To investigate the 12–month effectiveness of Diverse Segmented Defocus Optics (DSDO) and Defocus Incorporated Multiple Segments (DIMS) spectacle lenses in a real–world clinical population in myopic and pre–myopic Chinese children.
MethodsAbout 364 subjects prescribed DSDO or DIMS were enrolled. Axial length (AL) and cycloplegic spherical equivalent refraction (SER) changes over 12 months were measured. The subjects were further divided into age sub–group (6–9; 10–14) and SER sub–group (+0.75D≤SER<–0.50D; –0.50D≤SER<–2.00D; –2.00D≤SER<–4.00D; SER≤–4.0D). Contrast sensitivity and visual experience were also reported. The rate of myopia progression was compared with historical single–vision spectacles (SVS) lenses data to evaluate the effectiveness of the regime.
Results317 subjects were analyzed. At 12–month, AL changes in the DSDO and DIMS group were 0.16±0.16 mm and 0.21±0.22 mm, respectively (P = 0.0202). DSDO spectacle lenses had better control effect in +0.75D≤SER<–0.50D and SER≤–2.0D sub–groups. The proportion of participants had no greater than 0.20 mm AL elongation was 65.00% and 55.41% of in DSDO and DIMS group separately. Myopia control effect in DSDO group was 47%–69% and 33%–62% in DIMS group compared to historical SVS lenses.
ConclusionsBoth DSDO and DIMS spectacle lenses retarded AL elongation. DSDO showed more stable myopia control effect comparing to DIMS, especially in groups of SER≤–2.0D sub–groups and older patients. DSDO showed initial potential myopia prevention effect in pre–myopic children compared with historical SVS lenses data. However, the small sample and no control group in pre–myopes of this study are key limitations. Further research is needed to confirm and understand DSDO's role for pre–myopic children.
Myopia (nearsightedness) is characterized by the axial length of an eyeball being too long for its optical power, has become a global public health concern with a dramatically increasing prevalence in recent decades.1–3 The trend toward lessening hyperopia or increasing myopia during childhood continues with a rising prevalence of myopia each passing year, especially in East Asian populations such as in China, Japan, and Singapore, etc.4–7 Myopic axial elongation is associated with various pathological changes such as glaucoma, myopic macular degeneration, retinal detachment, and choroidal and scleral thinning, all of which will cause irreversible vision loss, even results in irreversible blindness.6,8 It is estimated that 49.8% of the population will be myopic worldwide by 2050 and 938 million people with high myopia.4
Myopia has been commonly believed as a multi–factors ocular disorders, interplayed by genetic, ethnic, and visual environment conditions.6,7,9 The major element contributing to faster progression and severe complications is younger age of onset. Globally, the onset of myopia has been reported to be at the age of 6,6,7,10 and more commonly occurs between 8∼13years.11 Children younger than 10 years of age are reported prone to developing rapid myopia progression in comparison with children over 10 years old.6,7,12,13 Preventing the onset of myopia and stopping the progression of myopia has the potential to positively affect visual quality and quality of life.14–16 Currently, multiple interventions have been implemented for myopia prevention and control in children,12,17 low–concentration atropine,18 orthokeratology lenses,19 dual–focal or multifocal soft contact lenses20,21 and specifically designed spectacle lenses,22,23 and low‑intensity red‑light therapy,24 etc. The efficacy of different treatment regimens for myopia control varies, with the reported range being between 25% and 70% when compared to single–vision spectacle lenses (SVS).25 However, one way, the regulatory approved indication and adverse events such as myopia rebound, photophobia, corneal complications, allergic reaction, and economic consideration limit their widespread application.26,27 Another way, due to the limited treatment regimen choices for younger children between 6 and 9 years old, the myopia control effect of spectacle lenses draws more attention for all relevant parties, including healthcare provides, children and guardians, and even government urging effort to restore public ocular health issue.
