To measure lens vault (LV) and to assess its correlation with various ocular parameters in healthy eyes, using for all measurements the same high-resolution swept-source optical coherence tomographer (SS-OCT).
MethodsWe prospectively recruited 67 Caucasian healthy patients whose mean age was 41.9 ± 12.4 years; only their right eye was included in the study. Data were all recorded with the ANTERION SS-OCT and comprised, for each patient, 5 consecutive measurements of LV, anterior chamber depth (ACD), lens thickness (LT), axial length (AL), white-to-white (WTW) distance, central corneal thickness (CCT), anterior chamber volume (ACV) and spur-to-spur (STS) distance.
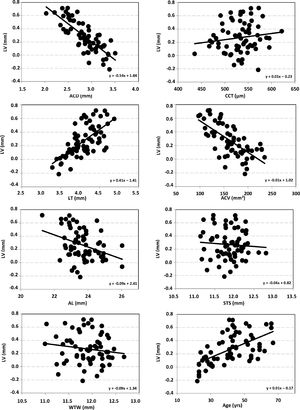
ResultsMean LV was 0.26 ± 0.23 mm (ranging from -0.24 to 0.78 mm). Data analysis revealed a statistically significant negative correlation between LV and ACD (R=-0.80, p < 0.001), AL (R = -0.36, p = 0.002), and ACV (R = -0.68, p < 0.001), and a positive correlation between LV and LT (R = 0.67, p < 0.001), and age (R = 0.53, p < 0.001). In contrast, no statistically significant correlation was found between LV and WTW (R=-0.17, p = 0.15), CCT (R = 0.11, p = 0.36) or STS (R=-0.10, p = 0.41).
ConclusionsTaking into account our findings about intra-parameter correlation levels, we believe that LV should be measured and analyzed together with other ocular parameters in clinical routine practice both for diagnosis and for some refractive surgeries.
The analysis of different anterior-segment parameters is mandatory for the prevention, treatment, and diagnosis of angle-closure pathologies but also for several refractive surgery procedures. The potential applications of anterior-segment imaging, which can provide a number of cross-sectional images (i.e., slices) of the anterior segment, have increased in recent years. Optical coherence tomography (OCT) and ultrasound biomicroscopy (UBM) are the most common imaging techniques being used in the clinical setting. These systems overcome several limitations inherently associated with gonioscopy; in fact, it has been suggested that enhanced versions of OCT and UBM will end up becoming the gold standard for angle screening and management.1 As a matter of fact, OCT is already being widely used due to its non-invasiveness and the quality of the objective images of different anterior chamber structures it provides, not only for angle analysis but also for the estimation of several distances that are relevant for refractive-surgery planning. OCT has evolved, from the first versions that were time-domain based and had low resolution to the current models that rely on swept-source technology (SS-OCT) ,2 and have a faster scanning speed, higher resolution and automated-measurement capabilities.3
Crystalline lens rise (CLR) or lens vault (LV) are two important distances that can be measured using either OCT or UBM. The former (CLR) is defined as the perpendicular anteroposterior distance between the anterior crystalline lens surface and the angle recess–to–angle recess line,4,5 whereas the latter (LV) is defined as the perpendicular distance between the anterior crystalline lens surface and the horizontal line joining the two scleral spurs.6 These two parameters have been widely measured and used for clinical diagnosis for the last 15 years. This enables us to define how is the convexity of the iris-crystalline lens and may be useful, for example, to know which type of phakic intraocular lens can be choose and its dimension, or to diagnose some ocular pathologies. It has been recognized greater LV as an anatomic parameter that is independently associated with angle closure.6 There are many studies in the literature that have reported LV and CLR data for different patient cohorts and making use of various measuring instruments.4–31 Some of these studies also measured, in the same series, other parameters such as anterior chamber depth (ACD), lens thickness (LT), axial length (AL), white-to-white distance (WTW), central corneal thickness (CCT), anterior chamber volume (ACV) or spur-to-spur distance (STS). However, in some of these cases two or more instruments had to be used to record the complete data set. As far as we are aware, there are not any studies in the literature where all these ocular parameters were measured with just one instrument and in the same group of patients in search of potential correlations with LV. Therefore, the purpose of this study was to bridge this gap and measure LV and assess its correlation with other ocular parameters by characterizing the dimensions of the eye's anterior segment in a group of healthy eyes using the same instrument; namely, a high-resolution SS-OCT platform.
MethodsParticipants and proceduresPatients participating in this study were recruited at the Oftalvist Clinic from November 2019 to February 2020. The clinical research followed the tenets of the Helsinki Declaration, and all procedures were approved by our Institutional Review Board. All patients, prior to their being recruited, underwent a comprehensive ophthalmic examination, which included anterior-segment slit-lamp assessment, fundus inspection, intraocular pressure measurement, visual acuity, and refraction. Considering the reported similarities between a given person's left and right eyes,32 only the participants’ right eye was included in the study. The inclusion criteria were to be a phakic subject aged 20–70 years. The exclusion criteria were having an ocular or systemic disease, poor fixation, a history of ocular surgery, or visual acuity below 20/25 Informed consent was obtained once they had been given an explanation about the purpose of the study, and details on the measurement technique and on data handling and processing.
