Pupillary dilatation with three types of eye drops is used regularly in the clinic; however, a mixture of these drops in a single bottle may be more beneficial in reducing workloads and resources. This study compared the efficacy in pupillary dilatation between two protocols of dilating drop instillation.
MethodsThis prospective, randomized, comparative study included 30 eligible Thai patients. The patients randomly received preoperative pupillary dilatations by either the conventional protocol (1% tropicamide (T), 10% phenylephrine (P) and 0.1% diclofenac (D) in three separate bottles) or the fixed combination (TPD) protocol which had the three types of eye drops mixed in a single bottle in a ratio of 4:3:3. The chi-square test and independent t-test were used to analyze the data.
ResultsThe conventional protocol group and TPD protocol group each had 15 patients. Sixty minutes after the initial instillation, all patients in the TPD protocol and 13 patients (86.7%) in conventional protocol achieved at least 6mm in the shortest diameter. The mydriatic rate between protocols showed no difference. In patients who received the TPD protocol, the systemic effects on the mean arterial blood pressure and pulse rate decreased over time.
ConclusionThe mixture of tropicamide, phenylephrine and diclofenac had a comparable efficacy for a pupillary dilatation to the conventional dilating drops in separate bottles. The systemic complications on blood pressure and arterial pulse of the TPD mixture were less than the conventional protocol.
Trial registrationTCTR20130325001.
La dilatación de la pupila con tres tipos de gotas oculares se utiliza normalmente en la práctica clínica; sin embargo, la mezcla de dichas gotas en un único envase puede resultar más beneficiosa a la hora de reducir las cargas de trabajo y los recursos. Este estudio comparó la eficacia entre dos protocolos de dilatación de pupilas.
MétodosEste estudio prospectivo, aleatorizado y comparativo incluyó a 30 pacientes tailandeses elegibles. A dichos pacientes se les dilató aleatoria y preoperatoriamente la pupila utilizando el protocolo convencional (1% tropicamida (T), 10% fenilefrina (P) y 0,1% diclofenaco (D) en tres envases separados), o el protocolo de combinación fija (TPD), que contenía los tres tipos de gotas oculares mezclados en un único envase, a un ratio de 4:3:3. Se utilizaron las pruebas de χ2 y la prueba independiente t para analizar los datos.
ResultadosTanto el grupo de protocolo convencional como el grupo TPD incluyeron a 15 pacientes. A los sesenta minutos de la instilación inicial, todos los pacientes del protocolo TPD y 13 pacientes (86,7%) del protocolo convencional lograron un mínimo de 6mm en el diámetro menor. La tasa midriática entre ambos protocolos no reflejó diferencia alguna. En los pacientes del protocolo TPD, los efectos sistémicos sobre la presión sanguínea media y el índice de pulso disminuyeron con el tiempo.
ConclusiónLa mezcla de tropicamida, fenilefrina y diclofenaco mostró una eficacia comparable a la de las gotas para dilatación de pupilas suministradas en envases separados. Las complicaciones sistémicas sobre la presión sanguínea y la presión arterial de la mezcla de TPD fueron menores a las del protocolo convencional.
Registro del ensayoTCTR20130325001.
Pupillary dilatation is necessary for a fundus examination and ophthalmic procedures. The mydriatic agents that are generally available in the eye clinic are tropicamide and phenylephrine. For an intraocular examination or operation, a non-steroidal anti-inflammatory drug is additionally required to prolong the mydriatic effect and lessen post-operative inflammation.1,2 The conventional formula of pre-operative pupillary dilatation commonly used in the hospital is 1% tropicamide, 10% phenylephrine, and 0.1% diclofenac in separate bottles. Each eye-drop bottle contains preservatives to inhibit microbial contamination. However, these preservatives have been linked to unwanted ocular surface side effects, such as stinging, redness and corneal punctate epithelial erosion. A combination of pre-operative eye drops in one bottle may not only reduce the frequency of multiple eye drop administrations but may also reduce the ocular and systemic complications.3–5 There were some reports on the efficacy and safety of a combination of tropicamide and phenylephrine,4–6 but there is no report on a combination of tropicamide, phenylephrine and diclofenac.
The objective of this study is to compare the mydriatic efficacy of a mixture of tropicamide, phenylephrine and diclofenac in a single eye-drop bottle to the conventional practice of applying the eye drops from separate bottles.