Numerous animals and human data demonstrate that impose optical myopic defocus apposite in the mid–peripheral retina slow the emmetropization process and control the eyeball elongation in myopic eyes in different extent. Provision of appropriate specially designed spectacle lenses are one of the simplest, safety and cost–effective strategies to improve vision and control myopia progression for children. Actually, the use of spectacle lenses for myopia control has a decades–long history. Clinical studies on under–correction, bifocal eyeglasses, peripheral addition progressive eyeglasses, newly designed positive–microstructure–based myopia defocus, and contrast–reduced spectacles technologies have demonstrated mixed effectiveness. (For detailed reviews, please refer to other systematic reviews28–31). Greater myopia control success has been reported with Defocus Incorporated Multiple Segments (DIMS) spectacle lens (MiyoSmart, Hoya Co., Japan) in different randomized clinical trials (RCTs),23,32,33 which showed around 50∼60% myopia control effect comparing to single–vision spectacle (SVS) lenses after 2–year follow–up.22,23,30,34 Nevertheless, RCTs are usually used as initial studies conducted to establish the safety and efficacy of an investigational device or drug, which present highly internal validity among a specific range of subjects and clinical application circumstance.35 One of the limitations for RCTs is the “extrapolation” problem, which may occasionally compromise generalizability to broad–spectrum patient–level population.36 Real world evidence (RWE) generated from real–world data (RWD), may complement the drawback of RCTs with occlusions generalizing from universal population rather than specialized population in controlled clinical trials.
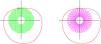
Recently, newer spectacle lenses technology with Diverse Segmented Defocus Optics (DSDO) spectacle lenses have been designed and applied in clinical practice (Fig. 1). All these designs incorporated relatively more positive power microstructure segmentations compared to the central distance correction refractive power. There microlenses array was arranged surrounded of central optic zone. However, there is still no real–world research result on the control effect of DSDO spectacle lenses on the axial length growth of patients with myopia and pre–myopia. Therefore, the present study is designed to investigate the 24–month effectiveness of DSDO and DIMS spectacle lenses in a single center real–world clinical population for myopia management in myopic and pre–myopic Chinese children, and the current report showed the initial 12–month results.
Representative diagram of intervention devices.
The present study is a 24–month, prospective, intervention cohort clinical study conducted in the Department of Ophthalmology and Optometry Centre, Peking University People's Hospital, Beijing, China. The study complied with the Declaration of Helsinki and approved by the Ethics Committee and approved by Institutional Review Board of the Peking University People's Hospital (2022PHB354–001).
The medical records of subjects aged 6–14–year–old with non–diseased eyes who were prescribed DSDO or DIMS between July 2022 and December 2022 were reviewed, and all the subjects will be expected to finish a 2–year follow–up. Subjects were allocated into either wearing DSDO or DIMS spectacle lenses according to the decision from eye care professionals, subjects, and their guardians.The purpose and details of the study were explained to all the subjects and their parents, and they all signed the informed consent form. The inclusion criteria include: (1) age between 6 and 14 years old (both inclusive); (2) diagnosed of pre–myope (+0.75D≤SER<−0.50D) or myopia (−0.50D≤SER≤−8.00D) condition under cycloplegic spherical equivalent refraction (SER); (3) best–corrected visual acuity (BCVA) of at least 20/20 (0.0 logMAR); (4) astigmatism ≤ 2.0D of each eye and anisometropia ≤1.5D between bilateral eyes; (5) subjects must be without strabismus, amblyopia, or other ocular or significant systematic abnormalities. The subjects who discontinued or changed the original treatment strategy (e.g., combined with atropine eye drop, changed to orthokeratology lenses, etc.) or did not complete both the 6– and 12–month follow–up visits were excluded. For the pre–myope condition (+0.75D≤SER<−0.50D) subjects, plano or positive refractive power spectacle lenses (+0.50D ≤ spectacle lenses power ≤ 0.0D) were prescribed according to the experience of the professionals. All the subjects were required to mandatorily complete follow–up visit every 6–month, and recommended to conduct a 3–month interval follow–up visit.
