Many sports-related concussion (SRC) outcomes can be prevented by removing affected athletes and allowing return after full recovery. Diagnosing concussions on the side-line is challenging, as tools often rely on visual performance assessment. Since acute exercise can affect vision, it is vital to determine if exercise can mask potential brain injury if visual performance assessments are used. The aim of this study was to determine the effect of a single bout of maximal aerobic exercise on acute visual performance.
MethodsEighty previously sedentary females were randomly assigned to an experimental group (N = 40) or control group (N = 40) and completed an identical visual task test battery. Two weeks later, participants returned to participate in the test battery, with the experimental group doing so immediately following a maximal treadmill exercise protocol.
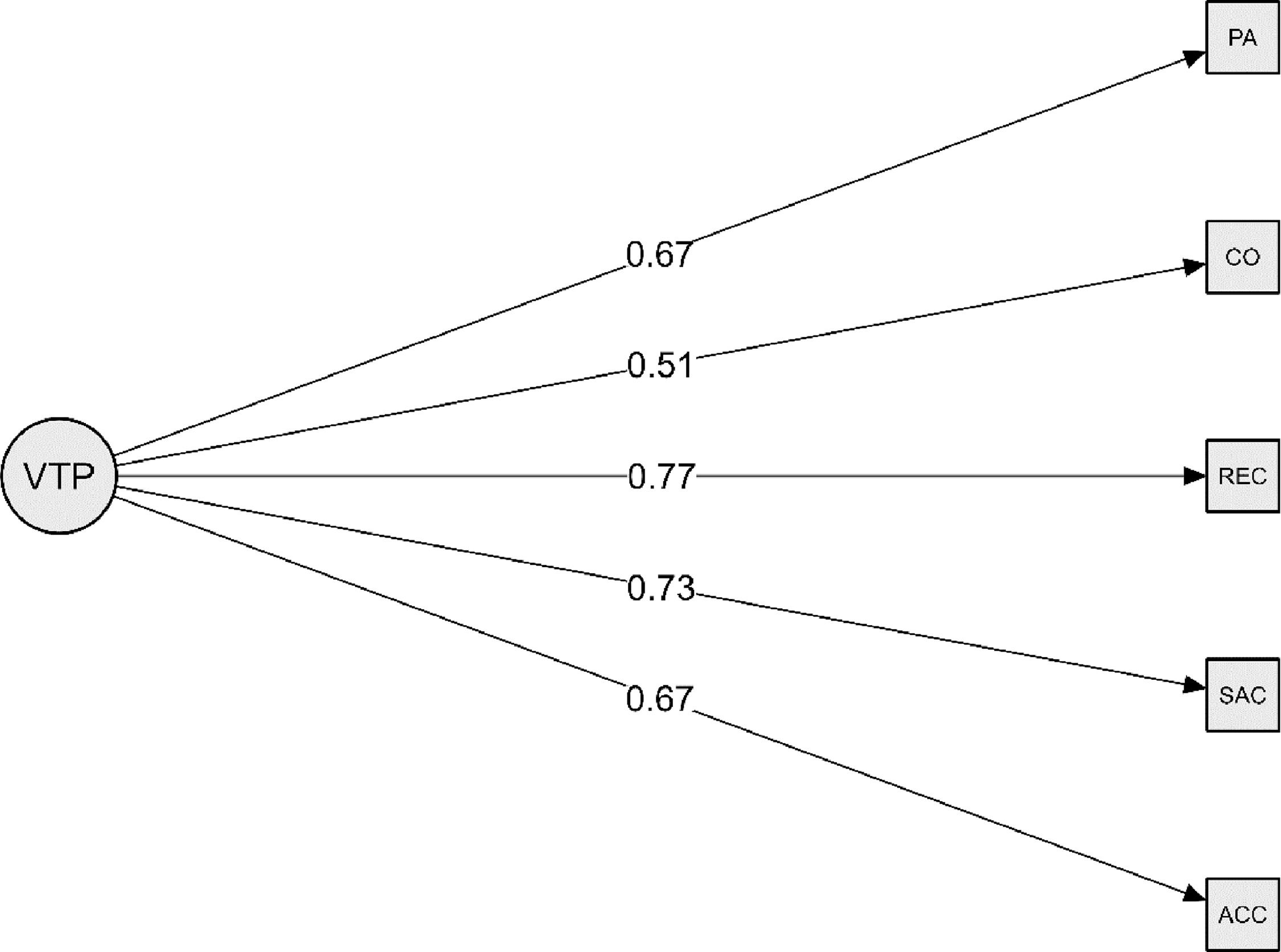
ResultsSignificant (P < 0.05) improvements were found in accommodation facility, saccadic eye movement, speed of recognition, peripheral awareness, and hand-eye coordination (P < 0.001 for all), but not in visual memory (P = 0.556). All visual tasks, barring visual memory, loaded onto a single factor and approximated the root mean square error of approximation (RMSEA) threshold value (RMSEA = 0.112 [90 %CI: 0.047–0.182]), and a significant large main effect was found on all factors as a universal visual task performance (P < 0.001; d = 1.01).