The high-resolution SS-OCT ANTERION platform (Heidelberg Engineering GmbH, Heidelberg, Germany) was the measuring instrument used in this research, which has shown high repeatability for different ocular parameters and useful for measurement different distances.33–35 It relies on a 1300 nm (infrared) light source to produce several B-Scans of the eye with an axial resolution <10 μm. The use of a long wavelength makes it possible to image the whole anterior segment and the lateral scanning SS-OCT allows for cross-sectional imaging providing data of different parameters to be studied. The device also includes an infrared camera. Horizontal cross-sectional images are used to measure CCT, ACD, LT, ACV, AL and STS (defined as the distance between opposite scleral spurs within one B-scan), while the infrared camera enables en-face imaging of the subject's eye that provides horizontal WTW data. For each participant, a total of 5 consecutive measurements series—each including all 8 parameters—were carried out within the same session by the same examinar. The patient was positioned correctly on the chin rest, with their forehead leaning on the ANTERION SS-OCT. Prior to each measurement session, the instrument was calibrated according to the manufacturer's recommendations. One trained observer was in charge of marking in each image the scleral spurs (which are defined as the inward protrusion of the sclera where a change in curvature of the corneoscleral junction is observed36). Based on these locations, LV was then automatically calculated.
Data analysis and statisticsThe statistical analysis was carried out using the SPSS software (version 22.0, IBM Corp., Armonk, New York, USA). All the measurements are given in the form of mean value ± standard deviation (SD). Pearson correlation analysis was used to evaluate the level of correlation between different parameters based on their corresponding Pearson's correlation coefficient (R). Moreover, linear regression analysis was also performed, and a regression equation was obtained for each correlation. The resulting p values were considered to be statistically significant if they were below 0.05.
ResultsPrior to data analysis, a literature review was carried out by searching the following databases: PubMed (U.S. National Library of Medicine), Web of Science (Thomson Reuters), and Scopus (Elsevier, BV). The search was limited to publications in English— both peer-reviewed scientific reports and research books— but no date restriction was applied. The date of the last electronic search was March 20, 2020. This literature search produced 27 relevant contributions, which were subsequently analyzed. Table 1 shows those clinical studies that recorded CLR and LV data using either OCT or UBM.
Clinical studies reporting data for crystalline lens rise (CLR) or lens vault (LV).
| Author | Year | Eyes | Patients | Instrument | Condition |
|---|---|---|---|---|---|
| Goldsmith et al.4 | 2005 | 40 | White, Asian, black | CAS OCT* | Normal |
| Baikoff et al.5 | 2005 | OCT | Artisan IOL | ||
| All | 87 | ||||
| Hyperopic | 43 | ||||
| Myopic | 44 | ||||
| Yan et al.7 | 2010 | Chinese | SL-OCT | Normal | |
| All | 126 | ||||
| Young | 42 | ||||
| Adults | 42 | ||||
| Older | 42 | ||||
| Nongpiur et al.6 | 2011 | Chinese | Visante OCT | ||
| Angle closure | 102 | Angle closure | |||
| Normal controls | 176 | Normal controls | |||
| Kojima et al.8 | 2012 | Japanese | IOLMaster/Vumax II UBM† | ||
| Study | 45 | ||||
| Validation | 81 | ||||
| Sun et al.9 | 2012 | 388 | Korean | Visante OCT | Normal |
| < 50 | 41 | ||||
| 50 to 60 | 49 | ||||
| 60 to 70 | 140 | ||||
| 70 to 80 | 118 | ||||
| ≥ 80 | 40 | ||||
| Lee et al.10 | 2012 | 684 | Chinese, Caucasian, Filipo, Hispanic and African | Visante OCT | Open and narrow angle |
| Open angle | 385 | ||||
| Chinese | 82 | ||||
| Caucasian | 147 | ||||
| Filipo | 41 | ||||
| Hispanic | 43 | ||||
| African | 72 | ||||
| Narrow angle | 299 | ||||
| Chinese | 50 | ||||
| Caucasian | 124 | ||||
| Filipo | 40 | ||||
| Hispanic | 43 | ||||
| African | 42 | ||||
