The digital transformation of daily routines has increased visual demands, especially at intermediate and near distances. The Devil multifocal intraocular lens (MIOL) design was developed to enhance intermediate visual performance. This design is a novel trifocal intraocular lens inspired by the fractal structure known as the 'Devil's staircase'. The aim of this research is to evaluate the visual performance of the Devil MIOL design in real patients.
MethodsThe visual acuity defocus curve was obtained from 25 eyes of healthy volunteers using the Visual Adaptive Optics Simulator (VAO, Voptica SL, Murcia, Spain). Additionally, images of optotypes simulating those seen through the Devil MIOL design were captured to qualitatively illustrate optotype perception at different vergence values.
ResultsMean visual acuity values of the evaluation were 0.03 logMAR at 0.00 D vergence, 0.10 logMAR at +1.50 D vergence, and 0.11 logMAR at +3.00 D vergence, corresponding to far (optical infinity), intermediate (66.7 cm), and near foci (33.3 cm), respectively. The experimental outcomes were indeed slightly better than numerical results obtained previously in a model eye.
ConclusionsThe Devil MIOL design provides satisfactory visual acuity across three primary foci and produces extended depth of focus between the intermediate and near foci.
In recent years, there has been a significant increase in the number of presbyopic patients due to the growing aging population. The digital transformation of our daily routines has led to a substantial rise in visual demands, especially at intermediate and near distances. Additionally, the use of surgical interventions to address presbyopia is increasingly common. One such intervention involves replacing the crystalline lens with a multifocal intraocular lens (MIOL). Currently, multiple designs of MIOLs are available on the market, each with different optical designs and features.1
Multiple clinical studies have assessed visual acuity (VA) following the implantation of commercially available trifocal MIOLs.2-7 Typically, the best corrected-distance VA tends to be poorer at intermediate distances compared to that achieved at far or near distances.3-6 Therefore, although patient satisfaction levels among those implanted with modern trifocal intraocular lenses are very high—even for intermediate vision—there is potential to improve visual performance at intermediate distances with a lens designed to provide extended depth of focus between intermediate and near foci.
Recently a new trifocal design has been conceived to meet these visual demands.8,9 This design is composed of 7 diffractive rings distributed its optical zone according to the fractal structure known “Devil's staircase”. Its optical performance was evaluated in a model eye, providing numerical evidence that this design has the potential to overcome the shortcomings of some commercial lenses, particularly in the intermediate range of vision.9 In this experimental preclinical evaluation, under photopic conditions, it worked like an extended depth of focus lens over almost the entire defocus range. Moreover, it was demonstrated that the Devil MIOL design was very robust to decentration.9
The aim of this work was to evaluate the visual performance of the Devil MIOL design9 in a simulation using real patients with the Visual Adaptive Optics Simulator (VAO, Voptica S.L., Murcia, Spain).10 This instrument enables the simulation of optical profiles of different MIOL designs, allowing an assessment of their visual performance before implantation.11,12 Employing visual simulators to study MIOL designs before implantation confers a significant advantage mainly because this approach allows both the patient and the ophthalmologist to preview the visual performance of the MIOL before surgery, facilitating the verification of whether the patient's visual expectations are met.
MethodsPatients and methodsThis study received approval from the ethics committee at the Universitat de València and adhered to the principles in the Helsinki Declaration. Each participant provided written informed consent. The inclusion criteria comprised healthy individuals with no ocular pathology, including no degree of cataract, who had not undergone refractive surgery, were not taking medications affecting vision or the eye, were aged between 18 and 40 years, and had astigmatism less than or equal to 0.75 D. The eye with the best corrected-distance VA was chosen for the evaluation.
Examination protocol and MeasurementsThe VAO instrument combines a Hartmann-Shack sensor to objectively measure refraction and wavefront aberrations of the eye. In terms of adaptive optics, the device has the capability to simulate optical profiles and designs of MIOLs using a liquid crystal on silicon spatial light modulator. Additionally, it allows for the precise positioning of stimuli at the desired vergence. A micro display featuring an organic light-emitting diode was employed to present visual stimuli (optotypes) to the patient.10,13-16
The examination started with three objective refraction measurements taken using the Hartmann-Shack sensor of the VAO, and the mean of these measurements was used as a starting point for subjective refraction. For the visual examination, two drops of cycloplegic solution (cyclopentolate hydrochloride, 10 mg/ml) were administered in both eyes. The protocol to obtain the subjective refraction followed standard steps (initial myopization, sphere refinement in ±0.25 D steps, and the use of Jackson cross-cylinders to refine the power and axis of the astigmatism).17 The optotype used to obtain the subjective refraction and then the VA defocus curve was a tumbling E optotype with 5 letters with 4 random orientations per line and steps of 0.10 logMAR between lines. This optotype was presented to the examined eye by means of the organic light-emitting diode integrated into the instrument, with an average photopic luminance of 80 cd/m².
