The efficacy of intraocular pressure reduction in retarding the progression of glaucoma has been demonstrated. This review examines the potential for prognostic advantage for glaucoma suspects in reducing their optic nerve head exposure to elevated intraocular pressure associated with activities which have been shown to elevate intraocular pressure. In this observational study, patients examined at the Centre for Eye Health (University of New South Wales) with a diagnosis of glaucoma suspect were surveyed to determine their histories for participation in activities which are known to elevate intraocular pressure. The evidence regarding the pathological significance of these sources of elevation in susceptible patients was examined. Apart from the universality of sleep-related intraocular pressure elevations, the histories from 183 confirmed glaucoma suspects indicate a wide range and variation in frequency of participation in other intraocular pressure elevating activities. A reduction in exposure to elevated intraocular pressure may improve the prognosis for glaucoma suspects. Additional patient specific assessment of the results of this screening could provide an indication of the degree (frequency, intensity level and duration) of exposure to elevated intraocular pressure. Such information may provide the basis for improving a patient's prognosis by helping them to identify opportunities to reduce such exposure to elevated intraocular pressure. Any benefit of reduction of such exposure appears likely to be greater if activities which elevate intraocular pressure are of long duration, occur frequently, occur over a long period of time, and/or involve high levels of intraocular pressure elevation.
Se ha demostrado la eficacia de la reducción de la presión intraocular para retardar la progresión del glaucoma. Esta revisión examina la posible ventaja de reducir la exposición de la cabeza del nervio óptico a la presión intraocular elevada asociada a las actividades que han demostrado que elevan dicha presión intraocular, en pacientes con sospecha de glaucoma. En este estudio observacional, se evaluó a los pacientes examinados en el Centro de Salud Ocular (University of New South Wales) con un diagnóstico de sospecha de glaucoma, para determinar sus antecedentes de participación en actividades, de las que se sabe que incrementan la presión intraocular. Se examinó la evidencia relativa a la significación patológica de estas fuentes de elevación en pacientes susceptibles. Aparte de la las bien conocidas elevaciones de la presión ocular relacionadas con el sueño, las historias de 183 sospechas de glaucoma confirmadas indican un amplio rango de variación de la frecuencia de participación en otras actividades que elevan la presión intraocular. La reducción de la exposición a la presión intraocular elevada puede mejorar el pronóstico de sospecha de glaucoma. La evaluación adicional y específica del paciente, de los resultados de esta revisión, podría aportar una indicación del grado (frecuencia, nivel de intensidad y duración) de la exposición a la presión intraocular elevada. Dicha información puede proporcionar la base de mejora del pronóstico del paciente, ayudándole a identificar las oportunidades para reducir dicha exposición a una presión intraocular elevada. Es probable que cualquier beneficio de reducción a tales exposiciones pueda ser superior si las actividades que elevan la presión intraocular se prolongan en el tiempo, y/o se producen con frecuencia y/o se producen durante un periodo prolongado y/o implican altos niveles de elevación de la presión intraocular.
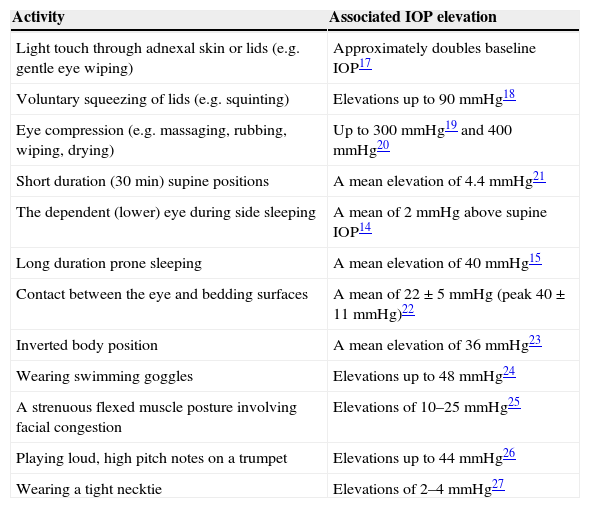