Intervention devicesDIMS (MiyoSmart, Hoya Co., Japan) and DSDO (AURA, Zhuhai Fitlens Ltd, China) were used in the present clinical study. The detailed spectacle lenses design and representative diagrams were shown in Fig. 1 and described in Discussion section. The result of cycloplegic subjective refraction was chosen as the final prescription to avoid over– or under–correction. It is recommended to replace the spectacle lens with update prescription when the change of cycloplegic SER was more than 0.5D.
Ophthalmic measurementsThe measurement of distant visual acuity was carried out with a standard logMAR tumbling E chart at a fixed distance of five meters. Best–corrected distant visual acuity (BCVA) was determined subsequently to a subjective refraction examination. Slit lamp biomicroscope was performed to identify ocular abnormalities and disorders. An experienced pediatric ophthalmologist or optometrist performed the cover–uncover and alternating cover test, Hirschberg test to assess the presence of strabismus. Refraction was measured before and after cycloplegia using autorefractor (model RM–800; Topcon, Tokyo, Japan). Axial length (AL) was measured before cycloplegia using the SW–9000 (SUOER, Tianjin, China), and the average of five recordings was taken for further analysis. Cycloplegia was attained using 1% cyclopentolate (Cyclogyl; Alcon, Fort Worth, TX, USA). Initially, each subject received a drop of 0.5% proparacaine hydrochloride in each eye, followed by two drops of 1.0% cyclopentolate, administered five minutes apart. Cycloplegia was confirmed by measuring papillary light reflex when reflex was disappeared. SER was calculated based on the algebraic sum of sphere and half the cylinder (SER = sphere + 0.5 × cylinder). Contrast sensitivity test (CSV–1000, VestorVision Ocular Health, Ohio, US) was conducted at 6–month follow–up visit.
Data categoriesOutcomes measured changes in AL and SER were reported over a period of 6 months and 12 months. Further subgroups were divided according to the age and SER. Baseline age was stratified as younger age (6– to 9–year–old) and older age (10–14–year–old). Baseline SER was stratified as pre–myopia group (PM, +0.75D≤SER<−0.50D), low myopia group (LM, −0.50D≤SER<−2.00D), moderate myopia group (MM, −2.00D≤SER<−4.00D) and higher myopia group (HM, SER≤−4.0D). Proportion of subjects with an AL elongation of each 0.1 mm in step of the DSDO and DIMS group was calculated. The rate of myopia progression was compared with historical SVS lenses data to evaluate the effectiveness of the regime.
Statistical analysisThe analysis was conducted using the GraphPad Prism (version 10.1.1, GraphPad Software, LLC.). Only the right eye of each subject was selected for analysis. Continuous variables were described as mean±standard deviation (SD). Group comparisons of continuous variables utilized the t–test. Statistical significance was set at a two–sided P value of less than 0.05, with 95% confidence intervals. The analysis was performed for all the subjects as a whole group, and for different subgroups defined by age and SER stratification.
ResultsBaseline demographic characteristicsA total of 364 subjects’ records with cycloplegic SER between +0.75 diopter (D) and −8.0D were reviewed during the recruitment period from July 2022 to December 2022. Among the above children, in total, 317 participants (82.55%) successfully completed the study: 160 (50.47%) in the DSDO group and 157 (49.53%) in the DIMS group. The mean age, SER and AL of the participants were 8.86±2.09 years old, −1.35±1.49 D, 23.94±1.04 mm in the DSDO group, and were 9.18±1.82 years old, −1.62±1.15, and 24.13±0.89 mm accordingly in the DIMS group. These data collected at baseline were no statistically significant in myopia group (−0.50D ≤SER≤−8.0D). Table 1 and Table S1 showed the demographic and ocular characteristics data of each group and subgroup at screening baseline. During the follow–up process, 47 participants (12.91%) were dropped–out from the current analysis, and the reasons for discontinuation included: lost to follow–up (18), change treatment strategies (10 change to orthokeratology, 9 combined with 0.01% atropine eye drops, 4 change to soft contact lenses), not interested in participating (4), and not seeing well during near–work reading condition (2). When comparing the baseline characteristics between drop–out subjects, no significant difference was found. Due to the nature of the myopia development process, there are only a few of children in HM group of 6–9–year–old group and in PM group of 10–14–year–old group.
