Acanthamoeba keratitis (AK) is a sight-threatening disease, commonly associated with contact lens use. Early diagnosis is challenging due to its varied clinical presentation. This study aimed to describe the clinical characteristics of AK and evaluate the utility of corneal scraping for early diagnosis.
MethodsThis retrospective study included 14 eyes from 13 AK patients diagnosed by detecting Acanthamoeba cysts in corneal scrapings between July 2019 and August 2023. Clinical history, presentation, treatment, and outcomes were analyzed.
ResultsThe mean age of the 13 patients was 28 ± 13 years. Ten patients used soft contact lenses and 3 patients used orthokeratology lenses. The mean time from symptom onset to diagnosis was 18.2 ± 12.9 days. The most common sign was elevated superficial punctate keratitis (SPK), followed by dot infiltrates, radial perineuritis, pseudodendrites, stromal edema, and ring infiltrates. Nine eyes were treated with polyhexamethylene biguanide (PHMB) eye drops, while the remaining five eyes received a combination of two or three anti-amoebic agents. Thirteen eyes improved with treatment, but one eye—initially misdiagnosed and treated with steroids—required therapeutic keratoplasty. LogMAR visual acuity in eleven eyes improved from an initial 0.51 ± 0.44 to 0.11 ± 0.10 after treatment.
ConclusionElevated SPK is the most frequent early feature of AK and may mimic contact lens-related sterile keratitis. Corneal scraping is a valuable diagnostic tool that enables earlier detection and improved outcomes. PHMB monotherapy is effective in early AK, while delayed diagnosis and prior steroid use may lead to disease progression and necessitate surgical intervention.
Acanthamoeba are ubiquitous, free-living amoebae found in air, soil, dust, tap water, pond water, and seawater. They exist in two forms: active trophozoites and dormant, double-walled cysts.1,2 Acanthamoeba primarily cause ocular infections in humans, most notably Acanthamoeba keratitis (AK)—a rare but potentially sight-threatening condition with a prolonged course. The incidence of AK has recently increased, particularly among contact lens (CL) users. Other risk factors include trauma involving vegetation or soil and exposure to contaminated water.1–3 Patients with AK may present with symptoms such as red eye, disproportionate eye pain, photophobia, tearing, and blurred vision, although symptomatology can vary.4,5 Because these manifestations overlap with those of CL-associated infiltrative keratitis and other types of keratitis—such as herpetic, bacterial, or fungal keratitis—accurate diagnosis is essential.6,7 AK is often initially misdiagnosed, further complicating diagnosis and delaying appropriate treatment. Treatment of AK remains challenging and is not yet standardized. Although combination therapy is generally considered more effective than monotherapy, some studies have reported promising outcomes with biguanide monotherapy.2,8 This study underscores the importance of prompt and accurate diagnosis through smear examination in patients suspected of having early-stage AK. It also describes the clinical manifestations of both early and late stages, as well as treatment outcomes achieved with either topical polyhexamethylene biguanide (PHMB) monotherapy or combination therapy involving PHMB, chlorhexidine gluconate (CHX), and/or voriconazole (VCZ). We further present a strategy for optimizing therapeutic approaches.
Materials and methodsPatient enrollmentThe study was approved by the Institutional Review Board of Cathay General Hospital (IRB number: CGH-P113032) and was conducted in accordance with the Declaration of Helsinki guidelines. A retrospective observational case series was conducted involving patients diagnosed with AK, confirmed by the presence of Acanthamoeba cysts in corneal scraping smears, between July 2019 and August 2023 in the Department of Ophthalmology at Cathay General Hospital, Taipei, Taiwan.
