with a long-term effect, whereas continuous application of commercial drugs would be necessary to induce a sustained effect on IOP.
This long-lasting reduction of IOP may be one of the most attractive features of this new approach for the treatment of ocular hypertension and glaucoma since it could allow reducing the frequency of instillation and improving patient compliance. Moreover, taking into account that the frequent application of some commercial drugs currently used at high concentrations to achieve the desired therapeutic effects, can lead to adverse side effects due to systemic absorption (e.g., timolol can generate deleterious effect in the heart)8 the use of siRNA therapy could minimize these side effects. Likewise, it has been demonstrated that, on the contrary to an isolated compartment as the eye, siRNA stability in serum is decreased as a consequence of RNases activity.18 Thus, a reduced siRNA half-life in serum also would contribute to reduce the likelihood of systemic side effects. A potential limitation of our study is that we have not evaluated those possible systemic side effects. However, previous reports suggest the safety of siRNA treatment. In animal biodistribution studies18 only trace amounts of siRNA were detected in systemic organs (lung, liver and kidney) and the absence of 5′-phosphorylated antisense strand of siRNA (a marker of intracellular delivery of the siRNA) indicated that the siRNAs were not internalized by the cells and accordingly they are not biologically active. In addition, the analysis of several parameters (clinical signs, electrocardiogram, hematology) in toxicity studies performed with animals that received ocular administration of siRNA did not reveal any alterations, indicating the safety of siRNA treatment.18 Supporting these data, in a study with humans using a siRNA targeting the β2 adrenergic receptor,19 no significant changes were observed during the comprehensive clinical evaluations, monitoring of vital signs or electrocardiograms.
On the other hand, although the eye content of RNases is lower than in other tissues and, indeed, a long-lasting effect was achieved by simple topical administration of a naked siRNA, perhaps the use of a non-viral vector carrier, preventing siRNA degradation, could even prolong the hypotensive action of the siRNA. Finally, the siRNA application did not alter the integrity of ocular structures and neither other ocular side effects were observed.
In summary, our results support a new generation of drugs to treat ocular hypertension and glaucoma based on RNAi therapy, which allows targeting of specific sequences of single genes and local delivery into the eye. The long-term effect on IOP reduction induced by siRNA could make possible to reduce the dose of siRNA minimizing side effects in other tissues as well as simplifying patient compliance.
Conflicts of interestThe authors report no conflicts of interest.
This work has been supported by the research grants SAF2013-44416-R, SAF2016-77084-R, RETICSRD12/0034/0003 and Universidad ComplutensePR1/07-14890.