Demographic and ocular characteristic of each group at baseline.
DSDO, diverse segmented defocus optics; DIMS, defocus incorporated multiple segments; SER, spherical equivalent refraction; PM, pre–myope; LM, low myopia; MM, moderate myopia; HM, higher myopia; SD, standard deviation; D, diopter; mm, millimeter; y, year.
Table 2, Table 3 and Fig. 2 showed the change of AL and SER in two groups over 6–month and 12–month. At 6 months, the annualized mean progression in AL and SER were 0.07±0.09 mm and −0.09±0.23D in the DSDO myopia group and 0.11±0.14 mm and −0.15±0.37D in the DIMS myopia group. A statistically significant (P < 0.05) effect was found in AL but not in SER mean progression. At 12 months, the annualized mean progression in AL and SER were 0.16±0.17 mm and −0.22±0.41D in the DSDO myopia group and 0.20±0.22 mm and −0.29±0.46D in the DIMS myopia group. There was no statistically significant effect in both AL and SER mean progression in the myopia group. Both at 6– and 12–month, the DSDO pre–myopia group showed slower mean progression by 0.09 mm and 0.12 mm differences compared to DIMS group, separately (P = 0.0041 at 6–month; P = 0.0056 at 12–month).
Annualized axial length changes over 6– and 12–month of each group from baseline.
Annualized spherical equivalent refraction changes over 6– and 12–month of each group from baseline.
Annualized adjusted changes in axial length and spherical equivalent refraction over 6 and 12 months in all groups adjusted axial length mean progression in all of the participants, including myopia children and pre–myopes; (B) and (C) represent the AL–adjusted mean progression in each sub–group at 6–month follow–up visit and 12–month follow–up visit; (D) adjusted spherical equivalent refraction (SER) mean progression in all of the participants, including myopia children and pre–myopes; (E) and (F) represent the SER–adjusted mean progression in each sub–group at 6–month follow–up visit and 12–month follow–up visit.
No. of DSDO group subjects: ALL=160; Myopia =122; Pre–Myope=38; ≤−0.5, >−2.0 = 83; ≤−2.0, >−4.0 = 27; ≤−4.0 = 12.
No. of DIMS group subjects: ALL=157; Myopia =142; Pre–Myope=15; ≤−0.5, >−2.0 = 91; ≤−2.0, >−4.0 = 41; ≤−4.0 = 10.
DSDO showed initial potential myopia prevention effect in pre–myopic children compared with historical SVS lenses data. It was shown in the previous study that among children in the pre–myopia stage, the one–year axial length (AL) elongation was 0.24 [0.18, 0.34] mm with the single vision spectacle lens.37 Thus, based on the median data of the 12 months axial length growth of those wearing single vision glasses in previous studies, we further performed a one sample Wilcoxon rank–sum test using the axial length change data in this study. The results indicated that there was a significant statistical difference (p < 0.001) in the axial length growth over 12 months between the DSDO group and the single vision spectacle group for pre–myopic children (Table S3). However, there was no statistical difference in the 12 months axial length growth between children in the pre–myopia stage wearing DIMS and the single vision spectacle group (p = 0.39, Table S3).