Data collectionMedical records, external eye photographs, and smear images were reviewed. Data collected included contact lens usage history, initial symptoms, the interval from symptom onset to diagnosis, clinical presentation, pre- and post-treatment visual acuity, anti-AK medications administered, and final outcomes. For all patients presenting with corneal infiltrative erosion, ulcer, or suspected AK, corneal scraping smears for Gram staining and corneal swabs for bacterial culture were routinely performed. Diseased cells on the surface of the corneal ulcer were removed with a sterile #15 surgical blade under topical anesthesia using 0.5 % proparacaine, particularly at the peripheral borders of the infiltrate. Corneal scrapings were thinly spread on clean microscope slides. Air-dried specimens were then transported for Gram staining. Patients with incomplete medical records or confirmed alternative diagnoses (e.g., bacterial or fungal keratitis) were excluded from the study. Anti-amoebic treatment was initiated when Acanthamoeba cysts were identified in corneal scraping smears under light microscopy. This included either monotherapy with topical PHMB 0.02 % eye drops (Aseptic Innovative Medicine, Taoyuan, Taiwan), administered hourly, or combination therapy consisting of topical CHX 0.02 % eye drops (Aseptic Innovative Medicine, Taoyuan, Taiwan) administered hourly, VCZ 1 % eye drops, or oral VCZ 200 mg once or twice daily. Treatment strategies were determined based on the clinical severity of AK and response to antibiotics. Most patients presenting with elevated superficial punctate keratitis (SPK) or pseudodendrites initially received PHMB monotherapy. Patients presenting with perineuritis, stromal infiltrate, ring infiltrate, or inadequate response to PHMB monotherapy received combination therapy. Histopathological examination of corneal button specimens from penetrating keratoplasty (PKP) was performed using hematoxylin–eosin staining.