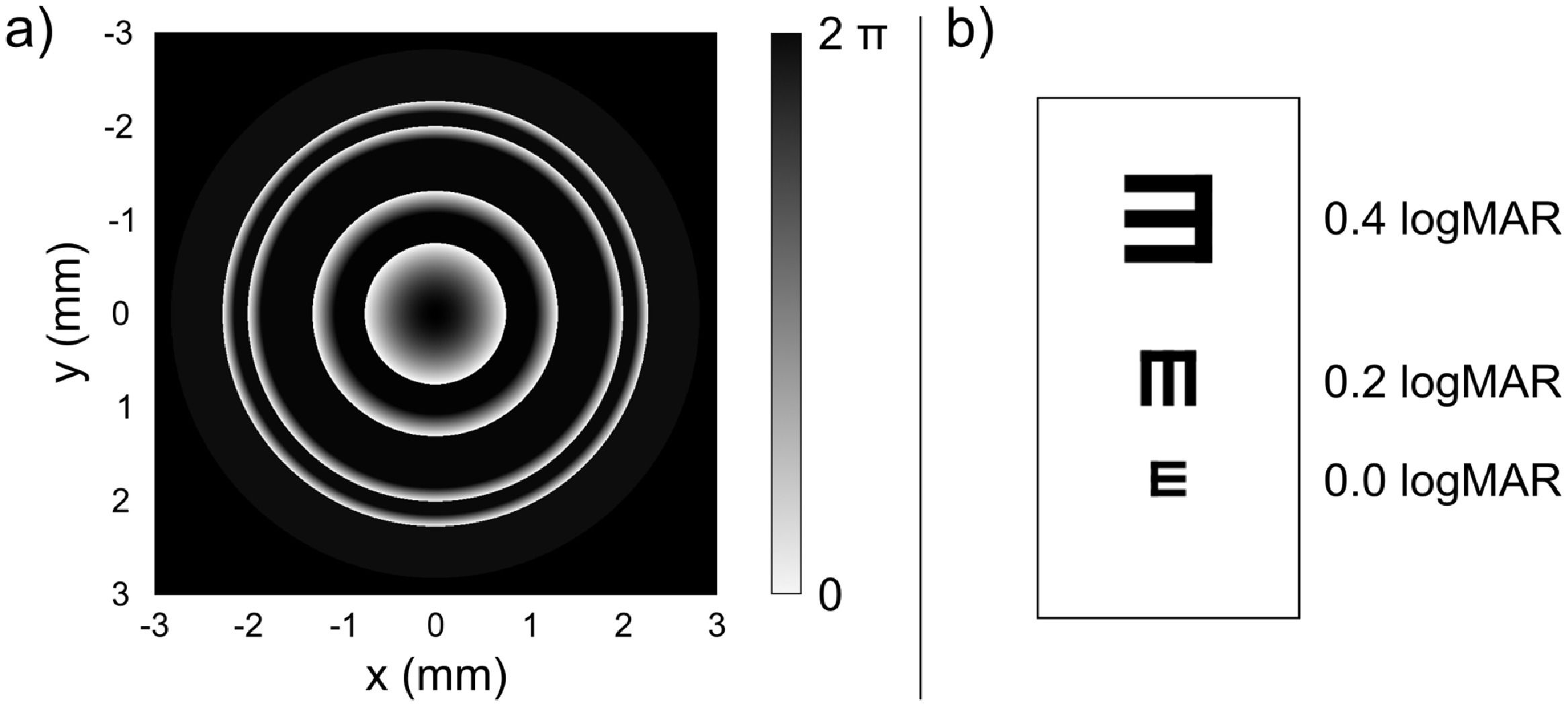
Subsequently, the phase profile of the Devil MIOL (Fig. 1a), which features a + 3.00 D addition for near vision and a + 1.50 D addition for intermediate vision, was projected onto the patient's pupil using the liquid crystal on silicon spatial light modulator. The assessment of visual performance was carried out through the VA defocus curve with a 4.50 mm pupil diameter (only one available in the standard instrument VAO V.1). The tumbling E optotype, as previously described, was virtually placed within a range of vergences from −0.50 D to +3.50 D, with increments of 0.50 D. The last line of VA that could be correctly recognized was documented, with errors in letter identification being discounted (each letter assigned a value of +0.02 logMAR).