| Tan et al.11 | 2012 | 1464 | Chinese, Malay, Indian and Other | IOLMaster/Visante OCT‡ | Open and narrow angle |
| Narrow angle | 315 | ||||
| Open angle | 1149 | ||||
| Chinese | 1317 | ||||
| Malay | 27 | ||||
| Indian | 102 | ||||
| Other | 18 | ||||
| Shabana et al.12 | 2012 | 148 | Chinese, Malay, Indian and Other | Sonomed/Visante OCT‡ | PAC |
| Ozaki et al.13 | 2012 | Japanese | US-800/Visante OCT‡ | Angle closure and normal | |
| Angle closure | 109 | ||||
| Normal | 68 | ||||
| Congdon et al.14 | 2012 | 884 | Chinese | IOLMaster/Visante OCT‡ | Normal |
| Moghimi et al.15 | 2013 | 189 | Iranian | Echoscan/Visante OCT‡ | AACG, CACG, PACS and normal |
| AACG | 40 | ||||
| CACG | 42 | ||||
| PACS | 40 | ||||
| Control | 27 | ||||
| Kim et al.16 | 2014 | Korean | IOLMaster/Visante OCT‡ | PAC (G) and normal | |
| PAC (G) | 101 | ||||
| Normal | 101 | ||||
| Moghimi et al.17 | 2014 | 115 | Iranian | Echoscan/Visante OCT‡ | PACG and PACS |
| Fellow eyes | 40 | ||||
| PACG | 39 | ||||
| PACS | 36 | ||||
| Lee et al.18 | 2015 | 166 | White, Chinese, African and Hispanic | Visante OCT | Open and narrow angle |
| Open angle | |||||
| White | 105 | ||||
| Chinese | 91 | ||||
| African | 62 | ||||
| Hispanic | 39 | ||||
| Narrow angle | |||||
| White | 61 | ||||
| Chinese | 41 | ||||
| African | 28 | ||||
| Hispanic | 36 | ||||
| Moghimi et al.19 | 2015 | Iranian | Echoscan/Visante OCT‡ | PhAC, AAC and normal | |
| PhAC | 28 | ||||
| AAC | 54 | ||||
| Control | 52 | ||||
| Moghimi et al.20 | 2016 | Iranian | Echoscan/Visante OCT‡ | PACG and PACS | |
| Fellow eyes | 40 | ||||
| PACG | 39 | ||||
| PACS | 36 | ||||
| Hong et al.21 | 2016 | Korean | Visante OCT | PAC NBG and PAC BG | |
| PAC NBG | 43 | ||||
| PAC BG | 89 | ||||
| Shimizu et al.22 | 2017 | Japanese | IOLMaster/Casia 1000 SS-OCT§ | Children and adults | |
| Children | 50 | ||||
| Adults | 50 | ||||
| Xu et al.23 | 2017 | 20 | NA | Casia2 SS-OCT/Spectralis OCT | Normals |
| Xu et al.24 | 2018 | 22 | NA | Casia2 SS-OCT | Normals |
| Li et al.25 | 2018 | Chinese | LenStar 900/MD-300 L UBM‖ | PAC | |
| <22.5 | 70 | ||||
| ≥22.5 to <23.5 | 53 | ||||
| ≥23. | 15 | ||||
| Chansangpetch et al.26 | 2018 | 53 | NA | Casia2 SS-OCT/Visante OCT | Open andnarrow angle |
| Yan et al.27 | 2018 | 35 | Chinese | Casia 1000 SS-OCT | |
| Nakamura et al.28 | 2018 | Japanese | Casia2 SS-OCT | ICL | |
| Development study | 46 | ||||
| Validation study | 35 | ||||
| González-López et al.29 | 2019 | 111 | White | Casia 1000 SS-OCT | ICL |
| Ghoreishi et al.30 | 2019 | 58 | Iranian | Casia 1000 SS-OCT | Normals |
| Moghimi et al.31 | 2019 | Iranian | Visante OCT | Fellow eyes of PAC, PhAC and normals | |
| Fellow eyes of PAC | 55 | ||||
| Fellow eyes of PhAC | 48 | ||||
| Normals | 52 | ||||
| Current study | 2020 | 67 | White | Anterior SS-OCT | Normals |
UBM: ultrasound biomicroscopy; OCT: optical coherence tomographer; SS: swept-source; IOL: intraocular lens; ICL: implantable collamer lens; PAC: primary-angle closure; AACG: acute angle closure glaucoma; CACG: chronic angle closure glaucoma; PACS: primary-angle closure glaucoma suspect; PACG: primary angle-closure glaucoma; PhAC: phacomorphic angle closure; AAC: acute angle closure; NBG: non-basal insertion; BG: basal-insertion.
A total of 67 healthy patients were consecutively recruited for this prospective study (46 female and 21 male) were Caucasian. Their mean age was 41.9 ± 12.4 years (range: 22–67 years). For all participating patients, their measurement session was completed uneventfully, and the recorded data were fed into the database for analysis (for each parameter, the mean of the 5 measurements was used for statistical-analysis purposes). Table 2 shows in the bottom row mean ± SD values and range for each parameter measured in the present study with the ANTERION SS-OCT; namely, LV, ACD, LT, AL, WTW, CCT, ACV and STS. Furthermore, for comparison purposes, the table also summarizes the most relevant findings of those studies stemming from the abovementioned literature search (i. e., papers where CLR or LV was measured using either OCT or UBM).