Statistical analysesStatistical analyses were performed using SPSS version 29 (IBM Corp., Armonk, NY, USA). Given the small sample size, the Shapiro–Wilk test was used to assess normality. As the data were normally distributed, a paired t-test was applied to compare pre- and post-treatment LogMAR visual acuity. A p-value of ≤ 0.05 was considered statistically significant.
ResultsPatient characteristicsThis study enrolled 14 eyes of 13 patients, with a mean age of 28 ± 13 years (range: 11–50 years). All patients had a history of CL use, including 10 with soft CLs and 3 with orthokeratology lenses. The 3 patients using orthokeratology lenses were aged 11, 14, and 15 years. The primary symptoms included eye pain, redness, and blurred vision. The mean interval from symptom onset to confirmed diagnosis was 18.2 ± 12.9 days (range: 3–49 days). The initial LogMAR visual acuity (VA) was 0.52 ± 0.45 (range: 0.05–1.0).
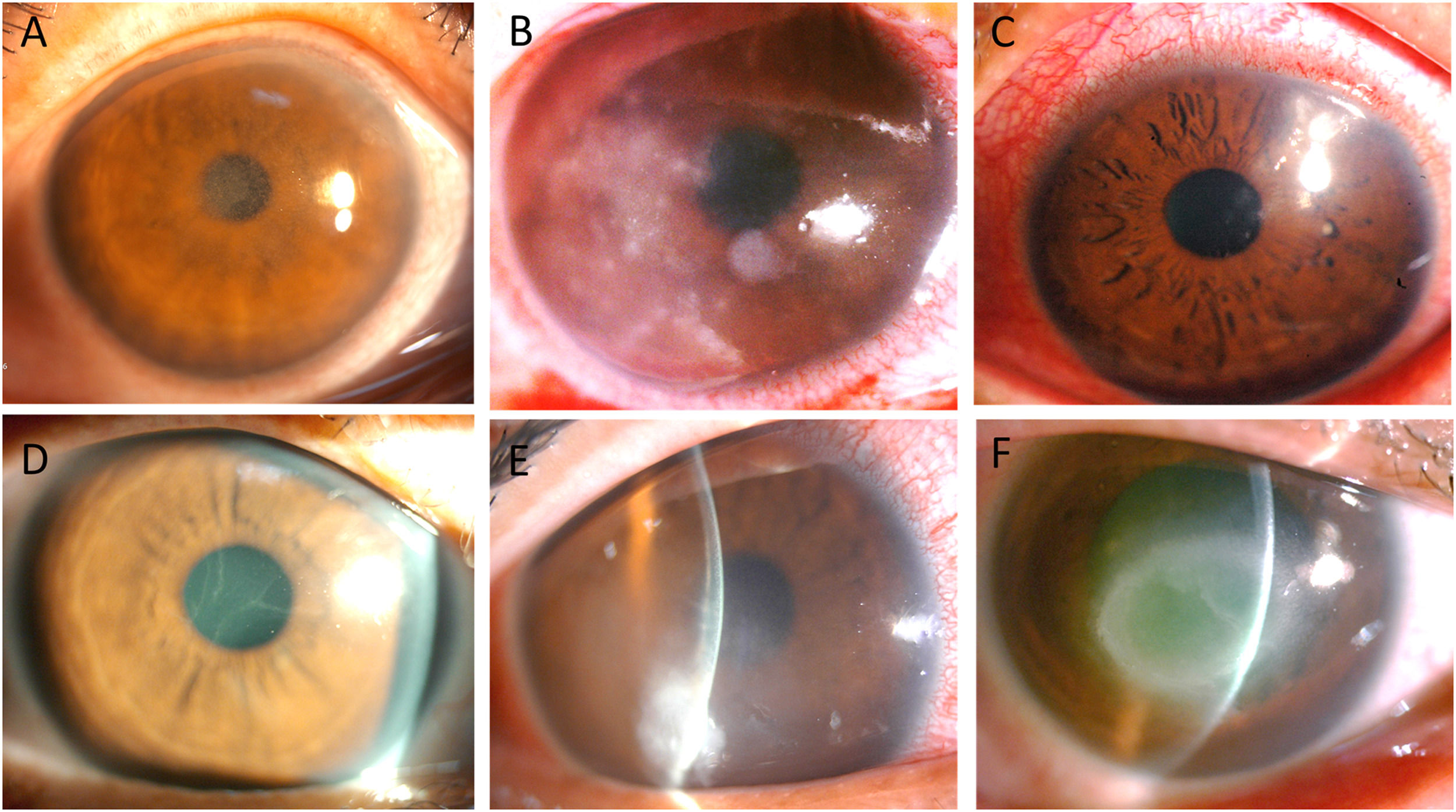
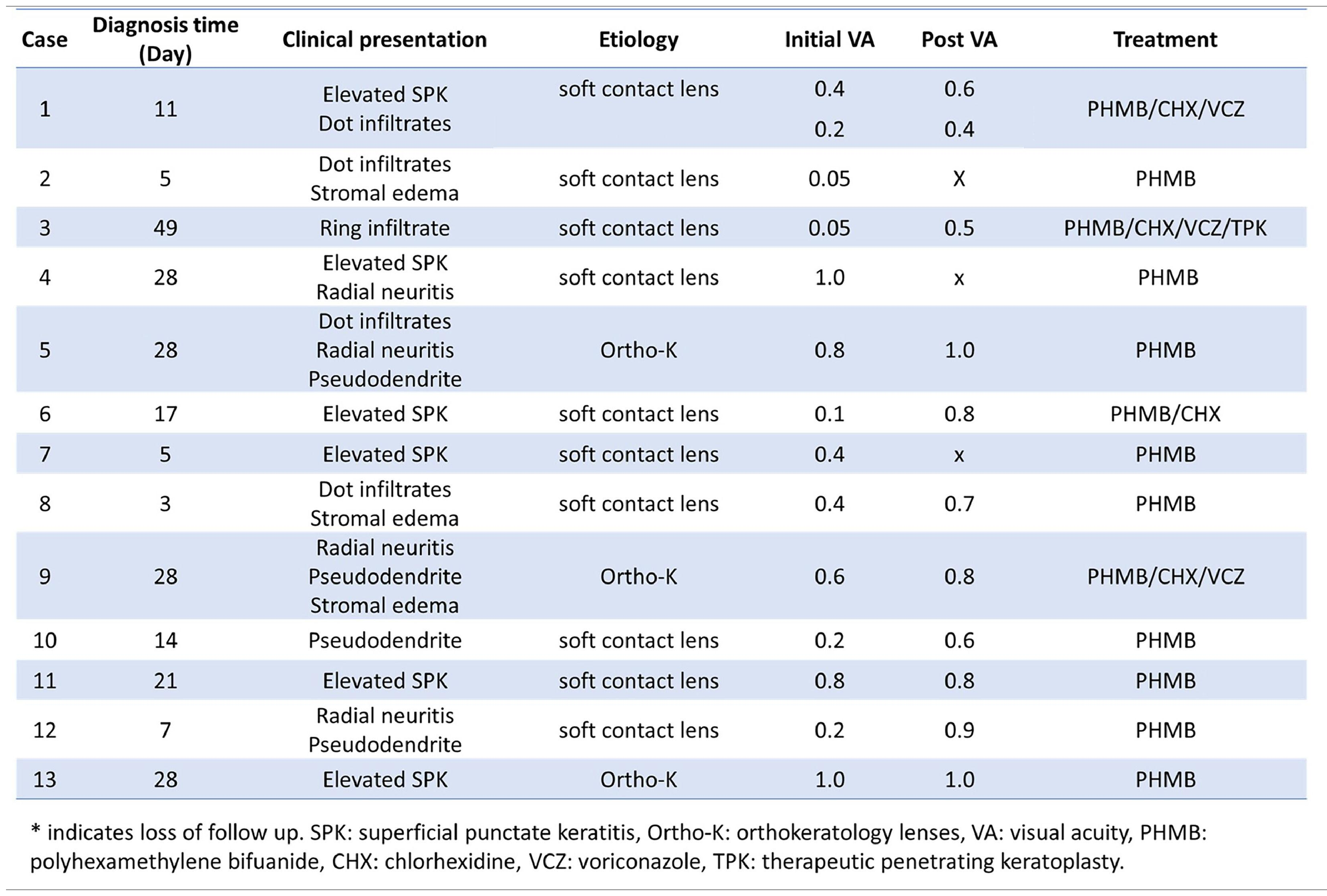
Clinical presentationClinical manifestations included elevated SPK in 7 eyes (Fig. 1A), dot stromal infiltrates in 5 eyes (Fig. 1B), radial perineuritis in 4 eyes (Fig. 1C), pseudodendrites in 4 eyes (Fig. 1D), stromal edema in 3 eyes (Fig. 1E), and a ring infiltrate in 1 eye (Fig. 1F). Among these manifestations, elevated SPK and pseudodendrites were frequently misdiagnosed elsewhere as corneal erosion and herpes simplex virus (HSV) keratitis, respectively.