In addition to the subjective VA defocus values recorded for the patients, objective images of optotypes (Fig. 1b) were capture by a camera affixed to the instrument, serving as an artificial eye. This camera comprised an achromatic doublet (AC254–050-A-ML, Thorlabs Inc., Newton, NJ, USA) equipped with a CMOS sensor (EO-10012C LE, 8 bits, 3840 × 2748 pixels, 6.41 × 4.59 mm).
Statical analysisThe statistical analysis was conducted using IBM SPSS Statistics for Windows software (version 28.0.1.1(14), IBM Corp., Chicago, IL, USA). Descriptive statistics were calculated, and the mean ± standard deviation (SD) values of VA for each vergence were obtained.
ResultsThe clinical study included 25 eyes of 25 volunteers (52 % in the right eye and 48 % in the left eye). 68 % of the volunteers were female and 32 % were male. The descriptive findings for the 25 assessed patients are presented in Table 1.
Descriptive findings for the 25 patients.
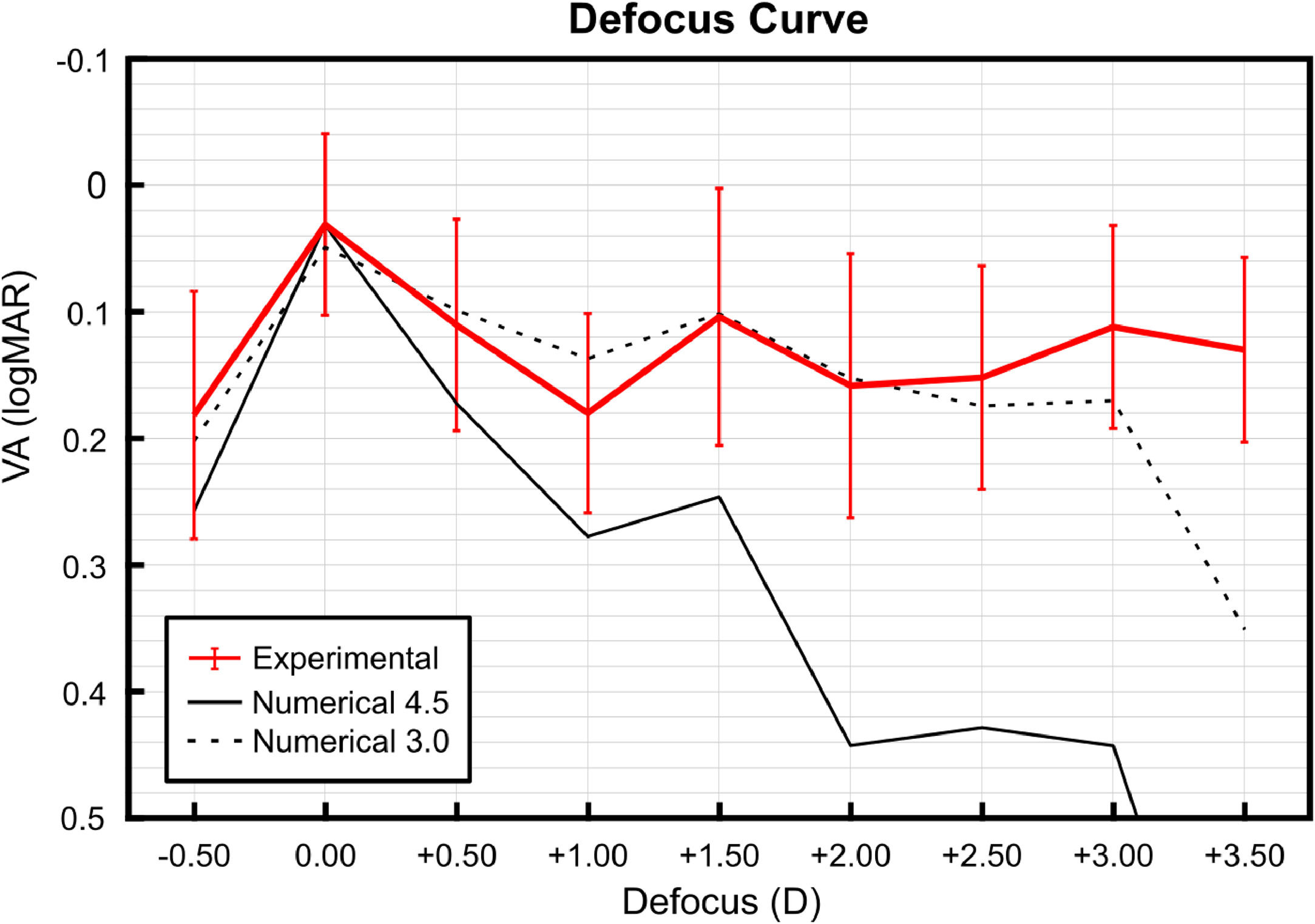
The mean ± SD VA data for vergences between −0.50 D and +3.50 D, simulating the phase profile of the Devil MIOL, are depicted in Fig. 2. The achieved VA mean values in the three main foci of vision, were 0.03 ± 0.07 logMAR at 0.00 D vergence, 0.10 ± 0.10 logMAR at +1.50 D vergence, and 0.11 ± 0.08 logMAR at +3.00 D vergence, corresponding to far, intermediate, and near foci, respectively. Note that the valley between far and intermediate vision foci is more pronounced than the valley between intermediate and near vision foci. In the valley between far and intermediate vision foci, the worst mean VA achieved is 0.18 logMAR, while in the valley between intermediate to near vision foci, the worst mean VA achieved is 0.16 logMAR.