Elevated intraocular pressure (IOP) is a positive risk factor for the development of glaucomatous optic nerve damage and visual field loss.1 Lowering IOP slows the rate of progression of glaucomatous optic nerve damage, regardless of the state of the disease.1 Nevertheless, some individuals with apparently controlled IOPs experience glaucomatous progression.2 This well-known clinical observation suggests that patients may have differing susceptibilities to IOP levels or that other variables related to IOPs are associated with progression.2,3 One such variable is fluctuation in IOP either over the 24-h diurnal period or across visits.2–4 For example, the Advanced Glaucoma Intervention Study found that large variations in IOP from visit to visit were associated with open-angle glaucoma progression.5 However, in-office tonometry, even when supplemented by assessments in a sleep laboratory, does not indicate the degree to which ocular tissues are exposed to IOP elevations which occur outside those settings3,6 during different activities.7 As yet no simple method exists for assessing IOP across 24-h periods in humans without placing restrictions on the type of activities in which participants can be engaged. For example, a wireless ocular telemetry sensor involving a micro-electromechanical system embedded in a contact lens has been used experimentally in humans.8 The antenna for this device is mounted around the monitored eye and has a bulkiness similar to a swimming goggle.8 The antenna greatly impairs participation in many activities which are known to elevate IOP such as eye rubbing, eye wiping, prone and lateral decubitus sleeping, wearing swimming goggles etc. In assessing diurnal IOP variations the greatest interest has been in relation to supine sleep positions.9–12 Side sleep positions have been shown to elevate IOP to higher levels than supine positions13,14 and prone positions to even higher levels.15 However, there are numerous other activities which are associated with elevated IOP in addition to those which are sleep-related (Table 1). Although apparently innocuous in normal eyes (most people do not develop glaucomatous pathology), these forms of elevation may be relevant to a patient whose eyes are susceptible to increased IOP distending forces.
An indication of the IOP elevation levels which have been recorded during activities known to cause such responses and which may contribute to glaucoma development or progression. (This is a supplemented version of a table16 which is reproduced here with the kind permission of John Wiley & Sons and Clinical and Experimental Optometry.).
| Activity | Associated IOP elevation |
|---|---|
| Light touch through adnexal skin or lids (e.g. gentle eye wiping) | Approximately doubles baseline IOP17 |
| Voluntary squeezing of lids (e.g. squinting) | Elevations up to 90mmHg18 |
| Eye compression (e.g. massaging, rubbing, wiping, drying) | Up to 300mmHg19 and 400mmHg20 |
| Short duration (30min) supine positions | A mean elevation of 4.4mmHg21 |
| The dependent (lower) eye during side sleeping | A mean of 2mmHg above supine IOP14 |
| Long duration prone sleeping | A mean elevation of 40mmHg15 |
| Contact between the eye and bedding surfaces | A mean of 22±5mmHg (peak 40±11mmHg)22 |
| Inverted body position | A mean elevation of 36mmHg23 |
| Wearing swimming goggles | Elevations up to 48mmHg24 |
| A strenuous flexed muscle posture involving facial congestion | Elevations of 10–25mmHg25 |
| Playing loud, high pitch notes on a trumpet | Elevations up to 44mmHg26 |
| Wearing a tight necktie | Elevations of 2–4mmHg27 |
A Bayesian joint regression model combining structure and function was found to provide more accurate and precise estimates of the rate of glaucomatous progression.28 It makes good sense to combine all information relevant to glaucoma,29 and consideration of the degree of participation in IOP elevating activities may be found to be a valuable inclusion in any model of glaucoma progression. For example, exposure to activities which are known to elevate IOP were found to be significantly more prevalent in normal tension glaucoma patients compared to those with primary open angle glaucoma.30 In the same way that IOP reduction, using medication and/or surgery for example, slows the rate of progression of glaucomatous optic nerve damage,1 IOP reductions achieved by avoiding or moderating activities involving exposure to elevated IOP may improve glaucoma prognoses.3,14,23–26,30 The key issue for any particular patient is the degree to which that patient is involved in any IOP elevating activities. This review examines the potential for prognostic advantage from reducing exposure to activities which elevate IOP by collecting histories from patients who have a confirmed diagnosis of glaucoma suspect.