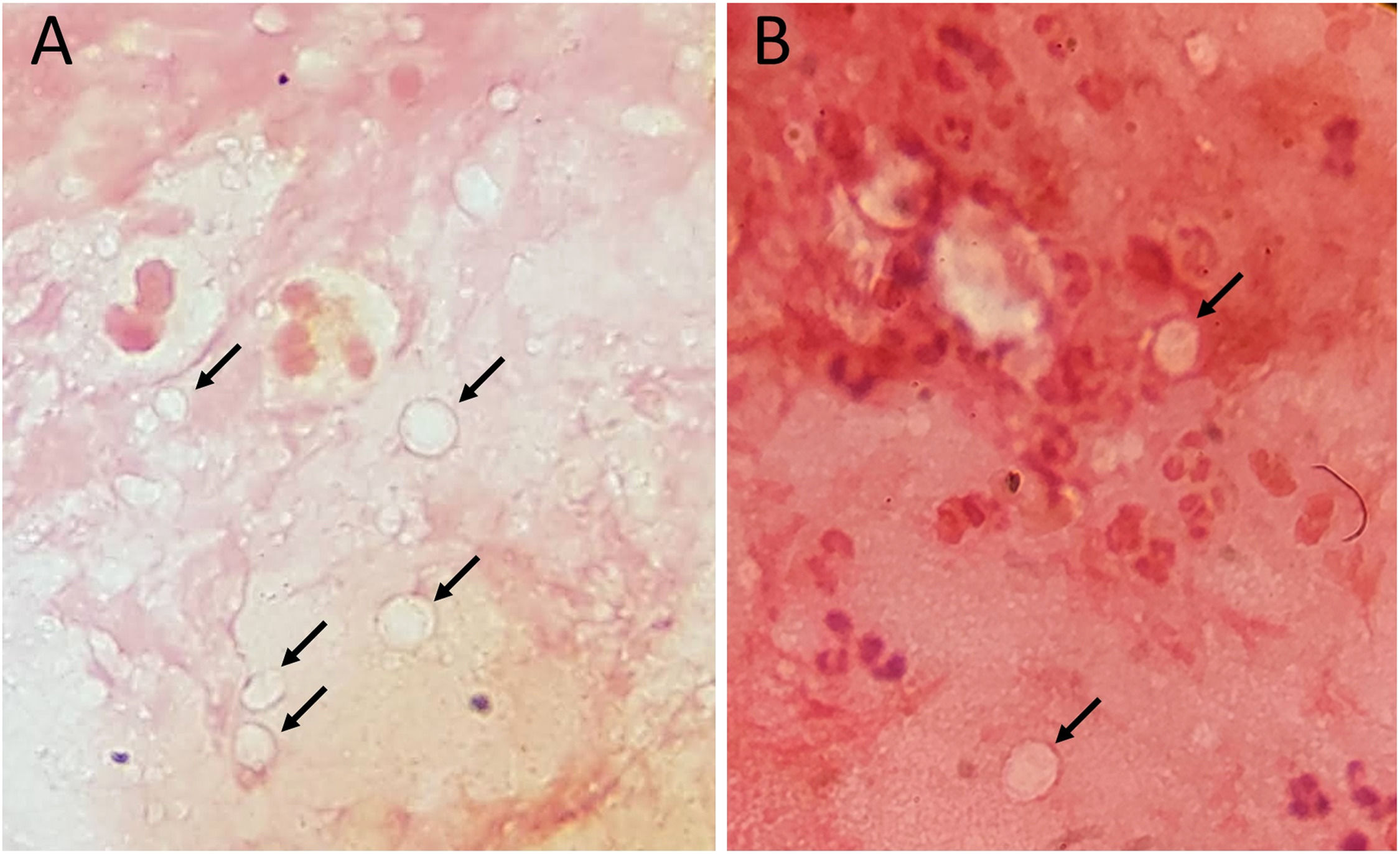
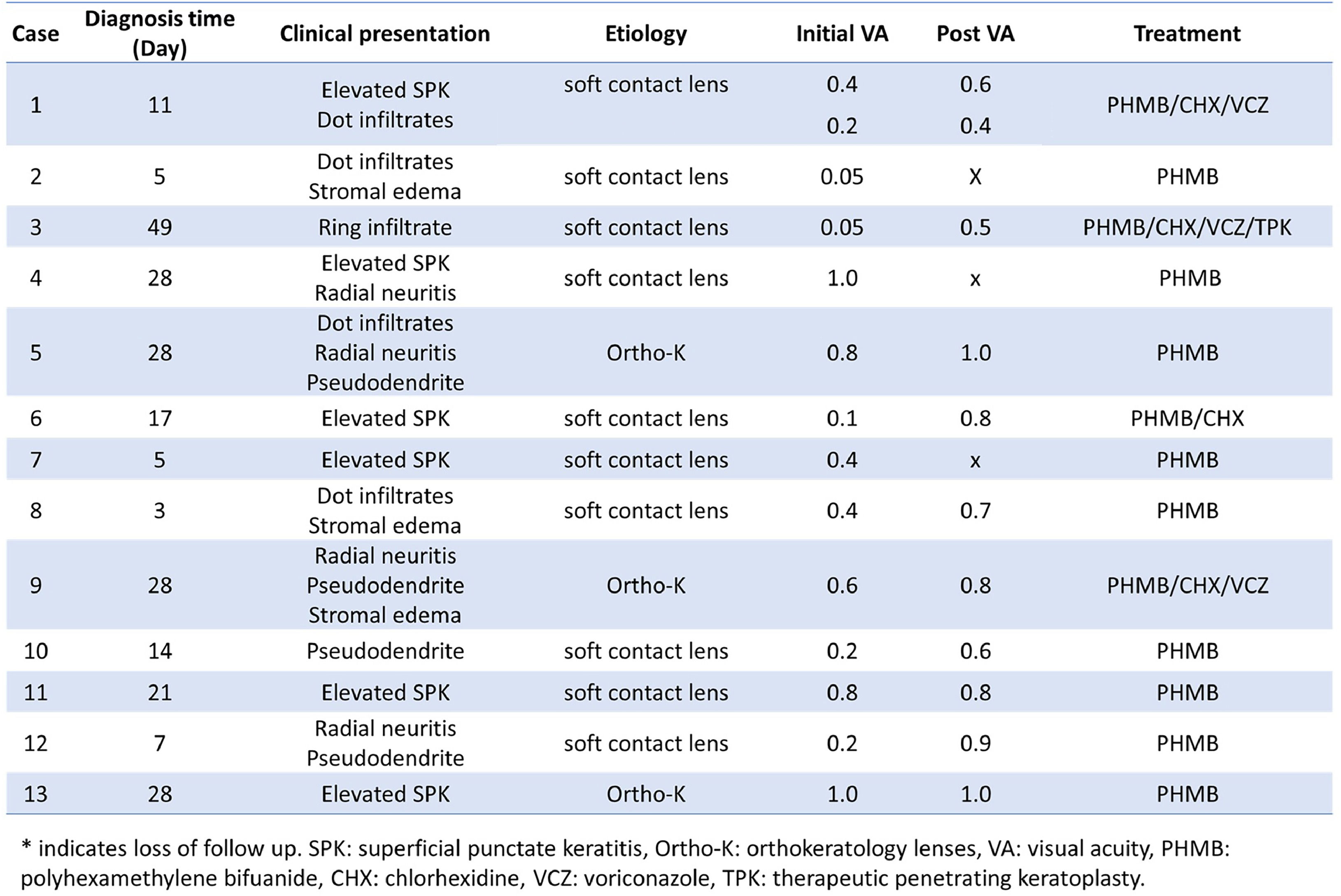
Diagnosis and treatmentDouble-walled Acanthamoeba cysts were identified in corneal scraping smears from all 14 affected corneas by Dr. Hou and experienced technicians in the Department of Clinical Pathology (Fig. 2A and 2B). Anti-Acanthamoeba treatment included PHMB monotherapy in 9 eyes, combination therapy with PHMB and CHX in 1 eye, and a triple-drug regimen comprising PHMB, CHX, and oral VCZ in 4 eyes. One patient with severe bilateral AK received topical PHMB, CHX, and topical VCZ due to intolerance to oral VCZ caused by elevated liver enzymes. Thirteen eyes responded to medical treatment, while one eye required therapeutic PKP. However, three patients were lost to follow-up after one month. Clinical manifestations and treatment outcomes for all 14 eyes are summarized in Table 1.