Mean ± standard deviation (range) values obtained in previous published literature for crystalline lens rise (CLR) and lens vault (LV). Anterior chamber depth (ACD), lens thickness (LT), axial length (AXL), white-to-white (WTW), central corneal thickness (CCT), anterior chamber volume (ACV) and spur-to-spur (STS) values were included when available.
| Author | Age (ys) | CLR (mm) | LV (mm) | ACD (mm) | LT (mm) | AXL (mm) | WTW (mm) | CCT (mm) | ACV (mm2) | STS (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Goldsmith et al.4 | NA | 0.39 ± 0.27 (−0.16 to 0.97) | 2.99 ± 0.32 | 11.78 ± 0.57 (10.83 to 12.83) | ||||||
| Baikoff et al.5 | ||||||||||
| All | 0.298 ± 0.288(−0.276 to 1.126) | 3.54 ± 0.39(2.89 to 6.68) | ||||||||
| Hyperopic | 44.5 ± 12.3(18 to 67) | 0.461 ± 0.254(−0.050 to 1.126) | 3.31 ± 0.27 (2.89 to 3.77) | |||||||
| Myopic | 36.5 ± 8.7(22 to 54) | 0.140 ± 0.226(−0.276 to 0.783) | 3.77 ± 0.35(2.89 to 3.40) | |||||||
| Yan et al.7 | ||||||||||
| All | 30.7 ± 16.8(6 to 60) | 0.11 ± 0.23(NA) | ||||||||
| Young | NA(6 to 20) | −0.12 ± 0.14(NA) | ||||||||
| Adults | NA(21 to 40) | 0.13 ± 0.17(NA) | ||||||||
| Older | NA(41 to 60) | 0.33 ± 0.11(NA) | ||||||||
| Nongpiur et al.6 | ||||||||||
| Angle closure | 65.3 ± 9.1 | 0.901 ± 0.265 | 2.66 ± 0.37 | 4.20 ± 0.92 | 22.86 ± 0.93 | |||||
| Normal controls | 54.2 ± 7.9 | 0.316 ± 0.272 | 2.95 ± 0.37 | 3.90 ± 0.73 | 23.93 ± 1.37 | |||||
| Kojima et al.8* | ||||||||||
| Study | 34.3 ± 6.6(22 to 45) | 0.31 ± 0.18(−0.04 to 0.69) | 3.25 ± 0.27(2.77 to 3.81) | 27.9 ± 1.3(25.8 to 31.3) | ||||||
| Validation | 35.6 ± 7.2(25 to 48) | 0.35 ± 0.20(0 to 0.82) | 3.08 ± 0.26(2.63 to 3.61) | NA | ||||||
| Sun et al.9 | 66.0 ± 11.3 | 0.459 ± 0.377 | 2.834 ± 0.522 | 24.00 ± 1.81 | ||||||
| < 50 ys | 0.170 ± 0.212 | 3.337 ± 0.363 | 26.01 ± 2.97 | |||||||
| 50 to 60 yrs | 0.349 ± 0.310 | 2.936 ± 0.416 | 24.18 ± 1.37 | |||||||
| 60 to 70 yrs | 0.432 ± 0.372 | 2.849 ± 0.507 | 23.95 ± 1.85 | |||||||
| 70 to 80 yrs | 0.561 ± 0.375 | 2.693 ± 0.495 | 23.51 ± 0.99 | |||||||
| ≥ 80 yrs | 0.684 ± 0.378 | 2.554 ± 0.535 | 23.31 ± 0.64 | |||||||
| Lee et al.10 | ||||||||||
| Open angle | ||||||||||
| Chinese | 59.86 | 0.265 ± 0.288(0.574 to 0.722) | 2.81 ± 0.32 | |||||||
| Caucasian | 65.89 | 0.431 ± 0.248(0.375 to 0.486) | 2.86 ± 0.38 | |||||||
| Filipo | 68.58 | 0.302 ± 0.213(0.216 to 0.388) | 2.76 ± 0.28 | |||||||
| Hispanic | 64.29 | 0.304 ± 0.263(0.193 to 0.415) | 2.79 ± 0.28 | |||||||
| African | 61.14 | 0.200 ± 0.237(0.127 to 0.273) | 2.91 ± 0.34 | |||||||
| Narrow angle | ||||||||||
| Chinese | 74.40 | 0.648 ± 0.198(0.574 to 0.722) | 2.36 ± 0.23 | |||||||
| Caucasian | 67.59 | 0.784 ± 0.232(0.729 to 0.839) | 2.40 ± 0.28 | |||||||
| Filipo | 70.82 | 0.799 ± 0.282(0.674 to 0.924) | 2.23 ± 0.27 | |||||||
| Hispanic | 66.71 | 0.691 ± 0.216(0.600 to 0.782) | 2.35 ± 0.24 | |||||||
| African | 64.92 | 0.659 ± 0.198 | 2.40 ± 0.26 | |||||||
| Tan et al.11† | ||||||||||
| Narrow angle | 0.775 ± 0.190 | 2.20 ± 0.20 | 23.09 ± 0.88 | |||||||
| Open angle | 0.386 ± 0.251 | 2.73 ± 0.29 | 24.13 ± 1.36 | |||||||
| Chinese | 0.471 ± 0.290 | |||||||||
| Malay | 0.479 ± 0.308 | |||||||||
| Indian | 0.444 ± 0.266 | |||||||||
| Other | 0.504 ± 0.239 | |||||||||