ConclusionThis study shows that exercise can affect performance across multiple, but not all, visual tasks and should be utilised with caution in comprehensive side-line SRC assessments. Visual memory may be more stable to the effects of acute exercise and be considered for inclusion in side-line SRC assessments.
Concussions, particularly those related to sports (SRCs), are common forms of mild traumatic brain injury (mTBI) resulting from direct head trauma or mechanical forces transmitted to the head.1 These injuries manifest with immediate and delayed symptoms and impairments across somatic, cognitive, or emotional, balance, behavioural, sleep/wake, cognitive, and physical domains.1 Oculomotor disturbances are a significant concern post-concussion, this is because over half of the brain's circuits are engaged in vision and eye movement, and many of these circuits are in areas that are prone to shear damage.2
The consequences of SRCs are severe, ranging from post-concussion syndrome, post-traumatic epilepsy, psychosocial effects, chronic traumatic encephalopathy (CTE), to even “fatal second-impact syndrome.”3 Preventing these consequences necessitates timely removal of concussed athletes from play and thorough appropriate side-line concussion screening.3 However, despite advances in concussion assessments, the diagnosis of concussion on the side-line remains challenging.4
The most common tools used to assess athletes who have suffered a concussion are the King-Devick Test (KDT), Post-Concussion Symptom Scale (PCSS), Standard Concussion Assessment (SAC), and Standard Concussion Assessment Tools (SCATs).5 While many current side-line protocols evaluate cognition, balance and/or vision, there has been calls to include and develop more inclusive acute or side-line concussion measures utilising primarily visual performance measures or ophthalmic abnormalities.2,5
Disturbances in oculomotor function represent significant indicators after sports-related concussions (SRCs) and are pivotal for sideline evaluations. The accommodation facility, responsible for adjusting eye focus, is frequently compromised in concussions, impacting visual clarity at varying distances.6 This impairment, known as accommodative insufficiency (AI), manifests as difficulty focusing on nearby objects and is observed in approximately 50 % of concussion cases, necessitating thorough assessment.2 Saccadic eye movements, involving rapid shifts between visual targets, play a crucial role in concussion diagnosis. The King-Devick test (KDT) assesses saccadic performance by evaluating reading difficulties with progressively spaced numbers after an SRC.7 Speed of recognition, denoting the capacity to make rapid decisions based on visual cues, may be compromised post-concussion.8 While the Immediate Post-Concussion Assessment and Cognitive Test (ImPACT) gauges speed of recognition, concerns have been raised about its validity in non-clinical, large-group settings, and specific populations, such as individuals with Attention Deficit-Hyperactivity Disorder (ADHD).8 Hand-eye coordination, integral to post-concussion assessment, involves visual perception and motor coordination. Tasks like the Nine-hole Peg Test and Box and Blocks Test assess cognitive-motor integration to evaluate concussion severity.9 Peripheral awareness, encompassing the perception of objects beyond the central field of vision, can be impacted by mild traumatic brain injury (mTBI), leading to tunnel vision. Various tests, including binocular (i.e., Useful Field of View (UFOV) and Peripheral Perception Test (PP)) and monocular methods (i.e., Humphrey Field Analyzer 630 (HFA)), have been developed to assess peripheral awareness.10 Additionally, previous observations have highlighted associations between SRCs and visual memory, emphasizing the immediate recall ability of the eyes.11
Based on the existing literature, it is essential to acknowledge that conducting visual function tests during sporting events could yield different results due to the physical demands of sports, impacting the visual system. This is because during exercise or sport, the human body undergoes a plethora of changes, such as modifications in blood pressure, blood glucose, blood oxygenation, intraocular pressure, and ocular perfusion pressure, all of which can impact the visual system.12,13 Acute exercise can either enhance or deteriorate visual performance, further complicating assessments conducted immediately post-head trauma.14 Thus, this study aimed to explore the effect of a single bout of maximal aerobic exercise on acute visual performance. Based on the existing literature, this study hypothesised that a single bout of maximal aerobic exercise would significantly improve accommodation facility, saccadic eye movement, speed of recognition, peripheral awareness, hand-eye coordination, and visual memory. These findings could have clinically significant implications and provide cautionary evidence when visual tasks are utilised in comprehensive side-line SRC assessments for concussion and subsequent determination of return-to-play decisions.