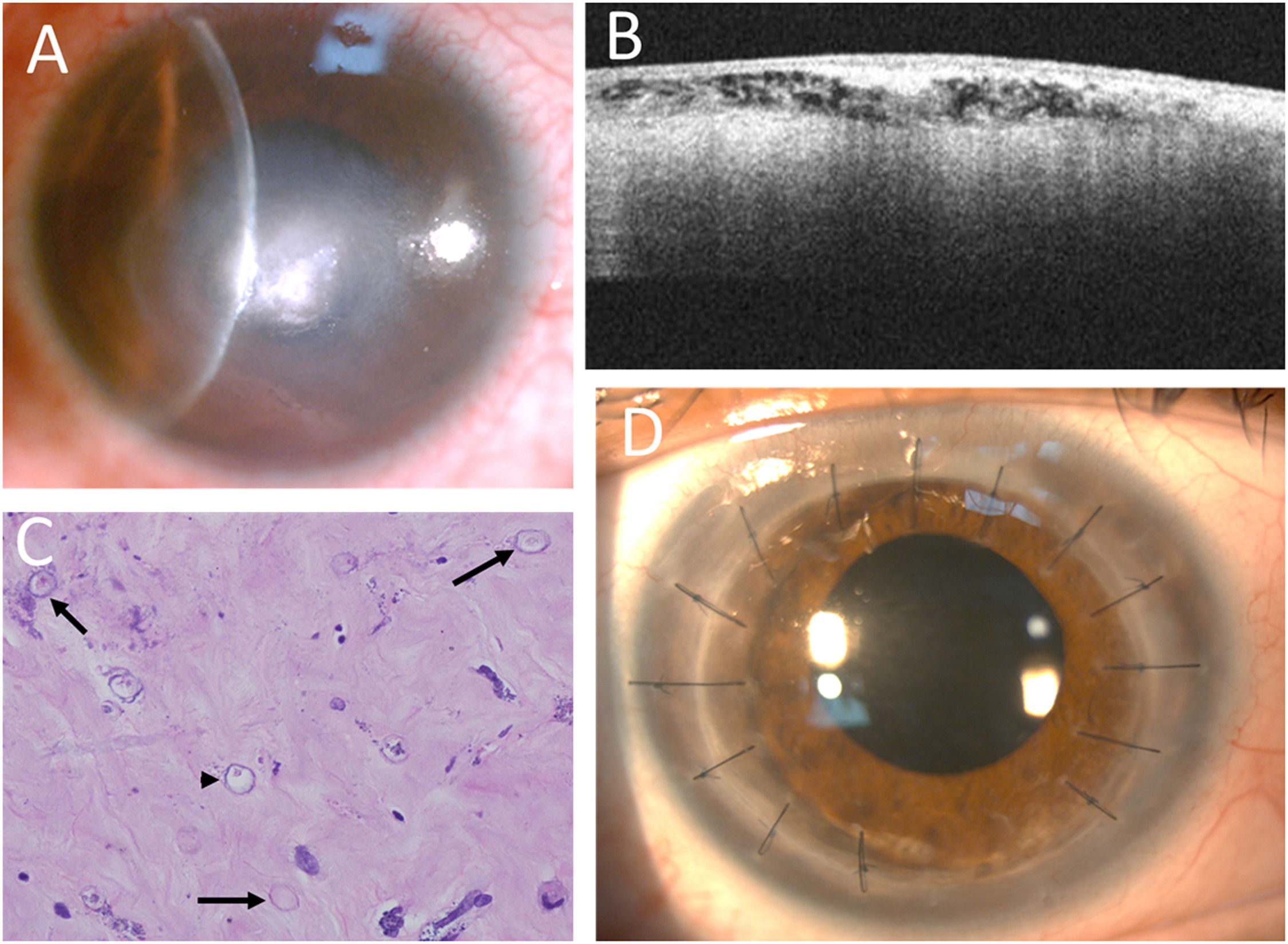
A 42-year-old male patient, diagnosed 7 weeks after symptom onset, required PKP. Initially misdiagnosed with HSV keratitis, he had been treated with topical 3 % acyclovir ointment and steroid solution at a local clinic. He subsequently developed a ring infiltrate (Fig. 1F), and Acanthamoeba cysts were later identified in the corneal smear at our clinic. Topical steroid therapy was discontinued, and treatment was initiated with topical PHMB, CHX, and oral VCZ (200 mg twice daily). Although the ring infiltrate regressed, central deep stromal infiltrates persisted after 10 months of treatment (Fig. 3A). Anterior segment optical coherence tomography (AS-OCT) revealed multiple subepithelial hyperreflective dots within hyporeflective vacuoles in the anterior stroma, accompanied by acoustic shadows (Fig. 3B). Repeated smears confirmed the presence of numerous cysts. Therapeutic PKP was performed, and histopathology revealed several cysts and trophozoites within the stroma (Fig. 3C). One and a half years postoperatively, the corneal graft remained clear, and visual acuity improved from 0.05 to 0.5 following cataract surgery (Fig. 3D).
Decreased ring infiltrates with central whitish infiltrates (A). AS-OCT showing numerous hyperreflective dots or lines within the hyporeflective superficial stroma, with some acoustic shadows (B). Histopathology revealing numerous double-walled cysts (arrows) and some trophozoites (arrowhead) (Hematoxylin and eosin stain, 400x) (C). A clear corneal graft after therapeutic keratoplasty (D).
Although three eyes were lost to follow-up between 8 and 32 days, all demonstrated clinical improvement during their respective short observation periods. Excluding these three cases, post-treatment LogMAR VA in the remaining 11 eyes significantly improved to 0.11 ± 0.10, compared with a pre-treatment LogMAR VA of 0.51 ± 0.41 (p = 0.004).