MethodsAn observational study of confirmed glaucoma suspect patients who had been referred to the University of New South Wales Centre for Eye Health (CFEH) was undertaken to determine their histories of participation in activities which are known to elevate intraocular pressure. The CFEH provides a service to optometrists and ophthalmologists involving advanced ocular imaging technology and clinical expertise focused on early detection of eye disease. CFEH patient records were accessed using Sunix record management software with inclusion criteria being that these patients were referred by their practitioner as glaucoma suspects requiring further examination and evaluation of that provisional diagnosis. The examination protocols employed by the referring practitioners are likely to have varied widely as would have the diagnostic criteria used, especially as access to some of the instruments and examination methods used at the CFEH would not be available to them. However, for the patients included in this study, the glaucoma suspect diagnosis had been confirmed at the CFEH following strict adherence to the examination protocol recommended by the National Health and Medical Research Council31 and indicated in Table 2. Surveys for the purpose of screening histories of participation in IOP elevating activities were sent to 259 confirmed glaucoma suspects who had previously indicated that they were willing to be contacted for research purposes. Patients diagnosed at the CFEH as low risk (normals) are referred back to their original practitioner and patients diagnosed with glaucoma are referred to an ophthalmologist. These patients were excluded from this study because this form of contact would be inappropriate. Only patients who remained under the care of the CFEH were surveyed. All procedures were approved by the University of New South Wales Human Research Ethics Advisory Panel and conducted in accordance with the Declaration of Helsinki. The names of patients cannot be associated with their responses.
Guidelines of the CFEH for examination and confirmation of glaucoma suspects.
| All patients were diagnosed as being at risk for having or developing glaucoma by the practitioner who referred them to the CFEH for further assessment where the examination followed the protocol recommended by the National Health and Medical Research Council Guidelines31 (NHMRC) which is described below. Patients subsequently diagnosed at the CFEH as normal or low risk, or who were referred to an ophthalmologist for treatment, were not included in the sample surveyed in this study. By these exclusions, only those patients diagnosed as confirmed glaucoma suspects were included. |
| Consideration of glaucoma risk factors such as: elevated or fluctuating intraocular pressure; family history of glaucoma (especially first degree family); advanced age; gender; african or Asian ethnicity; current diabetes; myopia (especially higher degrees); rural location; significant enlargement in cup/disc ratio and/or asymmetry; nerve fibre layer defects; optic disc haemorrhage; reduced corneal thickness; evidence of pseudo-exfoliation or pigmentary glaucoma; current smoking; current migraine and peripheral vasospasm; long term steroid use; previous eye injury; relevant comorbidities such as hypertension, thyroid disease, depression, asthma, liver and renal disease.31 |
| A full eye examination including anterior segment evaluation; Volk G4 Goniolaser or Ocular Sussman 4 mirror gonioscopy; dilated slit lamp funduscopic assessment of the optic nerve and retinal nerve fibre layer (see below) with a permanent record of subsequent stereo-photography assessments; Goldmannapplanation tonometry and DGH-550-Pachette 2 corneal thickness measurements. With reference to increased risk for eyes with corneas found to have significantly less thickness than the normal population mean of 535±31μm,31 IOP>24 was classified as extremely high risk and IOP 21–24mmHg was classified as high risk.31 However, IOP should be considered as a continuum of risk rather than as specific thresholds for concern.31 Although 95% of the population have pressures between 10 and 21mmHg, IOP within this range may be associated with a diagnosis of glaucoma or suspect glaucoma.31 |
| Standard automated perimetry (white on white) including comparison with age-corrected normals on a point-wise, regional (e.g. glaucoma hemifield test) and global basis using Humphrey Matrix (frequency-doubling threshold) perimetry (pattern standard deviation and mean deviation).32 |
| Heidelberg Retinal Tomograph III evaluation including linear cup/disc ratio, cup shape, rim area, rim volume, mean thickness of retinal nerve fibre layer, high variation contour of retinal nerve fibre layer, and glaucoma probability score classification.33 |
| Zeiss GDx-pro evaluation of the retinal nerve fibre layer for average temporal, superior, nasal, inferior, thickness and standard deviation, superior average, inferior average, internal eye symmetry and the nerve fibre indicator with scores between 30 and 50 indicating a glaucoma suspect classification.34 |
| Zeiss Cirrus Optical Coherence Tomography assessment of rim area, average cup/disc ratio, cup volume, and average retinal nerve fibre layer thickness. For example, this instrument provides an indication of the 5% and 1% levels of probability that RNFL thickness is glaucomatous. Glaucoma suspect diagnostic criteria included, for example, eyes with cup-disc ratio or cup-disc asymmetry ≥97.5th percentile for the normal population or a neuroretinal rim width reduced to ≥0.1 cup disc ratio but not having a visual field defect consistent with glaucoma.35 |
| Based on the NHMRC criteria for risk of developing glaucoma31 and glaucoma probability scores provided by these instruments, tripartite case classification resulted in diagnoses of: (1) Patients who are found to be normal or low risk who are referred for follow-up by their original (referring) practitioner; (2) patients who are found to be glaucoma suspects and scheduled forfurther CFEH assessment; (3) patients who are found to be at high risk of being glaucomatous and who are referred for treatment. |
Of the 259 surveys sent 183 replies were received and the number of positive responses to each part of each question is shown as a percentage of the number of responses to that question in Table 3. Apart from sleep, the most common activity reported was eye rubbing (85%) with 18% reporting that it was only a monthly event while 2% reported that eye rubbing occurred more often than hourly. The least common activity reported was playing a wind resistance musical instrument (0.5%). The number and range of types of activity reported varied widely between patients.