MethodsParticipantsEighty adult female participants were randomly assigned using a random numbers table to an experimental group (N = 40) or a control group (N = 40) after meeting the inclusionary criteria of not having previous experience with sport vision testing, previously sedentary (self-reporting no regular physical activity for the past six months), not using any medication that could have affected the physical or visual assessments, and having a minimum of 20/20 vision (either unaided or corrected for near and distant vision during the experiment).15 Participants were excluded if they had any relative or absolute contraindication to exercise and/or testing, any systemic condition (i.e. hypertension, cerebrovascular accident, cancer, parkinsonism, arthritis, myocardial infarction, congestive heart failure, pulmonary problems), visual disease or infection, such as blindness, physical disability and/or psychosocial distress.15 In addition to a comprehensive health survey consisting of questions concerning health, physical activity, medication usage, and general nutrition to screen for inclusionary and exclusionary criteria in line with scope of practice for Clinical Exercise Physiologists, descriptive questions were posed and recorded about the participant (name, date of birth, age, etc.), their previous sporting history (level, primary/secondary sport, position), vision correction, and concussion history.
This study made use of sedentary participants since research has demonstrated that engaging in athletic activities, such as running, can have a positive impact on vision, and may even be associated with a reduced risk of cataracts.16 Further, athletes engaging in sport have also previously demonstrated enhanced visuomotor skill recovery.17 Given that athletes might exhibit superior visual abilities compared to non-athletes, it follows that superior athletes, in turn, might possess even greater visual capabilities than their less proficient counterparts. While these enhancements are not unequivocal, should they exist, they may be specific to particular aspects of visual acuity and can vary across different sports.15 This study made use of a female-only sample as females experience more concussions, exhibit different symptoms, and have distinct brain functioning compared to males.18 Moreover, physiological, and anthropometric distinctions, such as increased head mass in females, may lead to varying responses to traumatic or mechanical head forces.19 Males typically possess greater neck strength, which plays a vital role in concussion prevention by providing additional support during impact preparation, mitigating head acceleration/deceleration effects, and facilitating the diffusion of impact forces, potentially reducing concussion severity.19
This study complied with the tenets of the Declaration of Helsinki as revised in 2013. The study protocol received approvals from the Institutional Review Boards of the University of Zululand (UZREC 171,110–030-PGD-2021/27). Prior to participation, all participants provided informed consent.
ProceduresTo ensure that participants met the inclusionary criteria, they underwent an optometric assessment to ensure that they had 20/20 vision using Spectrum Eyecare software (Version 6.0.0, Digital Optometry, South Africa). Spectrum Eyecare software was utilised to conduct the visual field examination to identify visual field areas of zero sensitivity and its levels throughout the visual field. As standard oculomotor tests often don't mirror real-life situations for athletes, focusing solely on isolated eye movements through constrained assessments, this study aimed to explore how a single exercise session impacts more demanding tasks akin to those encountered by athletes. All participants took part in these tests in identical sequential order listed below. All tests were performed following a nine- to 12-hour fast in the same Visio-Spatial Intelligence (VSI) Laboratory at the University of Zululand with room light approximately 700 lux, and a controlled temperature and humidity of approximately 22 °C and 55 %, respectively between the hours of 10:00 and 16:00.