DiscussionAK is a rare but potentially sight-threatening ocular infection, occurring predominantly in CL users, who account for 80–85 % of cases.2,7,9,10 Poor CL hygiene is the primary contributing factor.2,9 The incidence of AK is higher among soft CL users compared to those using rigid gas-permeable (RGP) lenses.2,10 Orthokeratology—a type of overnight RGP lens—has been associated with a rising incidence of AK.11,12 The increasing use of orthokeratology among young individuals with myopia may contribute to a higher AK incidence, potentially due to overnight wear and microbial contamination related to orthokeratology lens care systems.12,13 In our study, all patients wore either soft CLs or orthokeratology lenses, with orthokeratology users being relatively young. This suggests that both lens type and age may contribute significantly to AK risk.
The clinical presentation of AK varies between early and advanced stages. Common features include epithelial irregularity, elevated SPK, stromal edema, pseudodendrites, radial perineuritis, limbitis, and dot or patchy stromal infiltrates, as well as the characteristic ring infiltrate. In more advanced cases, less frequent manifestations—such as anterior uveitis, hypopyon, glaucoma, cataract, and scleritis—may also occur. In our study, elevated SPK was the most common early sign but was often misinterpreted as contact lens–related corneal erosion in clinical practice. Early-stage SPK may also be misdiagnosed as Thygeson's superficial keratitis or microsporidial keratitis, both of which can delay diagnosis and necessitate combination therapy. Pseudodendrites or possible co-infection with HSV frequently led to misdiagnosis as HSV keratitis, further delaying appropriate treatment. Ring infiltrates were indicative of late-stage disease. AK should be considered, particularly in CL users, when there is no clinical response to initial treatment for bacterial or HSV keratitis. Misdiagnosis or delayed initiation of anti-amoebic therapy—especially when corticosteroids are prescribed prior to diagnosis—can result in disease progression, as seen in our patient who ultimately required PKP. Early corneal scraping in cases presenting with elevated SPK or pseudodendrites can support differential diagnosis and guide appropriate therapeutic decisions.