SER– and Age– sub–group analysisDuring analysis among LM, MM and HM sub–groups, DSDO showed better myopia control efficacy compared to DIMS in subjects with a SER of over −2.0D at 6– and 12–month follow–up visits (Table 4, Table 5andFig. 2), and there was a significant difference between the groups. Analyzing the data by age groups, we found a statistically significant difference in the myopia control effect of DSDO lenses in the 10–14–year–old group, with a mean AL difference of 0.09 mm (P = 0.0067) and 0.09 mm (P = 0.0053) at 6– and 12–month in myopia kids. The SER mean progression was slowed by 0.18D and 0.24D at 6– and 12–month in the DSDO group. There was no significant difference in AL elongation between the two groups of younger age myopia patients. Caution should be considered because of the limited number of subjects in older age with PM and in younger age with HM.
Annualized axial length changes in subgroup from baseline.
Annualized spherical equivalent refraction changes in subgroup from baseline.
In DSDO spectacle lenses group at the 12–month follow–up, 65.00% (104/160) of all the participants, 60.32% (76/126) of 6–9–year–old, and 82.35% (28/34) of 10–14–year–old patients had myopia progression of no greater than 0.20 mm AL progression. In DIMS spectacle lenses group at the 12–month follow–up, 55.41% (87/157) of all the participants, 53.47% (54/101) of 6–9–year–old, and 58.93% (33/56) of 10–14–year–old patients had myopia progression of no greater than 0.20 mm AL progression (Table S2 and Fig. 3).
Distribution of myopia axial length progression between DSDO and DIMS spectacle lessens in different sub–groups
adjusted axial length mean progression in 6– to 9–year–old group in myopia subjects; (B) and (C) represent the annualized AL–adjusted mean progression in younger and older age groups at 12–month follow–up visit; (D), (E) and (F) describe the distribution of axial length progression in 0.10 mm step at 12–month follow–up visit comparing to baseline in DSDO lenses group; (G), (H) and (I) represent the distribution of axial length progression in 0.10 mm step at 12–month follow–up visit comparing to baseline in DIMS lenses group.
No. of DSDO group subjects: ALL=160; Myopia =122; Pre–Myope=38; ≤−0.5, >−2.0 = 83; ≤−2.0, >−4.0 = 27; ≤−4.0 = 12.
No. of DIMS group subjects: ALL=157; Myopia =142; Pre–Myope=15; ≤−0.5, >−2.0 = 91; ≤−2.0, >−4.0 = 41; ≤−4.0 = 10.
The questionnaire was adopted from a previous published article.38 As for subjective rating of vision experience (3–day phone call follow–up visit), it was reported by 92.06% (116/126; 126 parents/guardians answered) in DSDO group and 89.39% (118/132; 132 parents/guardians answered) in DIMS group had clear vision at all viewing distances, and they could adapt lens wearing within 3 days. The mean contrast sensitivities scores at 6–month of 3cpd, 6cpd, 12cpd and 18cpd were 5.35, 5.16, 5.33, 5.21 in DSDO group, and were 5.33, 5.15, 5.22 and 5.22 in DIMS group, separately. The average cumulative daily spectacle lenses wearing time for DSDO and DIMS groups were 12.03 ± 1.24 and 11.82 ± 1.16 h, respectively, and the difference was not statistically significant (P = 0.07). The average cumulative daily spectacle lenses wearing time for PM and Myopia groups were 11.94 ± 1.29 and 11.92 ± 1.19, respectively, and the difference was not statistically significant (P = 0.84). A total of 6 adverse events (3.75%) were reported for the DSDO group and 7 adverse events (4.46 %) for the DIMS group. The adverse events including itching of the eye, dry eye, allergic conjunctiva. None of the adverse events was related to a medical device. There were no adverse events in the PM group, and the patients had good acceptance.
DiscussionThe myopia management effects of DSDO and DIMS spectacle lenses in Chinese children were compared in the present real–world study, and the novel findings include: (1) confirmed both DSDO and DIMS eyeglasses provided myopia control effect, and 55% of the subjects in DIMS group and 65% in DSDO group had no greater than 0.20 mm AL elongation at 12–month follow–up; (2) subjects with moderate and higher myopia or subjects with older age would benefit more from DSDO lenses treatment; (3) DSDO spectacle lenses showed more retardation effect in AL elongation of pre–myopic subjects compared with historical SVS lenses data; (4) the subjective rating of vision experience in both spectacle lenses are both well–tolerated and comparable in both groups.