Indicating the survey questions and responses (in rounded brackets) from 183 glaucoma suspects (47–79 years, mean 64±8.2 years, 58% female). Also shown (in squared brackets) are the responses to the questionnaire from 100 consecutive non-glaucomatous patients attending for the correction of refractive error (13–78 years, mean age 52.5±14, 54% female)36 (which are reproduced here with the kind permission of Optometric Association Australia).
| 1. How often do you rub your eyes? For example when they feel itchy? |
| Never (15%) [14%]; Monthly (18%) [7%]; Weekly (26%) [22%]; Daily (34%) [47%]; Hourly (4%) [9%]; More than hourly (2%) [1%]. |
| 2. If you rub your eyes do you mostly rub the: |
| Right eye? (22%) [14%]; Left eye? (14%) [4%]; Both equally? (64%) [68%]. |
| 3. How often do you wipe your watery eyes with a tissue or handkerchief? |
| Never (48%) [45%]; Monthly (18%) [16%]; Weekly (14%) [17%]; Daily (16%) [17%]; Hourly (3%) [5%]; More than hourly (1%) [1%]. |
| 4. Which eye do you need to wipe the most frequently? |
| Right? (21%) [10%]; Left? (24%) [4%]; Both equally? (54%) [41%]. |
| 5. What is your favourite position for sleeping? |
| On your back? (11%); On your right side? (45%); On your left side? (33%); On your stomach? (10%). |
| 6. How often do you sleep on your stomach with your head to the side? |
| Never (67%) [49%]; Monthly (10%) [7%]; Weekly (11%) [12%]; Daily (12%) [32%]. |
| 7. When sleeping on your stomach do you prefer to have your head turned to the |
| Right side? (28%) [20%]; Left side? (20%) [22%]; Both equally? (52%) [10%]. |
| 8. How often do you do physical exercises like sit-ups, or push-ups, and/or weight lifting? |
| Never (48%) [41%]; Monthly (7%) [14%]; Weekly (36%) [28%]; Daily (9%) [17%]. |
| 9. Do you ever do yoga exercises such as head stands, back bend, or lying-down poses etc.? |
| Never (88%) [94%]; Monthly (4%) [3%]; Weekly (6%) [2%]; Daily (2%) [1%]. |
| 10. Do you wear spectacles and/or contact lenses for seeing in the distance? Yes or No |
| How often do you wear spectacles for seeing in the distance? |
| Never (25%) [58%]; Sometimes (8%) [4%]; Fairly often (3%) [2%]; Frequently (4%) [2%]; Very frequently (4%) [5%]; Routinely (56%) [29%]. |
| 11. How often do you swim wearing swimming goggles? |
| Never (78%) [81%]; Monthly (11%) [13%]; Weekly (9%) [4%]; Daily (1%) [2%]. |
The patient history items in the survey did not involve clinically sensitive or otherwise threatening subjects. For this reason, the validity of this form of self-report is likely to be at least satisfactory if not high.37 However, as discussed below, for the purpose of clinical management of an individual patient, it is necessary to further examine these responses. Discussion of them with the patient allows for an estimate of the degree (duration, frequency, intensity level) and potential significance of exposure to particular forms of IOP elevation indicated by the survey responses. However, all patients are exposed to sleep-related IOP exposure with the degree of elevation and its possible significance depending on the frequency and duration of time spent in particular sleep positions38 (or activities involving similar postures). For example, previous studies have indicated that IOP elevation during supine sleep21 are lower than elevations during side sleep,14 which are lower than those during prone sleep.15 Thus, reducing or avoiding exposure to the greater IOP elevation during prone sleep or reducing exposure to greater dependent eye IOP elevation for the more suspect or advanced eye by avoiding side-sleep which places that eye in the dependent position might be positive for glaucoma prognoses.38
That glaucoma suspects are more exposed to IOP elevating activities than normals is not a premise for this study. Notwithstanding widely varying individual differences between individuals, there does not appear to be any reason, other than by chance, why the group results for one class of patients would be greatly different from any other. Also shown in Table 3 are the responses to the questionnaire from 100 consecutive non-glaucomatous patients attending for the correction of refractive error (13–78 years, mean age 52.5±14, 54% female).36 These findings provide an indication of the sporadic prevalence of these activities for comparison with the glaucoma suspects in this study.36 As might be expected, differences