MethodsInclusion criteriaThis randomized prospective study was performed in accordance with the declaration of Helsinki and was approved by the Ethics Committee of the Faculty of Medicine, Prince of Songkla University, Thailand (EC 56-153-02-1-2). We followed our institution protocols regarding patient data protection. Written informed consents were obtained from 30 consecutive, eligible patients before the study began. The inclusion criteria were patients of 18 years old or older and scheduled for an operation at Songklanagarind Hospital, Prince of Songkla University, Thailand from April to July 2013. The pre-operative fasting plasma glucose of all diabetic patients was less than 200mg/dl. The resting systolic pressure was ≤160mmHg and the resting diastolic pressure was ≤90mmHg.
Exclusion criteriaPatients with a risk of angle-closure, uncontrolled blood pressure, pregnancy, iris or pupil abnormality, or a history of allergic reactions to any of the investigating drugs were excluded from the study.
Sample size of patientsThe sample size of 30 patients was calculated from the formula n>2×(Zα/2×σ/d1)2, where Zα=1.96, σ (sigma)=0.6, d1 (delta)=8.0–7.0mm (mean=8.0mm, lower limit=7.0mm). The calculated number of recruited patients was at least 14 for each group with an anticipated 20% drop-out allowance.
Dilating eye dropsThe eye drops for the conventional protocol included three commercial preparations: 1% tropicamide (Mydriacyl®, Alcon-Couvreur, Puurs, Belgium), 10% phenylephrine (Phenylephrine HCL®, Silom Medical Co., Ltd, Thailand) and 0.1% diclofenac (Voltaren Ophtha Oftalmico®, Novartis PharmaAG, Basel, Switzerland) in separate bottles. The mixture of the fixed combination protocol was a combination of those commercial preparations in a ratio of 4:3:3 that resulted in a final concentration of 0.4% tropicamide (T), 3% phenylephrine (P) and 0.03% diclofenac (D). This reduced concentration mixture (TPD) was labeled for the TPD protocol. A pharmacist (PA) of the hospital was responsible for the preparation of this TPD mixture. The mixture was prepared from commercially available eye drops instead of using the primary preservative-free pharmaceutical products. This mixture was easily prepared in the office and dispensed to several units. The precipitation of tropicamide, which may occur when mixed with phenylephrine, was less likely to occur at a concentration less than 0.5%.6 The fixed combination of these three medications was chemically stable.
Study protocolsEligible patients were randomized into two groups and received either a conventional or TPD formula for pupillary dilatation. Patients in the conventional protocol received eye drops every 5min (Table 1). Patients in the TPD protocol received the TPD mixture every 15min. Other eye drops, such as topical anesthetics, were not allowed before the study. The measurements of pupillary diameters, pulse and blood pressure were taken at baseline, 10min, 25min and 60min after the first eye drop instillation of each protocol that was administered. The pupillary diameter was measured in both horizontal and vertical dimensions by an infrared pupillometer (Colvard®, OASIS Medical Inc., CA, USA) and was read by only one investigator (SS). The average of these two values was recorded for the analysis. The sitting arterial blood pressure and pulse were measured by an automatic sphygmomanometer (Spirit®, Spirit Medical Co., Ltd., Taiwan, ROC). The target pupillary size was at least 6mm in the shortest dimension within 60min. With a pupillary size of at least 6mm in diameter, the fundus examination along with most intraocular operations achieved a sufficient view of the lens, retina and vitreous.
Protocols of eye drop administration.
| Formula | Measurement | 0min | 5min | 10min | Measurement | 15min | 20min | 25min | Measurement | 30min | 35min | 40min | 45min | 60min |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional protocol | BP, P, pupil | D | T | P | BP, P, pupil | D | T | P | BP, P, pupil | D | T | P | D | BP, P, pupil |
| TPD protocol | TPD | – | – | TPD | – | – | TPD | – | – | TPD |
BP, blood pressure; P, arterial pulse; pupil, pupil diameter; D, 0.1% diclofenac; T, 1% tropicamide; P, 10% phenylephrine; TPD, Mixture consisting of 0.4% tropicamide (T); 3% phenylephrine (P) and 0.03% diclofenac (D).
The primary outcome was a difference of the mean pupillary diameters at 60min between the two protocols. The secondary outcomes were changes in the mean arterial blood pressure (MAP) and arterial pulse. A formula for the MAP calculation was [(2×diastolic value)+systolic value]/3.
Statistical analysisA comparative analysis of continuous data was performed by an independent t-test. A comparative analysis of categorical data was performed by a Chi-square test. SPSS version 16.0 was used for these analyses. A p-value of 0.05 or less was considered statistically significant.