Mean ± standard deviation (SD) of monocular visual acuity (VA) defocus curve illustrating the clinical results with the Devil MIOL (red line) and the numerical results of calculated VA with Devil MIOL in a Liou-Brennan model eye for 3.0 mm (black dotted line) and 4.5 mm pupil diameter9 (black line).
The clinical outcomes obtained were compared with those previously reported numerically,9 at the same focal points where the experimental evaluation is carried out (vergences between −0.50 D and +3.50 D, in steps of 0.50 D) using Zemax OpticStudio software (v. 18.7, LLC, Kirkland, WA, USA) with the MIOL virtually implanted in the Liou–Brennan model eye.18 Both numerical and experimental results are presented in Fig. 2. It becomes evident that the experimental results closely align with the numerical predictions, computed for 3 mm pupil diameter. Interestingly, for both far and near vision, clinical VA values are superior to the numerical predictions. The fact that the experimental results are closer to those calculated with a 3.0 mm pupil than to those obtained numerically with a 4.5 mm pupil in Ref. [9] indicates that, on the one hand, the Liou–Brennan model underestimates image quality at intermediate and near distances, and, on the other hand, it is likely that the pupil size of the simulator projected onto the eye is smaller than the physical pupil in the device.
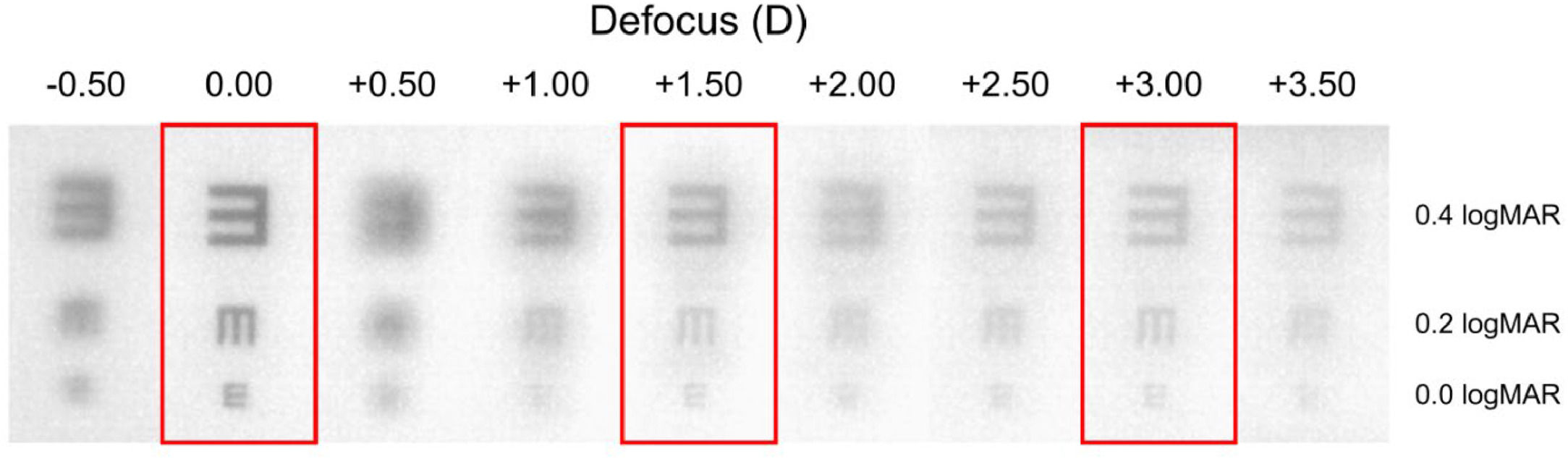
Finally, the images captured by the artificial eye of the tumbling E optotype (Fig. 1b) are presented in Fig. 3. In these images, one can discern the previously announced depth of focus. Optotypes exhibit higher resolution between vergences of +2.00 D and +2.50 D compared to those at +0.50 D and +1.00 D, despite having lower contrast.