in participation in IOP elevating activities does not appear to be remarkably different, other than might be expected by chance, especially for uncommon activities. In addition, whether any particular form of exposure to IOP elevation activity is common or not, is of no clinical consequence. Nor would it be of any clinical significance if participation in IOP elevations activities was not independent of clinical status. For an individual patient, the only relevant issues are the type of activities in which they engage and their susceptibility to the associated episodes of IOP elevation. However, normal tension glaucoma pathology suggests that increased optic nerve susceptibility to IOP could be the key factor in vision loss, unless there is exposure to elevated IOP during particular activities. For example, wearing swimming goggles is an uncommon activity but it may be clinically significant for a patient who is susceptible to the associated episodes of IOP elevation.24 The possible significance of wearing swimming goggles appears likely to depend on the type of goggle,24 as well as the duration and frequency of their use. People who swim multiple laps of a pool every day, for example, appear to be at greater risk. Apparently any form of exposure during IOP elevating activities can be innocuous and of no consequence for the majority who are not susceptible to associated IOP elevation.
However, the survey responses which appear to be the most significant behaviours for this sample of glaucoma suspects (Table 3) include the 4 (2%) who rub their eyes more often than hourly, 2 (1%) who wipe their watery eyes more often than hourly, 18 (10%) for whom prone was their favourite sleep position, 22 (12%) who slept prone on a daily basis, 17 (9%) who performed strenuous physical exercises daily, 4 (2%) who practised yoga exercises daily, 2 (1%) who swam wearing goggles daily and 80 (44%) who reported less than routine wear of a correction for distance vision and who, depending on their level of unaided vision may be elevating IOP by squinting stenopaically to clear their vision. Voluntary squeezing of lids was found to cause IOP elevations of the order of 90mmHg18 apparently due to increased tonus in the orbicularis muscle39 and associated increased compressive lid forces on the eye. Some patients did not report participation in the most significant levels of exposure listed above such as rubbing/wiping, but nevertheless did report exposure to sources of IOP elevation which, depending on individual susceptibility to them, may still represent a significant contribution to exposure of the optic nerve head to potentially pathological levels of IOP distending forces.
Elevated IOP remains an important risk factor in glaucoma and IOP reduction is still the only treatment of proven benefit40 having been demonstrated to decrease glaucoma incidence.41 Although activities involving IOP elevations are apparently innocuous in patients who are not susceptible to glaucomatous pathology, exposure to them in susceptible patients may help explain some cases of optimum treatment (including full compliance with medication and seemingly good control of daytime in-office IOP) which are nevertheless associated with unchanged rates of disease progression.2 Similarly, the great variation in glaucoma progression rates across diagnostic groups and also among patients in the same diagnostic group42 may be explained in part by variable levels of exposure to IOP-elevating activities such as has been found in this study. Differences in ability to limit glaucomatous progression found between alternative drugs may be due to different mechanisms of drug action but other risk factors could also play a significant role.43 For example, the evaluation of the results of treatment with drugs may be confounded by lack of control for exposures to IOP elevating activities. Some poor responses to drug therapy may be partly due to high levels of exposure to IOP elevation even when sitting IOP is maintained significantly below baseline levels. Primary open-angle glaucoma affects both eyes but often asymmetrically.44 As found for sleep-related asymmetry exposure to elevated IOP13 and, as discussed below, asymmetrical presentations of glaucomatous damage may be explained in part by corresponding asymmetrical exposures to IOP elevating activities unrelated to sleep.