Variables and instrumentsKinanthropometryFor descriptive purposes, participants were first evaluated for body composition as per the International Society for the Advancement of Kinanthropometry (ISAK) guidelines.20 Body weight was measured and recorded (to the nearest 0.1 kgme (kg)) using a digital scale (Jawon, Model IOI-353, Kyungsun City, Korea), while body height was measured (to the nearest 0.5 cm) using a wall-mounted stadiometer (HM210D, Charder Electronic Co, Ltd., Taichung City, Taiwan). Body mass index (BMI) was calculated by dividing the participant's body mass (kg) by stature squared (m²) and expressed as kilogrammes per square meter (kg/m2).
Accommodative facilityThe use of charts at two different distances, with fixation being rapidly alternated between the two charts, is commonly utilised to assess accommodative facility.21 While the Hart Chart is commonly utilised among athletes, the current study utilised the Hart Near Far Rock Test to stimulate the accommodation system for assessment under more natural viewing conditions.21 A large Hart Chart (Bernell Corporation, Mishawaka, Indiana, USA) was placed three meters away from the participants at head height.15 Participants were instructed to hold another smaller chart at arm's length away and were asked to read the first letter of the first line of the chart three meters away and then immediately read the first letter of the smaller chart at arm's length. This was followed by participants reading the second letter, third letter, and so onward for 30 s (sec). Final scores were calculated by subtracting errors from the total score.15
Saccadic eye movementSaccadic eye movement charts were used to evaluate saccadic eye movement.22 Two charts were placed on a board, one meter apart, and three meters away from each participant. Participants were then tasked with reading the first letter on the lateral side of the left chart, and then rapidly move their eyes and without moving their heads to reading the first letter on the lateral side of the right chart. This was followed by participants reading the second letter on the left then right chart, third letter on the left then right chart, and so onward for 30 s.22 Final scores were calculated by subtracting errors from the total score.
Speed of recognitionLinks have previously been established between clinical tests that are sensitive to dysfunction following concussion and digital sports vision tools.23 As such, the Batak Pro-and Evasion Programme (Quotronics Limited, Horley, Surrey, United Kingdom (UK)) as used to evaluate speed of recognition. This programme lights the Batak Pro's 12 light-emitting diode (LED) lights or targets randomly for one second. While lit, participants had to hit the target. If an LED light flickered, participants had to refrain from hitting the flickering target. If they did, five points were deducted from the final score.15 During the programme, if all the lights flickered simultaneously, participants were tasked with evading the small central infrared beam. If caught by the beam, five points were deducted from the final score. All scores were automatically established by the Batak Pro-with a maximum of 100 targets being lit.
Hand-eye coordinationThis study utilised the Ball Wall Toss Test to assess hand-eye coordination.9,15 This is since the small, quick eye movements associated with concussion, known as ocular flutter or opsoclonus, can cause eye-tracking problems, which affects hand-eye coordination.9 Due to this intersection between ocular and manual motor control,9 hand-eye coordination already forms part of comprehensive SRC assessment batteries, such the SCAT2 and side-line assessment of SRCs.9 In the Ball Wall Toss Test, participants stood on a mark placed two meters from a wall and were required to throw and catch a tennis ball with alternating hands for 30 s.15 Final scores were recorded as the total number of successful throws and catches.
Peripheral awarenessSimple objective, inexpensive, and rapid peripheral awareness tests can detect neurocognitive deficits and may assist in diagnosing more serious concussion cases.24 Peripheral awareness was measured using the Batak Pro's Accumulator Programme which randomly lit an LED target for 60 s or until struck by the participant. Once struck, another random LED target lit up.15 All scores were automatically established by the Batak Pro.