Recently, the Real world evidence (RWE) studies exhibited a growing impact on the healthcare activity, pharmaceutical and medical device manufacturers.39 Recent real–world studies evaluated the myopia control effect of DIMS spectacle lenses in different clinical practice situation, data showed that the 1–year mean AL elongation of 0.25 mm, 0.27 mm, 0.30 mm, which represented effectiveness of 30∼45% in slowing down AL growth.40–42 This present study revealed that the 12–month progression of axial elongation in DIMS group is 0.20±0.22 mm in myopic subjects (−0.50D≤SER<−8.00D), which indicated better overall myopia control effect than recent reported studies. Nevertheless, after sub–divided into different refractive error groups, the AL retardation effects was reduced as the SER increase gradually, especially in children with myopia over −4.0D: AL elongation was 0.15±0.18 mm, 0.26±0.21 mm and 0.44±0.35 mm in LM, MM and HM group, respectively. Thus, these findings alert the eye care professionals that close follow–up should mandatorily require for high–risk patient during routine observation, such as higher myopia patients. For DSDO spectacle lenses group, the AL progression was presented relatively well–controlled among different SER sub–groups. However, caution should be considered due to small sample size in HM group. Overall, comparing to historical SVS treatment effects at 12–month based on previous published articles (between 0.30 mm and 0.52 mm), the myopia control effect with reference to AL elongation is between 47%∼69% in DSDO group (0.16±0.17 mm at 12–month follow–up), and 33%∼62% in DIMS group (0.20±0.22 mm at 12–month follow–up), further confirmed the myopia control effect on children with various efficacy.
According to the report of CLEERE study (the large–scale collaborative longitudinal evaluation of ethnicity and refractive error43–45), the ocular AL growth rate in emmetropic eye showed an average of 0.1mm/year in children aged 6–14–year–old, with faster for age 6–9–year–old of 0.16mm/year and slower to less than 0.1mm/year in children older than 10 years old.9,13,44 Subsequently, several articles demonstrated the annual elongation rate without proper myopia control treatment strategy in Asian myopic eyes were about 0.3mm∼0.4 mm, which will lead to high risks of sight–threatening complications in their later–life phase.10,31,46 In this current study, the mean AL changes were 0.19±0.15 mm and 0.21±0.20 mm in DSDO and DIMS groups for age between 6– and 9–years old subjects after 1–year treatment; and were 0.07±0.15 mm and 0.21±0.25 mm of older age patients. Furthermore, there was a greater proportion of an AL slow progression rate of up to 0.2 mm (≤0.2 mm) in the DSDO group compared with DIMS group (60% and 53%, respectively) in the 6–9–year–old group, and the differences were higher in DSDO group in 10–14–year–old group (82% and 59%, respectively). More astonishing is that nearly 40% of the children in 6–9–year–old group from DSDO treatment group presented not more than 0.1 mm AL elongation, which was similar toemmetropic ocular growth condition.
Another noteworthy finding is that the application of plano and positive spectacle lenses (0.0D ≤ spectacle correction power ≤ +0.50D) in pre–myope subjects showed controlled axial length elongation rate in DSDO group. At 12–month follow–up visit, the AL elongation is 0.16±0.13 mm, and 76.32% (29/38) of the subjects exhibited not more than 0.2 mm axial growth. Previous study has shown that in children in the pre–myopia stage, the one–year axial length (AL) elongation was 0.24 [0.18, 0.34] mm with the single vision spectacle lens,37 there was a significant statistical difference (p < 0.001) in the axial length growth over 12 months between the DSDO group and the single vision spectacle group for pre–myopic children. Providing early intervention for kids who have not yet developed to myopia condition has been a debate for academic field. However, due to visual environmental factors and visual demand changes in modern daily life, the prevalence of myopia in primary school has achieved 35% in China, with the prevalence of 12% even for kindergarten children, indicating the trend of early onset is inevitable.47,48 Currently, preventing the onset of myopia is one of the hot topics in myopia scope in mainland China, including increase outdoor activity time, reduce intensive near–work, low–dose atropine eye drop application, etc. Optical intervention with spectacle lenses has been raised attention by several researchers recently.12,49 These results in the present study showed new clues of pre–myopia prevention strategy. However, given the absence of a strictly controlled cohort, matched baseline data, compounded by the fact that the limited sample size among pre–myopic participants in this research is a key limitation, more extensive and comprehensive clinical studies need to be conducted to demonstrate the reliability of the plano and positive DSDO spectacle lenses intervention modality in pre–myopes.