However, the survey only serves as a screening instrument. Follow-up discussion of the findings permits an estimate of the degree of exposure and its potential significance for an individual patient. For individual patients, the duration, frequency and intensity level of episodes of activities in which patients engage, and other factors that are not evident from the raw survey screening responses would need to be considered. For example, although all eye rubbing elevates IOP to potentially pathological levels, the intensity of eye rubbing (and associated level of IOP elevation) can vary widely17,19,20 as can the frequency and duration of rubbing episodes. Similarly, participation in yoga or other forms of exercising are more significant when horizontal or inverted body positions are assumed and even more so when strenuous muscular effort and altered breathing are involved.
Glaucoma suspects (and perhaps especially patients with a glaucoma diagnosis) may be more or less interested in knowing how modification to their behaviour might reduce their risk of glaucoma progression. However, quality of life needs to be taken into account when contemplating avoidance or moderation of participation in IOP elevating activities. Visual field loss in progressing glaucoma is independently associated with a loss of both disease-specific and generic quality of life.45 It is important to prevent progression, both in early and advanced glaucoma.40 The possibility that avoidance or moderation of exposure to IOP elevating activities may impair quality of life has to be considered and weighed up against the possibility of impaired quality of life associated with glaucoma progression. Practical matters might make some activities harder or easier to consider avoiding or moderating. Patient awareness that supine sleep is associated with lower IOP elevations and that prone sleep is associated with the higher elevations might aid the achievement of potentially favourable modification of sleep habits. A professional player of a high wind-resistance musical instrument would find reduction in playing time very difficult to achieve. However, it might be possible to avoid the highest IOP elevations which are associated with playing loud and/or high pitched notes.26 Ideally, such a patient who is at risk for glaucomatous progression would also have other avenues for reducing exposure to IOP elevating activities which could be more easily achieved. A study of adherence with glaucoma medication indicated that only 53% of those invited to participate agreed to do so but only 70% of those who participated completed the study.46 The participants may have self-selected on the basis of tending to be more conscientious and cooperative, but nevertheless only 66% of the 70% who completed the study demonstrated good adherence with prescribed drug doses, 19% frequently missed doses, 11% took frequent drug holidays, and 3% discontinued their therapy.46 It is not known whether medication adherent patients are also likely to be the most adherent to suggestions to modify their behaviour to reduce exposure to IOP elevating activities. However, hopefully some medication non-adherent patients might find adherence to recommendations to avoid or moderate exposure to some IOP elevating activities easier to achieve and sustain than being compliant with medication.
To illustrate another basis for further evaluation of survey responses, consider a subject who reported the habit of predominantly rubbing or wiping their right eye and/or sleeping on their right side and/or sleeping prone with their head turned to the left potentially placing their right eye in contact with bedding. These items of asymmetric (right eye) exposure to elevated IOP may have more significance if this patient is found to have evidence of greater glaucomatous change or risk of change in the right eye. For example, significant differences in retinal nerve fibre layer thicknesses have been detected in asymmetric primary open angle glaucoma.11 Alternatively, greater visual field losses and/or morphological optic nerve head changes may also indicate asymmetric disease progress. For example, in glaucoma patients IOP elevation asymmetry during side sleeping was found to be associated with corresponding asymmetric field losses.13 Correspondence between asymmetric exposure to elevated IOP and clinical findings suggest that appropriate behaviour modification to reduce exposure to IOP elevation for the more advanced eye could be more likely to be of benefit.