Visual memoryIt is well known that athletes demonstrate a significant decline in visual memory following a concussion.11 That is why the domain of visual memory is already integrated into comprehensive assessment packages for sports-related concussions (SRCs), like ImPACT, and the K-D Test, which serve as quick visual screening tools for SRCs.5 Visual memory was assessed using the Batak Pro's Flash Memory Programme.15 In the Flash Memory Programme, six random lights or targets were lit for ½ second and required participants to recall which six targets were lit and the order in which they were lit up.15 All scores were automatically established by the Batak Pro.
InterventionsFollowing a wash-out period of two weeks (within a 24-hour period), all experimental and control participants returned to the laboratory and were re-assessed for the inclusionary and exclusionary criteria. The control group only performed the visual test battery as at pre-test, while the experimental group engaged in a single bout of incremental maximal treadmill (JohnsonT8000 PRO treadmill, Johnson Health Tech. Co., Ltd, Taichung City, Taiwan) exercise.25 Participants initially walked at two kilometers per hour (km.h−1) and 1 % grade for two minutes. Following this, the speed incremented to 5.5 km.h−1 and 1 % grade. Every 15 s thereafter, treadmill speed increased by 0.2 km.h−1 (while grade was kept constant) until a maximum of 16 km.h−1. At this point, grade increments increased by 0.5 % every 30 s until test termination criteria were achieved. The total time of the treadmill test was also recorded. Heart rate was monitored continuously throughout the maximal treadmill test via by telemetry (Polar, Coventry, UK, and Jiangsu Dengguan Medical Treatment Instrument Co. Ltd., Changzhou, Jiangsu, China, respectively) to capture maximal heart rate (HRmax) achieved during the treadmill test. The test was terminated when at least two of the following three criteria were achieved: (a) volitional termination by the participant due to fatigue, (b) meeting or exceeding predicted HRmax (HRmax = 208 − 0.7 × age) for more than 15 s, and/or (c) participant rating of perceived exertion was ≥17 on Borg's 6–20 scale.25
Statistical analysisThe first analytic strategy involved repeated measures t-tests to examine differences in acute visual performance between experimental exercise and control to assess the relative impact of the single bout of maximal aerobic exercise. This statistical test was used to assess if a learning effect existed in each group. A repeated measures analysis of variance (rm-ANOVA) was performed to determine the acute effects of the single bout of maximal aerobic exercise on acute visual performance (i.e. exercise x visual task performance). Effect size for the interaction was also determined between visual measures pre- and post-exercise using Cohen's d, where 0.20≤d < 0.50 corresponded to a small effect size, 0.50≤d < 0.80 corresponded to a medium effect size, and d ≥ 0.80 corresponded to a large effect size. A multivariate ANOVA (MANOVA) was performed to assess the effect of a single bout of maximal aerobic exercise on multiple dependent variables (e.g., accommodation facility, saccadic eye movement, speed of recognition, peripheral awareness, hand-eye coordination, and visual memory). This repeated measures design was applied to assess change over time as the study involves multiple measures of the same variable taken on participants over two time periods (i.e. longitudinal design). This statistical test allowed for the analysis of the interaction effects between independent variables and within-subjects factors. Due to its exploratory nature, this study also made use of factor analysis to determine if there was a central trend or relationship throughout the dataset regarding the effect of acute exercise on visual task performance. This method was considered given the completeness of the data. Specifically, Exploratory Factor Analysis (EFA) was used to develop an overall hypothesis regarding the relationship between variables. The estimates of the parameters were obtained using the minimum residual method and included as items loading on factors based on Eigen values >1 and calculated the factor loading (λ) of each observed variable to assess the strength of its association with the proposed underlying (latent) factor. A cut-off value of λ ≥0.4 was used as the minimal level of practically significant factor loading. The comparative fit index (CFI), and Tucker Lewis Index (TLI) were used as indices of global fit to assess the goodness-of-fit of the hypothesised model to the data. Standardized root mean square residual (SRMR) and change in root mean square error of approximation (RMSEA) fit index was used to identify the number of factors to retain. A CFI >0.90 and a TLI >0.90 were used to indicate an acceptable fit if RMSEA ≤0.08. Additional fit indices with acceptable and good fit defined as SRMR ≤0.10. Factors were retained if their inclusion resulted in a change in RMSEA of ≤0.015. For all statistical analyses, the results were assumed to be significant at an alpha level of 0.05. Results are presented as means and standard deviations. The statistical analyses were conducted using Just Another Statistics Package (JASP) open-source software, version 0.15 (University of Amsterdam, Amsterdam, Netherlands).