| Shabana et al.12 | 68.3 ± 9.8 | 0.85 ± 0.02 | 2.14 ± 0.02 | 4.69 ± 0.84 | 22.78 ± 0.93 | 11.43 ± 0.03 | ||||
| Ozaki et al.13 | ||||||||||
| Angle closure | 73.5 ± 7.0 | 1.034 ± 0.257 | 2.51 ± 0.39 | 4.91 ± 0.54 | 22.22 ± 0.77 | |||||
| Normal | 72.6 ± 7.3 | 0.429 ± 0.236 | 3.14 ± 0.35 | 4.54 ± 0.47 | 23.28 ± 0.81 | |||||
| Congdon et al.14‡ | 52 ± 11.8 | 0.438 ± 0.280 | 3.21 ± 0.37 | 6.21 ± 0.51 | 23.6 ± 1.1 | |||||
| Moghimi et al.15 | ||||||||||
| AACG | 61.6 ± 9.3 | 1.067 ± 0.252 | 2.26 ± 0.22 | 5.05 ± 0.46 | 21.84 ± 1.17 | |||||
| CACG | 61.1 ± 8.7 | 0.847 ± 0.189 | 2.50 ± 0.24 | 4.85 ± 0.39 | 22.24 ± 0.80 | |||||
| PACS | 60.2 ± 8.1 | 0.890 ± 0.221 | 2.53 ± 0.28 | 4.92 ± 0.30 | 21.97 ± 0.73 | |||||
| Control | 62.4 ± 10.6 | 0.266 ± 0.308 | 3.15 ± 0.33 | 4.16 ± 0.49 | 22.46 ± 4.35 | |||||
| Kim et al.16 | 64.5 ± 6.2 | |||||||||
| PAC (G) | 1.06 ± 0.41 | 2.06 ± 0.40 | 22.96 ± 0.94 | |||||||
| Normal | 0.36 ± 0.37 | 2.94 ± 0.38 | 24.02 ± 1.33 | |||||||
| Moghimi et al.17 | ||||||||||
| Fellow eyes | 59.4 ± 9.2 | 0.977 ± 0.192 | 2.36 ± 0.21 | 4.96 ± 0.32 | 21.69 ± 1.13 | |||||
| PACG | 60.7 ± 9.0 | 0.851 ± 0.186 | 2.49 ± 0.25 | 4.86 ± 0.40 | 22.48 ± 0.82 | |||||
| PACS | 60.2 ± 8.1 | 0.890 ± 0.221 | 2.53 ± 0.28 | 4.92 ± 0.30 | 21.97 ± 0.73 | |||||
| Lee et al.18 | ||||||||||
| Open angle | ||||||||||
| White | 65.1 ± 13.1 | 0.41 ± 0.28 | 0.557 ± 0.034 | 161.3 ± 35.3 | 12.1 ± 0.5 | |||||
| Chinese | 62.0 ± 16.1 | 0.29 ± 0.33 | 0.051 ± 0.038 | 149.4 ± 34.8 | 11.7 ± 0.4 | |||||
| African | 61.7 ± 14.1 | 0.24 ± 0.26 | 0.537 ± 0.038 | 157.6 ± 34.5 | 12.0 ± 0.4 | |||||
| Hispanic | 67.4 ± 13.6 | 0.33 ± 0.28 | 0.054 ± 0.036 | 147.1 ± 31.9 | 11.8 ± 0.5 | |||||
| Narrow angle | ||||||||||
| White | 68.3 ± 12.6 | 0.76 ± 0.23 | 0.562 ± 0.033 | 123.0 ± 25.5 | 12.0 ± 0.5 | |||||
| Chinese | 70.4 ± 14.9 | 0.68 ± 0.22 | 0.555 ± 0.052 | 110.6 ± 19.2 | 11.5 ± 0.4 | |||||
| African | 64.9 ± 9.8 | 0.63 ± 0.21 | 0.540 ± 0.036 | 120.2 ± 20.4 | 11.8 ± 0.4 | |||||
| Hispanic | 66.7 ± 13.0 | 0.69 ± 0.21 | 0.556 ± 0.036 | 110.8 ± 18.9 | 11.7 ± 0.4 | |||||
| Moghimi et al.19 | ||||||||||
| PhAC | 76.6 ± 12.4 | 1.364 ± 0.351 | 1.43 ± 0.43 | 22.83 ± 1.21 | 11.36 ± 0.41 | |||||
| AAC | 61.4 ± 9.4 | 1.002 ± 0.271 | 1.86 ± 0.28 | 21.92 ± 1.10 | 11.19 ± 0.48 | |||||
| Control | 63.9 ± 10.8 | 0.391 ± 0.377 | 2.76 ± 0.44 | 32.12 ± 0.86 | 11.55 ± 0.45 | |||||
| Moghimi et al.20 | ||||||||||
| Fellow eyes | 59.4 ± 9.2 | 0.977 ± 0.192 | 2.36 ± 0.21 | 4.96 ± 0.32 | 21.69 ± 1.13 | |||||
| PACG | 60.7 ± 9.0 | 0.851 ± 0.186 | 2.49 ± 0.25 | 4.86 ± 0.40 | 22.48 ± 0.82 | |||||
| PACS | 60.2 ± 8.1 | 0.890 ± 0.221 | 2.53 ± 0.28 | 4.92 ± 0.30 | 21.97 ± 0.73 | |||||
| Hong et al.21 | ||||||||||
| PAC NBG | 62.7 ± 5.7 | 0.982 ± 0.20 | 2.10 ± 0.21 | |||||||
| PAC BG | 59.8 ± 7.3 | 0.889 ± 0.25 | 2.11 ± 0.30 | |||||||
| Shimizu et al.22 | ||||||||||
| Children | 7.1 ± 3.3 | 0.04 ± 0.22(−0.436 to 0.594) | 3.05 ± 0.27 | 22.21 ± 0.88 | 0.560 ± 0.032(0.503 to 0.635) | 11.65 ± 0.33(10.76 to 12.40) | ||||
| Adults | 73.7 ± 7.8 | 0.54 ± 0.31(−0.09 to 1.352) | 2.53 ± 0.43 | 22.34 ± 0.53 | 0.522 ± 0.030(0.464 to 0.582) | 11.20 ± 0.39(10.38 to 11.88) | ||||
| Xu et al.23 | 37 | |||||||||
| Casia2 | 0.08 ± 0.37 | 11.68 ± 0.21 | ||||||||
| Spectralis | 0.20 ± 0.37 | 11.88 ± 0.15 | ||||||||