The present study revealed that DSDO spectacle lenses showed more stable and potent myopia control effect and no significant difference in term of AL elongation among various SER and age sub–groups. The most likely reason of these findings could be the unique design technology of DSDO. Conclusions from the studies of animal model and patient, the insufficient myopic defocus and higher refractive error might compromise the myopia control effect.2,13,14,17 The design of both DSDO and DIMS spectacle lenses both are based on the principle of optical defocus theory, which by imposing optical myopic defocus stimulation on the mid–peripheral retina guides the eye growth to inhibit elongation. Comparing to the designs of the two technologies, the main optical differences include distinctive addition power distribution and radius arrangement of the microlenses segmentations. For DSDO spectacle lenses, the inner two continuous circle of +4.0D lenslets provide additional +0.5D power, that may exert more myopic defocus in the mid–peripheral retina comparing to DIMS lenses (consisting of +3.50D microlenses23). As reported by animal studies, myopia control effect is dose–depended, at least within a certain dioptric range,2,50–52 which may possibly explain DSDO exsert higher efficacy by projecting more myopia defocus closely to the surrounding area of the fovea, which is the core location that regulate ocular growth. In addition, the lenslet array geometry for DSDO is linearly arranged which surround the clear central zone. The radial “GAP” (function as optical correction) between microstructure segments are more in line with the characteristics of the eye rotation habitual, i.e., turning the eyeball to various clock positions with the visual axis as the center. However, more research on mechanisms of the spectacle lenses needs to be further explored, such as quantification the amount of myopic defocus, contrast modulation methods and other characteristics of the two spectacles both computationally and on–bench experimentally.53–55
Nevertheless, due to the nature of the real–world study, limitations in the current study are notable. First, limited subjects’ numbers in the moderate and higher myopia group, especially in the older age group, could potentially have an impact on the results interpretation. Second, the peripheral retinal myopia defocus and peripheral axial length was not measured. Third, there is no consensus on the treatment strategy on pre–myopic subjects, and there was a small sample size and no strictly controlled group. Furthermore, loss–of–follow–up subjects may have more confounding factors for explaining the myopia control effect. Future studies for enlarged sample size, balanced group participants, and long–term duration are needed to investigate the myopia and pre–myopia management effect.
ConclusionIn summary, both DSDO and DIMS spectacle lenses can significantly retard AL elongation compared to SVS lenses and both lens designs are worthy choices for myopia control. In addition, DSDO lenses showed more stable myopia control effect comparing to DIMS lenses, especially in sub–groups of SER≤−2.0D, and moreover presented more potent efficacy in patients older than 10 years old. DSDO demonstrated a potential for preventing myopia in pre–myopic children as contrasted with the previous study data of historical SVS lenses. Nonetheless, the small samples and the lack of a control group within the pre–myopic cohort of this study constitute some constraints. Supplementary research is essential to corroborate the part that DSDO plays in the context of pre–myopia. And, comprehensive large–scale future studies are needed to investigate the long–term myopia management effect.
Funding supportThis work was supported by the National Natural Science Foundation of China (Grant No. 82171092, 82371087), Capital's Funds for Health Improvement and Research (No. 2022–1G–4083), the National Key R&D Program of China (No. 2021YFC2702100).
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.