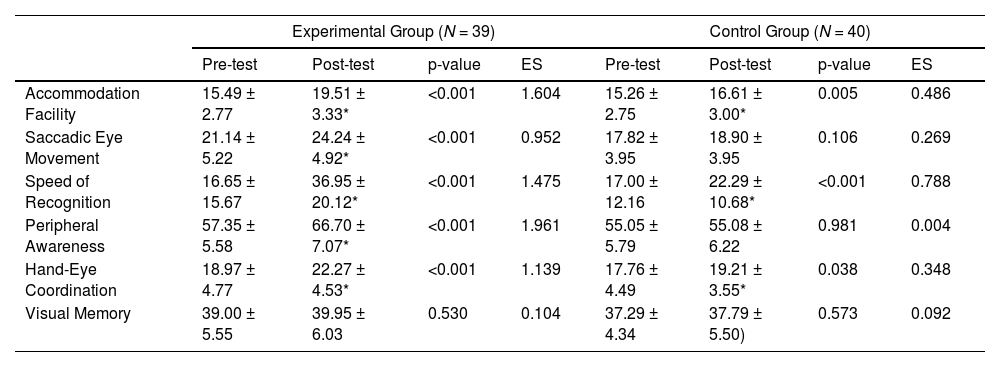
ResultsOf the 40 participants sampled in the experimental group, one participant failed to return for post-testing and her data was excluded from the final analysis (Table 1). T-testing revealed significant (P < 0.05) differences in all measured visual parameters, except visual memory (P = 0.556), following a single bout of maximal aerobic exercise (Table 2) with large effect sizes found for accommodation facility (d = 1.604), saccadic eye movement (d= 0.952), speed of recognition (d= 1.475), peripheral awareness (d= 1.961), and hand-eye coordination (d = 1.139), but was trivial for visual memory (d = 0.104). For the control group, significant (P < 0.05) differences were found for accommodation facility (P = 0.005, with small effect size; d = 0.486), speed of recognition (P < 0.001, with medium effect size; d = 0.788), and hand-eye coordination (P = 0.038, with small effect size; d = 0.348). This indicated that a learning effect did take place from baseline to re-test. However, no significant changes or learning effect were found for saccadic eye movement, peripheral awareness, and visual memory in the control group.
Participant demographics.
Data reported as means ± standard deviations (SD). cm: centimetres; kg: kilogrammes; kg/m2: kilogrammes per square metre.
Effects of a single bout of maximal aerobic exercise on acute visual performance.
| Experimental Group (N = 39) | Control Group (N = 40) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-test | Post-test | p-value | ES | Pre-test | Post-test | p-value | ES | |
| Accommodation Facility | 15.49 ± 2.77 | 19.51 ± 3.33* | <0.001 | 1.604 | 15.26 ± 2.75 | 16.61 ± 3.00* | 0.005 | 0.486 |
| Saccadic Eye Movement | 21.14 ± 5.22 | 24.24 ± 4.92* | <0.001 | 0.952 | 17.82 ± 3.95 | 18.90 ± 3.95 | 0.106 | 0.269 |
| Speed of Recognition | 16.65 ± 15.67 | 36.95 ± 20.12* | <0.001 | 1.475 | 17.00 ± 12.16 | 22.29 ± 10.68* | <0.001 | 0.788 |
| Peripheral Awareness | 57.35 ± 5.58 | 66.70 ± 7.07* | <0.001 | 1.961 | 55.05 ± 5.79 | 55.08 ± 6.22 | 0.981 | 0.004 |
| Hand-Eye Coordination | 18.97 ± 4.77 | 22.27 ± 4.53* | <0.001 | 1.139 | 17.76 ± 4.49 | 19.21 ± 3.55* | 0.038 | 0.348 |
| Visual Memory | 39.00 ± 5.55 | 39.95 ± 6.03 | 0.530 | 0.104 | 37.29 ± 4.34 | 37.79 ± 5.50) | 0.573 | 0.092 |
Data reported as means±standard deviations (SD).