| Xu et al.24 | 34.2 ± 8.4 | |||||||||
| Light AM | 0.09 ± 0.22 | 3.14 ± 0.27 | 12.07 ± 0.47 | |||||||
| Light PM | 0.07 ± 0.11 | 3.13 ± 0.25 | 12.08 ± 0.47 | |||||||
| Dark AM | 0.07 ± 0.26 | 3.13 ± 0.29 | 12.03 ± 0.46 | |||||||
| Dark PM | 0.08 ± 0.27 | 3.13 ± 0.29 | 12.03 ± 0.46 | |||||||
| Li et al.25§ | ||||||||||
| <22.5 mm | 62.7 ± 8.0 | 1.05 ± 0.18 | 1.81 ± 0.20 | 4.75 ± 0.43 | 22.00 | 0.537 ± 0.029 | 11.10 ± 0.35 | |||
| ≥22.5 to <23.5 mm | 64.0 ± 10.8 | 0.96 ± 0.18 | 2.01 ± 0.24 | 4.71 ± 0.37 | 22.98 | 0.542 ± 0.033 | 11.55 ± 0.44 | |||
| ≥23 mm | 69.3 ± 14.9 | 1.08 ± 0.17 | 1.99 ± 0.27 | 4.95 ± 0.36 | 23.80 | 0.556 ± 0.022 | 11.93 ± 0.46 | |||
| Chansangpetch et al.26 | 70.9 ± 8.4 | |||||||||
| Casia2 | 0.599 ± 0.301 | 2.477 ± 0.332 | 11.68 ± 0.38 | |||||||
| Visante | 0.607 ± 0.316 | 2.459 ± 0.333 | 11.74 ± 0.43 | |||||||
| Yan et al.27 | 26.1 ± 2.6 | 25.23 ± 1.01 | ||||||||
| Before exercise | −0.134 ± 0.198 | 3.14 ± 0.24 | 3.86 ± 0.32 | |||||||
| After exercise | −0.195 ± 0.198 | 3.16 ± 0.25 | 3.85 ± 0.32 | |||||||
| Nakamura et al.28 | ||||||||||
| Development study | 33.0 ± 6.6(22 to 47) | 0.080 ± 0.160(−0.270 to 0.410) | −0.14 ± 0.19(−0.52 to 0.29) | 11.72 ± 0.42(11.0 to 12.6) | 11.87 ± 0.43(11.08 to 12.96) | |||||
| Validation study | 30.7 ± 9.8(19 to 51) | −0.013 ± 0.200(−0.400 to 0.300) | −0.21 ± 0.21(−0.75 to 0.10) | 11.78 ± 0.30(11.0 to 12.3) | 11.94 ± 0.27(11.42 to 12.43) | |||||
| González-López et al.29 | ||||||||||
| Mydriasis | 0.106 ± 0.176 | |||||||||
| Miosis | 0.165 ± 0.173 | |||||||||
| Ghoreishi et al.30 | 28.95 ± 6.04(22 to 55) | −0.012 ± 0.161(−0.389 to 0.339) | 3.42 ± 0.22(2.98 to 3.89) | 11.87 ± 0.36(11.0 to 12.60) | 0.502 ± 0.041(0.401 to 0.588) | |||||
| Moghimi et al.31 | ||||||||||
| Fellow eyes of PAC | 61.4 ± 9.8 | 0.961 ± 0.203 | 1.96 ± 0.21 | |||||||
| Fellow eyes of PhAC | 72.5 ± 11.3 | 0.781 ± 0.294 | 2.21 ± 0.37 | |||||||
| Normals | 63.9 ± 10.8 | 0.391 ± 0.377 | 2.76 ± 0.44 | |||||||
| Current studyNormal | 41.9 ± 12.4(22 to 67) | 0.26 ± 0.23(−0.24 to 0.78) | 2.88 ± 0.33(2.02 to 3.53) | 4.08 ± 0.26(3.26 to 4.88) | 23.66 ± 0.86(21.28 to 26.04) | 11.90 ± 0.37(10.65 to 12.70) | 0.533 ± 0.034(0.434 to 0.622 | 164.2 ± 33.6(95.7 to 235.3) | 11.86 ± 0.36(11.08 to 12.86) |
NA: not available; AACG: acute angle closure glaucoma; CACG: chronic angle closure glaucoma; PACS: primary-angle closure glaucoma suspect; PAC: primary-angle closure; PACG: primary-angle closure glaucoma; PhAC: phacomorphic angle closure; AAC: acute angle closure; NBG: non-basal insertion; BG: basal-insertion.
The pairwise comparison between LV and each of the remaining ocular parameters under evaluation (ACD, LT, AL, WTW, CCT, ACV, STS, and age) is shown in Fig. 1 through a set of 8 graphs, with their corresponding regression equation. More specifically, the statistical analysis revealed a negative correlation between LV and ACD (R = −0.80, p < 0.001), AL (R = −0.36, p = 0.002), and ACV (R = −0.68, p < 0.001), while a positive correlation was observed between LV and LT (R = 0.67, p < 0.001), and age (R = 0.53, p < 0.001). Finally, no statistically significant correlation was found between LV and WTW (R = −0.17, p = 0.15), CCT (R = 0.11, p = 0.36), and STS (R = −0.10, p = 0.41).