ResultsPatient demographic dataThirty patients (16 male, 14 female) were enrolled in the study and were randomized to receive the conventional or the TPD protocol. The patients’ ages ranged from 23 to 83 years. Underlying diseases such as diabetes mellitus (DM), hypertension (HT) and dyslipidemia were reported in 60% of patients in the conventional group and in 40% of patients in the TPD group. Three patients (20%) in each group had diabetes mellitus. Eight patients (53.3%) in the conventional group and 5 patients (33.3%) in the TPD group had hypertension. Two patients (13.3%) in each group had dyslipidemia. However, none of these underlying diseases were statistically different between the groups (p=0.465 for overall conditions, p=1.00 for DM, p=0.46 for HT, and p=1.00 for dyslipidemia). Other baseline characteristics of the patients are shown in Table 2. There were no differences in the basic characteristics of the patients between the protocols.
Baseline demographic data.
| Characteristics | Conventional (n=15) | TPD (n=15) | p value |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 7 (46.7) | 9 (60.0) | 0.714 |
| Female | 8 (53.3) | 6 (40.0) | |
| Age (years, mean±SD) | 61.53±16.95 | 64.27±11.77 | 0.612 |
| Underlying diseases, n (%) | |||
| Absence | 6 (40.0) | 9 (60.0) | 0.465 |
| Presence | 9 (60.0) | 6 (40.0) | |
| Studied eye, n (%) | |||
| Right | 8 (53.3) | 8 (53.3) | 1.000 |
| Left | 7 (46.7) | 7 (46.7) | |
| Lens status, n (%) | |||
| Phakic | 14 (93.3) | 13 (86.7) | 0.595 |
| Pseudophakic | 0 (0) | 1 (6.7) | |
| Aphakic | 1 (6.7) | 1 (6.7) | |
| Previous ocular operation, n (%) | |||
| Never | 8 (53.3) | 6 (40.0) | 0.714 |
| Presence | 7 (46.7) | 9 (60.0) | |
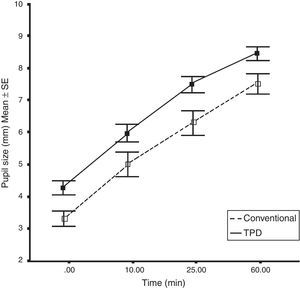
At baseline, the mean pupillary size of the patients in the TPD group was slightly larger than the conventional group. However, the average difference in the final pupillary size from baseline in both groups was equal. The pupil diameter increased by 4.12±1.06mm in the TPD group and by 4.10±0.80mm in the conventional group (Table 3). The average rate of mydriasis in the TPD group was 0.0687mm/min and 0.0683mm/min in the conventional group. There was no statistical difference of mydriatic rates between the two protocols (Fig. 1). All patients in the TPD group and 13 patients (86.7%) in the conventional group achieved their target pupillary size at 60min. Changes in the pupil diameter from baseline were not significantly different between protocols (Table 3). These changes also showed no differences between normal patients and patients with underlying diseases at every time point in the subgroup analyses of both protocols (Table 4).
Pupillary diameter, pulse and mean arterial blood pressure changes over time.
| Variable | Conventional (n=15) | TPD (n=15) | p value |
|---|---|---|---|
| Pupil diameter (mm, mean±SD) | |||
| Baseline | 3.45±0.89 | 4.33±0.82 | 0.009* |
| At 10min | 4.95±1.41 | 6.02±1.05 | 0.026* |
| (+1.50±0.95) | (+1.68±0.98) | (0.607) | |
| At 25min | 6.33±1.44 | 7.50±0.93 | 0.013* |
| (+2.88±0.95) | (+3.17±0.97) | (0.427) | |
| At 60min | 7.55±1.25 | 8.45±0.77 | 0.025* |
| (+4.10±0.80) | (+4.12±1.06) | (0.961) | |
| Pulse (permin, mean±SD) | |||
| Baseline | 65.87±13.56 | 66.60±11.54 | 0.876 |
| At 10min | 67.07±13.38 | 63.80±10.37 | 0.461 |
| (+1.20±3.49) | (−2.80±3.26) | (0.003*) | |
| At 25min | 67.93±13.46 | 63.00±10.64 | 0.275 |
| (+2.07±4.38) | (−3.60±4.10) | (0.001*) | |
| At 60min | 67.87±12.32 | 61.67±9.84 | 0.139 |
| (+2.00±7.19) | (−4.93±4.43) | (0.004*) | |
| MAP (mmHg, mean±SD) | |||
| Baseline | 88.91±8.26 | 91.62±8.00 | 0.369 |
| At 10min | 85.37±13.63 | 88.98±8.21 | 0.388 |
| (−3.54±12.01) | (−2.64±5.91) | (0.798) | |
| At 25min | 89.60±7.55 | 90.24±8.32 | 0.826 |
| (+0.69±5.15) | (−1.38±5.031) | (0.288) | |
| At 60min | 91.84±8.23 | 89.33±10.86 | 0.481 |
| (+2.93±7.84) | (−2.29±10.68) | (0.138) | |
MAP, mean arterial pressure.