rm-ANOVA revealed a significant main effect on accommodation facility (P < 0.001), saccadic eye movement (P < 0.001), speed of recognition (P < 0.001), hand-eye coordination (P = 0.029), and peripheral awareness (P < 0.001), but not visual memory (P < 0.942) due to the single bout of maximal aerobic exercise.
The single bout of maximal aerobic exercise was found to have a greater effect than no treatment on accommodation facility (F(17.167) = 22.445, P < 0.001), saccadic eye movement (F(12.642) = 15.290, P < 0.001), speed of recognition (F(24.686) = 37.298, P < 0.001), hand-eye coordination (F(9.476), P = 0.001), and peripheral awareness (F(31.900) = 56.659, P < 0.001). However, the single bout of maximal aerobic exercise was found to have the same effect as no treatment on visual memory (F(0.512) = 0.516, P = 0.475). An rm-ANOVA revealed a significant main effect on all factors as universal visual task performance (VTP) (P < 0.001) due to the single bout of maximal aerobic exercise, with a large effect size being found (d = 1.01).
Accommodation facility, saccadic eye movement, speed of recognition, hand-eye coordination, and peripheral awareness all loaded onto a single factor. This method was conducted because of the control group's small learning effect found for accommodation facility, speed of recognition, and hand-eye coordination to produce transformed features to show how much each input feature contributed to each transformed feature, or factor. This contextual information is used to determine if systematic inter-dependence exists in all the visual tasks to create a commonality. Loading for visual memory was acceptable (λ ≥ 0.3), meeting the criteria for inclusion in the model but this item loaded more strongly as a second, unique factor. This single-factor model based on five items, met the criteria for global fit (CFI = 0.957, TLI = 0.914, SRMR = 0.036). This model approximated the RMSEA threshold value (RMSEA = 0.112 [90 %CI: 0.047–0.182]) likely due to skewness in the of distribution of peripheral awareness scores (Figure 1). Highest loading was for speed of recognition (R2 = 0.77) and hand-eye coordination accounted for the lowest loading (R2 = 0.51). Visual memory was, therefore analysed separately. This continuous score was normally distributed and met the assumptions underlying parametric analysis.
DiscussionThe aim of this study was to determine the effect of a single bout of maximal aerobic exercise on acute visual performance. In this regard, the study found that a single bout of maximal treadmill aerobic exercise significantly improved accommodation facility, saccadic eye movement, speed of recognition, peripheral awareness, and hand-eye coordination, but not visual memory.
Side-line detection is the first and most important measure in identifying a potential concussion and removing an athlete from further harm. While most side-line protocols assess cognition and balance, there is a persistent call to include assessments of visual performance on a variety of visual domains as SRCs commonly affect the visual pathways.5 This study showed that a single bout of short maximal aerobic exercise improves accommodation facility, saccadic eye movement, speed of recognition, peripheral awareness, and hand-eye coordination, but not visual memory.
This is in contrast to the inferences that exercise-induced fatigue can negatively influence visual performance through central, or peripheral pathways, or both.26 Further, it has been speculated that visual performance may also be indirectly affected by declines in cognitive functioning as a result of mental fatigue associated with exercise-induced fatigue.26 However, substantial research has demonstrated a positive effect of a single bout of exercise on cognitive performance27 and that a single bout of exercise can have stimulatory effects on certain visual domains. While these findings may appear controversial, acute exercise increases circulating central or brain catecholamines, which, in turn, increase arousal by activating the reticular formation via the “catecholamines hypothesis.”28 Acute exercise has also been shown to increase the autoregulatory mechanisms for ocular blood flow and increase retinal and choroidal tissue circulation during exercise through several substances, such as in nitric oxide (NO), thus improving nutrients and gas exchange at the eye, concomitantly improving visual performance.29 When combined with findings that acute exercise stimulates brain regions involved in motor and cognitive processes, through multiple mechanisms, such as increased brain blood flow and oxygenation,29 it may be that exercise has an “exercise priming” or stimulatory effect on visual performance.