Scatter plots of relation between lens vault (LV) and the different ocular parameters measured: anterior chamber depth (ACD), lens thickness (LT), axial length (AL), white-to-white (WTW), central corneal thickness (CCT), anterior chamber volume (ACV), sulcus-to-sulcus (STS) and age. The solid line represents the best-fit line for each relationship (regression equation was included in each graph).
As was mentioned above in the Introduction, LV or CLR, are two important parameters that should be evaluated in the clinical setting. They may help to diagnose some ocular pathologies, but they are also necessary for specific surgeries (i.e. phakic intraocular lens implantation). In 2005, Goldsmith et al.4 and Baikoff et al.5 were the first groups to assess the vault of the crystalline lens and to apply these data for refractive surgery with phakic lenses. Years later, in 2011, Nongpiur et al.6 considered measuring LV to assess patients with angle closure, since crystalline lens position may play a role in this condition's pathogenesis. Table 1 shows that LV has been assessed with different instruments in quite a few studies. Some authors specifically assessed the effect of race, others the patients’ age and, in some cases, looking for correlations with a specific angle structure (open or narrow). In general, after a detailed analysis of the outcomes (see Table 2, summarizing the values found in each study) and the conclusions reported, we might consider that the measurement of LV is an important parameter that should be evaluated during clinical routine.
Our results revealed statistically significant correlations between LV and each of the remaining parameters, except for WTW, CCT and STS (p > 0.1). Lee et al.18 reported comparable CCT and STS values for different LV (0.41 and 0.76 mm, for open and narrow angle, respectively) in Caucasian patients (see Table 2 for CCT and STS values). Li et al.25 reported different STS values for similar LV in their cohort of 138 patients. It seems that the cornea (thickness and width) does not play a significant role in LV variations. On the other hand, negative significant correlations were found for ACD, AL and ACV, and positive significant correlations for LT and age. These findings are to be expected since, a shallow ACD—hence leading to a smaller ACV—results in a shorter AL; moreover, a thicker lens is linked to aging, and both parameters are correlated with significant positive LV. Note that eyes with shorter AL tend to have small ACD.37,38 A reduction in ACD, also related to age, is caused by forward protrusion of the crystalline lens’ anterior pole.39 In our study, R-values for ACD, ACV and AL were negative; i.e., LV was significantly high for small ACD and AL values. However, Li et al.25 found that there were no differences in ACD, LV, iris parameters, and ciliary body thickness among primary-angle closure patients belonging to different AL groups. Tan et al.11 also concluded that after adjusting for age and gender, significant associations with greater LV were shorter AL and shallower ACD. We consider that if we have a longer eye, this may result in large ACD, and, then, the crystalline lens may be posteriorly located resulting in lower LV.