DiscussionIn this work, the visual performance of a diffractive trifocal intraocular MIOL design whose profile is based on the Devil's lens8,9 was assessed 25 patients, by means of the VA defocus curve obtained using the Visual Adaptive Optics Simulator (VAO, Voptica S.L., Murcia, Spain), a technological tool that employs adaptive optics principles, to simulate and analyze visual performance in various optical conditions. Our results confirm that with this design, it is possible to achieve good levels of VA, enabling patients to meet their visual expectations in the range from intermediate to near distances. These findings suggest an improvement over the visual outcomes delivered by currently available intraocular lenses in the market. In fact, in most clinical studies involving various commercially implanted MIOLs, a decrease in VA was reported at the intermediate focus compared to that obtained at the far and near foci.3-6 Additionally, our experimental results validate our predictions published in a previous numerical study.9 According to these results (Fig. 2), we can speculate that the proposed design has the potential to provide VA values of 0.20 logMAR or better across the entire range of vision, with similar optical performance at intermediate and near focal points.
At this point, it is important to note that this feature meets the following criterion outlined by the American Academy of Ophthalmology Task Force Consensus Statement on EDoF IOLs: 'The monocular depth of focus for the EDoF-implanted eyes needs to be at least 0.5 diopters (D) greater than the depth of focus for the monofocal IOL controls at logMAR 0.2.'19 However, we recognize as a limitation that additional criteria from this Document should be tested in a clinical study with patients implanted with real lenses, rather than using visual simulators. On the other hand, according to the classification of extended depth of field IOLs, recently proposed by Fernández et al.20 our design can be included in the Continuous Full Range of Field (coined: FRoF-C) group.
Another important issue related to diffractive MIOLs are dysphotopic phenomena, such as halos, and glare.21,22 Several studies21,23,24 suggest that the higher the number of diffractive rings, the greater the incidence of dysphotopic phenomena tends to be. The Devil MIOL design features 7 diffractive rings, in contrast to other MIOLs that have between 16 and 29 diffractive rings, like the AT Lisa Tri (Carl Zeiss Meditec AG, Jena, Germany), Fine Vision (PhysIOL, Liège, Belgium), or Panoptix (Alcon Laboratories, Fort Worth, TX), among others.23,25 Therefore, the reduced number of diffractive rings could be an additional advantage of the new design. In future studies, we will verify whether this hypothesis holds true.
Another limitation of our study is that the assessment of visual performance was conducted monoculary using the single pupil diameter included in the simulator. According to the information provided by the manufacturer, the simulated phase of the MIOL is projected onto the pupil of the patient.13 Moreover, as the instrument lacked a chin rest, the evaluation position could potentially impact the alignment of the instrument with the eye of the patient (although we maintained careful control over the correct alignment during the test). It should be noted that when using these visual simulators, patients only experienced a brief period of time with the multifocal design. Therefore, it is expected that after the MIOL implantation and subsequent neuroadaptation process,26 the achieved VAs will be higher than those predicted using the instrument; but, on the other hand, the patients in the study have an average age of 24 years (range 20 to 40 years) with young and healthy corneas and retinas, while the potential patients, will be considerably older and therefore have more aberrated and irregular corneas, as well as somewhat reduced neural responses compared to the younger patients. For this reason, our clinical results may not be directly extrapolating to real clinical outcomes. However, in our opinion, they are sufficient to provide us with an insight into the visual quality that could be achieved after Devil MIOL implantation.
In conclusion, this simulation study in humans demonstrated that the Devil MIOL design enhances vision at intermediate distances, addressing the evolving lifestyle and visual demands of the current presbyopic population. Future studies would benefit from exploring the optical performance of the Devil MIOL design with varying pupil diameters and levels of higher-order corneal aberrations. Additionally, prototypes of the new MIOL will be constructed, and further studies will involve in vitro measurements of the optical quality of the MIOL.27,28
FundingThis work was supported by Ministerio de Ciencia e Innovación (PID2022–142407NB-I00); and Generalitat Valenciana (CIPROM/2022/30). A. M.-E. acknowledges financial support from Universitat de València programa Atracció de Talent 2021 (UV-INV-PREDOC21-1915492). D. M.-M. also acknowledges the Margarita Salas grant from the Ministerio de Universidades, Spain, funded by the European Union-Next Generation EU.