What is noteworthy is that this study found that a single bout of maximal aerobic exercise had no effect on visual memory. Interestingly, principal component analysis (PCA) further confirmed this by demonstrating that visual memory was not associated with the other five visual tasks in this study. Visual memory may be unaffected by acute exercise because it is supported by an extensive network of brain regions that are responsible for the relationship between perceptual processing and the programming, storage, and recall of the resulting neural representations.30 One explanation for this complex relationship is the two streams hypothesis which proposes that multiple specialised and distinctive posterior brain mechanisms are responsible for visual memory.30 While the ventral stream is responsible for visual object recognition, the dorsal stream is responsible for the visual-spatial location of objects. However, it must be noted that the two streams maintain intercommunication along their entire rostral course.30 Given the relative acute “stability” of visual memory following exercise, it may be that this visual task should be considered for inclusion in SRC concussion assessments as it is unlikely to be affected by previous exercise. However, further research is needed to confirm this. This is especially relevant in that each test battery took approximately 25 min, and it may be that any effect of exercise may have worn of by the end of the protocol, which is when visual memory was assessed.
Limitations included an initial lack of binocular testing at the pre-test optometric assessment, which could have led to some participants not being screened for conditions that could have impacted upon visual-motor integration, accommodation, and/or saccades. This study made use of a female only sample limiting application to male populations. However, this inclusion criterion was applied since this study aimed to determine the effect of a single bout of maximal aerobic exercise on acute visual performance in females. This study also utilised a single-sex population to recruit a considerably large sample size for an investigation of this type. Although this study utilised sedentary participants, any significant differences in cardiorespiratory fitness within the cohort may have had an impact on cognitive function and visual performance. While a multitude of visual domains exist and are constantly being linked to concussion, this study only assessed those visual domains most included, or proposed, in side-line SRC assessments. In addition, while SRC assessments using cognitive function and visual performance could be measured immediately following submaximal physical activity in a “real-world situation”, this study measured visual performance changes only following maximal effort.
Research may be needed to determine if these findings are different in athletes from different sports, and at different levels of play. This is because athletes may have better visual abilities than non-athletes, although controversy still exists in this regard.15 Athletes engaging in sport have also previously demonstrated enhanced visuomotor skill recovery.17 Research should also continue to investigate the effect of different exercise programme designs (i.e., with varying intensity, modes, and duration) or sports on visual task performance used to identify SRCs at side-line. Research may also need to extend the wash-out period between visual assessments to a month or more and thus further to eliminate any learning effect arising from certain tests. Further, research will also need to determine the latent effects of such exercise or sporting activities on visual tasks. This may provide an indication of how long the effects of a particular exercise or sport has on certain visual tasks, and this information could be utilised to design optimal side-line assessments, including the possibility of timing of follow-up side-line tests for athletes that have undergone an SRC assessment but have been returned to play. Finally, research also needs to determine those visual tasks that are not influenced by acute exercise or sporting activities to provide tests to be included in comprehensive side-line SRC assessments to minimise the possibility of a premature clearance to return to sporting activities. In this regard, future tests should also examine the feasibility and pertinence of including alternative tests for a particular visual task, such as examining the use of the Lens Flipper Test to measure the accommodation instead of Hart Charts.
While the control group also improved their performance through a learning effect on three visual tasks, such changes were considered clinically small to moderate, this study's findings show that a single bout of aerobic exercise can affect performance across multiple, but not all, visual tasks. Uniquely, this study does also demonstrate that some visual tasks, like visual memory, may be more stable, and thus more “resistant” to the effects of acute exercise and should have a predilection for inclusion in side-line SRC assessments. Clinically, these results provide caution for the use or inclusion of multiple visual tests within comprehensive and more complex side-line SRC assessments for return to play decisions. This is especially relevant given the subtle changes in ocular motor function that occur with concussion.