Frequent disc haemorrhages at follow-up were confirmed as an important sign which conferred an increased risk of glaucomatous progression.47,48 The mechanical theory for the pathogenesis of glaucoma hypothesises that the primary event is neurodegeneration of the optic nerve putting stress on blood vessels, eventually leading to bleeding.49 Some individuals may be more prone to blood vessel breakage.49 However a review indicates that disc haemorrhages do not appear to be related to the mean level of IOP.49 IOP elevating activities (Table 1) can add mechanical stress such as with eye massaging IOP elevations which have been recorded to be as high as 26 times greater than a baseline of 15mmHg.20 These very high IOP elevations may provide the precipitating mechanical trauma for a disc haemorrhage. For example, vigorous rubbing of an itchy eye appears likely to elevate IOP to higher levels. The associated risk of haemorrhage is consistent with the observation that IOP recorded during a clinical trial indicated that maximum findings may be a more important risk factor for functional progression than mean IOP over the same time period.50 The electroretinogram components which showed the greatest loss of ganglion cell function following IOP elevation to 50mmHg and 70mmHg in rats also displayed the slowest recovery.51 Both the degree and duration of IOP elevation were found to be significant determinants of functional loss, with the peak IOP being the critical determinant of the degree of loss.51 However, for a given peak level of IOP elevation, the duration of the elevation plays the key role.51 Apart from the degree and duration of IOP elevation episodes, other important parameters appear to be their frequency and the associated time for recovery between them. A new episode of IOP elevation prior to optic nerve head tissue recovery from previous exposure to IOP elevation may be more damaging, especially if tissue in a recovering phase is more susceptible to IOP-related trauma. In some patients with glaucoma, susceptibility to progression appears to be increased even after intervention has lowered IOP.52 Once damaged, the optic nerve head appears to be more susceptible to further damage despite lower IOP.52 The strong association between glaucoma and advancing age suggests that the ageing eye becomes more susceptible to glaucomatous injury.52 For many patients the ability to participate in some forms of IOP elevating activities may be reduced with increasing age (yoga, strenuous physical exercises, swimming for example). However, although episodes of related IOP elevation may be less frequent and of shorter duration with increasing age, any reduction in participation in other IOP elevating activities may be more relevant if susceptibility to the effects of other IOP elevating activities is increased by age or previous glaucomatous damage. Of course, some IOP elevating activities such as wiping watery eyes for example, may become more frequent with increasing age.
Glaucoma progression risk has been found to decrease by about 10% with each millimetre of mercury of IOP reduction from baseline.13,47 The findings of this study and review suggest that if any behaviour modification is feasible (a history of exposure combined with a willingness and/or ability to modify behaviour) there can be the potential to significantly reduce episodes of exposure to IOP elevation. A review of clinical trials of glaucoma treatment has demonstrated the efficacy of IOP reduction in retarding the progression of glaucoma for a wide spectrum of the disease from low to high IOPs and from early to advanced disease.53 Some level of sleep-posture-related IOP elevation occurs for all patients and similar postures may also occur during reading, television watching, sunbathing and in a gymnasium for example.38 Apart from sleep-posture-related elevations, the findings in this study of glaucoma suspects indicate varying levels of additional potential for reducing exposure to elevated IOP for many patients. As IOP remains the most important risk factor in glaucoma and because its fluctuation seems to play a role in the disease development and progression, even in cases of statistically normal pressures,1,54–56 it appears to be prudent to include a wider consideration of the potential role of all sources of IOP elevation in the development and progression of this disease as well as the possibility of beneficially reducing exposure to them.7 Because functional damage from high IOP is exponential, even transient elevations in IOP may cause significant glaucomatous damage.7 Patients who have periodic or sporadic pressure spikes can lose visual field due to cumulative effects.7 An important goal in therapy therefore, should be to prevent transient IOP elevations.7 The goal of detecting and reducing abnormal 24-h IOP fluctuations is warranted in all newly diagnosed glaucomatous patients as well as in patients who continue to progress at lower pressures.57 The treatment of ocular hypertension has been problematic in the few decades since it was recognised.58 A decision to not treat ocular hypertension might be easier to make when a history of participation in activities that elevate IOP provides an opportunity for avoidance or moderation of those activities and the associated exposure to episodes of IOP elevation. The same principles appear to apply to any glaucoma suspect and perhaps more so for patients with a glaucoma diagnosis. For example, the temporary and sometimes dramatic elevations and fluctuations in IOP observed in brass and woodwind musicians during common playing conditions may increase risk of developing glaucoma, especially for those with co-existing risk factors.59 Life hours of high resistance wind instrument playing was found to have a significant relationship to abnormal visual field and corrected pattern standard deviation scores.26 The same relationships may be clinically significant for susceptible patients in relation to any other IOP elevating activities. For example, the progression of glaucomatous pathology was found to cease when a patient stopped doing yoga headstands for 5min every day (with IOP elevations to 40mmHg),25 and another patient with glaucomatous changes, beneficially stopped his habit of regularly rubbing his eyes,60 an activity which is known to elevate IOP to very high levels (Table 1). Estimating risk of visual field loss in treated patients43 and assessing factors for glaucoma progression46 may be improved by the evaluation of histories of participation in IOP elevating activities.