In relation to age, our results agree with those by Yan et al.,7 who reported a positive significant correlation of CLR with age (R = 0.81, ages from 6 to 60 years). However, Nongpiur et al.,6 using a Visante OCT in Chinese eyes, found that LV and LT were poorly correlated (R = 0.17). They considered that it is likely that the increase in LV with age may be associated with changes in zonular laxity with age. In addition, the degree and type of cataract may influence these two variables and the sample analyzed (>40 years). Our sample was younger and only comprised healthy (i.e., non-cataractous). Our results agree also with those reported by Sun et al.9 who found, in 388 eyes from patients aged 30 to 89 years, that LV increased with age, thus explaining why aging is a significant risk factor for primary angle glaucoma.
LV has also been analyzed as a function of the ethnicity and the angle. Lee et al.10,18 and Tan et al.11 analyzed, in three studies with large samples (n = 684, 166 and 1464 eyes, respectively), the association of LV in eyes with narrow angles among different ethnic groups. Lee et al.10 showed that narrow-angle eyes have greater LV compared to open-angle eyes, regardless of the subject's ethnicity. They concluded that once LV goes above a given threshold for that particular ethnicity, angle narrowing tends to occur. Tan et al.11 found that there were no association between LV and race, and concluded that LV was independently associated with the presence of narrow angles and showed good diagnostic performance in detecting eyes with narrow angles. In contrast, Lee et al.18 reported that ethnic variation in anterior segment biometric parameters exists. ACV and LV were stronger than anterior chamber width in predicting variance of anterior chamber angle width in the four-ethnic groups they assessed (White, Chinese, African, and Hispanic). Africans subjects exhibited the lowest mean value for the LV, and Whites the highest mean values for LV (see Table 2). Their findings suggest that differences in anterior chamber area, ACV, and LV may be one of the main causes of differences in the prevalence of primary angle-closure glaucoma across ethnicities.
As stated by Nongpiur et al.,6 larger positive LV values indicate that a greater bulk of the lens is located anterior to this plane. A larger LV would thus probably worsen the angle crowding in an already at-risk. Several studies carried out on different ethnicities agree that LV is indeed a risk factor for angle closure.6,13,15 Moghimi et al.15 suggested that higher LV may play a particularly strong role in the development of an acute attack of angle closure, and may be used to differentiate acute angle closure, primary-angle closure glaucoma and eyes with suspected primary-angle closure.17 Specific analysis in phacomorphic angle closure and acute primary-angle closure eyes revealed that a greater LV is one of the main differences between these two types of eyes.19 In a recent cross-sectional study, Moghimi et al.31 found that in acute primary-angle closure cases, the fellow eye showed the greatest LV, followed by fellow eyes of phacomorphic angle closure cases. A multivariable analysis revealed that LV >0.786 mm is a factor that discriminated fellow eyes of acute primary-angle closure from phacomorphic angle closure. Other factors, such as diurnal variation, light or exercise have been also analyzed. Xu et al.,24 for example, concluded that no diurnal variation of LV measurements was detected in the light and dark, and Yan et al.27 assessed the impact of post-aerobic exercise on the lens and reported that LV decreased significantly.
As detailed in Table 1, different instruments have been used over the past 15 years to measure CLR or LV. The initial studies relied on time-domain OCT platforms (Visante) while the most recent ones resorted to SS-OCTs (Casia 1000 and Casia2), which use a longer-wavelength light source to better analyze the anterior segment of the eye.2 New high-resolution SS-OCT instruments may provide clinicians and investigators images with better resolutions. UBM8,25 has also been used but to a much lesser extent than OCT since it is not very widespread in the clinical setting, possibly due to it being an invasive and time-consuming technique. In addition, some of the previous studies have made use of more than one instrument to obtain several parameters (i.e. biometer + UBM or biometer + OCT). In our case, we used only one instrument, the ANTERION SS-OCT, which is suitable for both anterior segment analysis and biometry and which will become a versatile device to be used for clinical activity and research reporting all the variables.
One main limitation in our research was the relatively small sample size, especially taking into account that our population comprised only healthy Caucasian patients. Future studies using this instrument should include a higher number of patients, different ethnicities, and also eyes with narrow angle and also with primary-angle glaucoma. We want also to point out that despite the fact that in our study scleral-spur localization was done manually (i.e., relying on a subjective method), it was the same expert examiner who did all the examinations.
ConclusionThe ANTERION SS-OCT platform is a reliable and user-friendly device to evaluate LV, while making it possible as well to measure—with that same instrument—various anterior-segment parameters. Our findings revealed that LV is significantly correlated with different ocular parameters such as ACD, AL, ACV, LT, and age. We believe that LV measurement should be part of any clinical routine examination both for diagnosis and prior to certain refractive surgeries, such as phakic intraocular lens implantation.