Diagnostic methods for AK include direct microscopy of corneal scraping smears, cultures, polymerase chain reaction (PCR), confocal microscopy, and histopathological analysis of corneal biopsy specimens. Corneal scraping smears provide a rapid and accessible tool for differential diagnosis. Special stains—such as calcofluor white or immunoperoxidase—can assist in detecting trophozoites or cysts, although these techniques may not be readily available in all clinical settings. Our findings suggest that experienced ophthalmologists or trained technicians can identify the characteristic double-walled cysts using routine Gram staining and distinguish them from white blood cells, microsporidia, or other pathogens. The visibility of the cyst wall can be enhanced by slightly lowering the microscope condenser during examination. Acanthamoeba culture remains the diagnostic gold standard, requiring specialized media such as non-nutrient agar overlaid with Escherichia coli. However, its sensitivity ranges from 30 % to 60 %.1 While PCR and confocal microscopy offer higher sensitivity than culture, they are not widely available in all institutions.14 In this study, the mean interval from symptom onset to AK diagnosis was 18.2 ± 12.9 days, which is notably shorter than those reported in previous studies.15,16 Early corneal scraping examination may facilitate timely diagnosis and improve clinical outcomes.
AS-OCT can assist in identifying intrastromal hyperreflective bands or lines in cases of radial keratouveitis associated with AK, thereby aiding in its differentiation from HSV keratitis.17 In our case, the presence of multiple subepithelial hyperreflective dots and hyporeflective stromal vacuoles suggested active infiltrates, as confirmed by smear examination and histopathology. This study underscores the utility of AS-OCT in monitoring treatment response and evaluating the depth and activity of corneal infiltrates.
PHMB and CHX, both members of the biguanide class of cationic antiseptics, are recognized as the most effective treatments for AK.8,18 Both agents exhibit cytocidal activity, and monotherapy with either has demonstrated similarly favorable outcomes.8,18 Propamidine, miconazole, and VCZ serve as alternative options.8,18 Our findings suggest that PHMB monotherapy is particularly effective for early-stage AK and is better tolerated than CHX for long-term use. However, in one early-stage case included in this study, monotherapy with PHMB was insufficient, and clinical improvement was achieved following combination therapy. Typically, a biguanide is combined with propamidine as the initial topical treatment.19 Miltefosine, a unique alkylphosphocholine anti-amoebic agent,20 has shown efficacy as an adjuvant systemic therapy for refractory AK cases.21 However, topical propamidine and oral miltefosine are not currently available in Taiwan. Our findings further suggest that advanced-stage AK—or early-stage AK unresponsive to PHMB—may require combination therapy involving two or three anti-amoebic agents, such as PHMB, CHX, and VCZ. The use of topical corticosteroids remains controversial. However, their administration prior to anti-amoebic therapy has been associated with increased rates of therapeutic PKP and poor visual outcomes,22,23 as observed in one case in our study. Notably, only 1 out of 14 eyes (7 %) in our cohort required PKP—a substantially lower rate compared to previously reported ranges of 9.3 %–25.5 %.23–25 This reduction may be attributable to early diagnosis via smear examination and the application of combination therapy in advanced cases.
In conclusion, AK presents with diverse manifestations and is often misdiagnosed as corneal erosion or HSV keratitis. Corneal scraping is a rapid and accessible diagnostic method that enables early identification of Acanthamoeba cysts, allowing for timely and targeted treatment, which may reduce the risk of complications and improve visual outcomes. AS-OCT may serve as a valuable adjunct in diagnosing and monitoring infiltrate activity and treatment response. However, this study is limited by its retrospective design, single-center setting, small sample size, and lack of culture or PCR data to confirm Acanthamoeba infection. Further prospective research with microbiologically confirmed AK cases is warranted to evaluate the diagnostic performance of corneal smear examination and AS-OCT in a larger patient cohort.
Funding statementThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributionsAll authors contributed to the critical revision of the manuscript and approved the final version for publication. K.Y.W. and H.Y.C. performed clinical examinations; C.W.H. collected and analyzed the data; all authors discussed the results. C.W.H. and H.Y.C. drafted the manuscript.
Data statementThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Financial supportNone.
Conflict of interest disclosuresThe authors have no proprietary or commercial interest in any materials discussed in this article.
Part of this information was presented as “Clinical Manifestations and Outcomes of Early and Late Diagnosed Acanthamoeba Keratitis” at the 63rd Annual Meeting of the Ophthalmological Society of Taiwan, held on November 19–20, 2022, in Taipei, Taiwan.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We would like to thank the staff of the Department of Clinical Pathology for their assistance with smear staining and cultures, and the staff of the Department of Pathology for conducting the pathological examination of the corneal specimen removed during therapeutic PKP.