The mean changes from baseline are shown in italics and parentheses.
Pupillary diameter changes compared between normal patients and patients with underlying diabetes mellitus or hypertension.
| Variable | Conventional | p value | TPD | p value | ||
|---|---|---|---|---|---|---|
| Normal patients | Patients with DM or HT | Normal patients | Patients with DM or HT | |||
| Pupil diameter (mm, mean±SD) | ||||||
| Baseline | 3.42±1.11 | 3.53±0.81 | 0.827 | 4.42±1.03 | 4.25±0.43 | 0.739 |
| At 10min | 4.92±1.63 | 4.84±1.39 | 0.929 | 6.08±1.05 | 6.10±1.18 | 0.979 |
| At 25min | 5.88±1.69 | 6.59±1.34 | 0.390 | 7.61±0.93 | 7.40±1.10 | 0.708 |
| At 60min | 7.21±1.76 | 7.81±0.85 | 0.465 | 8.58±0.72 | 8.30±0.97 | 0.544 |
| Pupil diameter difference from baseline (mm, mean±SD) | ||||||
| At 10min – baseline | 1.50±0.63 | 1.31±1.08 | 0.712 | 1.67±0.86 | 1.85±1.32 | 0.756 |
| At 25min – baseline | 2.45±0.68 | 3.06±1.06 | 0.247 | 3.19±0.90 | 3.15±1.29 | 0.941 |
| At 60min – baseline | 3.79±0.68 | 4.28±0.89 | 0.284 | 4.17±1.13 | 4.05±1.15 | 0.857 |
DM, diabetes mellitus; HT, hypertension.
The changes in MAP of the patients at each time point after initiating the dilating drops were not significantly different between the protocols. However, the changes of arterial pulse from the baseline were strikingly different between the two protocols. While the mean arterial pulse of the patients who received the conventional protocol increased over time, the mean arterial pulse of the patients who were administered the TPD protocol decreased over time (p<0.005). Although the change in the MAP from baseline was not significantly different between the protocols, the values showed a tendency to decrease over time (Table 3).
DiscussionOur study included DM and HT patients: 60% in the conventional group and 40% in the TPD group. Although Table 2 demonstrates that patients with these underlying diseases were not statistically different, the underlying diseases did have a more or less effect on the pupil size at baseline (Table 3). The mean baseline pupil size of the patients in the conventional group was smaller than the TPD group with a significant difference. Therefore, concerning the rates of pupillary dilatation, it would be more useful to compare the effect of the eye drops between protocols than the sizes of the final pupillary diameter. At the end of the study, both protocols yielded an equal degree of pupillary dilatation and the rate of pupillary dilatation was not different between the two groups. There was also no statistical difference in the pupillary sizes between the patients with DM or HT who received either the conventional or TPD protocol at every time point (Table 4).
A mixture of reduced concentration of tropicamide and phenylephrine in patients with darkly pigmented irides was reported to be sufficiently effective for pupillary dilatation in indirect ophthalmoscopy.4,5 This study also showed that the lower concentration of dilating drops in the TPD protocol neither compromised the average gain in pupillary increment nor the mean mydriatic rate at the 60min mark. Diclofenac can be added into the mixture of tropicamide and phenylephrine without disturbing the mydriatic effects. However, the prolonged efficacy of pupillary dilatation during the intraoperative period may be required in a future study. The limitation of this study was the small number of subjects. Therefore, a subgroup analysis of mydriatic response between patients with underlying diseases and normal patients could not be provided.
In regards to the systemic effects on the MAP and pulse, phenylephrine has a sympathetic stimulation on the heart rate and, therefore, probably induces a raise of the arterial blood pressure and pulse. Each patient in the conventional protocol received 3 drops of 10% phenylephrine while each patient in the TPD protocol received 4 drops of 3% phenylephrine. Therefore, the total amount of phenylephrine in the conventional group of patients was much higher than the patients in the TPD group. This may be the reason the TPD protocol had fewer patients with cardiovascular side effects than patients who received the conventional protocol. The tendency of the mean arterial pulse to decrease over time in the TPD group may occur from a full rest of the patient with less sympathetic stimulation from the eye drops. A similar result was also reported in a previous study.5 Therefore, the TPD protocol may be more beneficial in patients with systemic hypertension or cardiovascular diseases.
Patients in the conventional protocol received the eye drops 10 times while patients in the TPD protocol received the mixture only 4 times. As a result, if the TPD protocol can yield an equal dilating efficacy compared to the conventional protocol while requiring less work, the TPD protocol is preferred in daily practice.
ConclusionsThe TPD protocol with the fixed combination of tropicamide, phenylephrine and diclofenac is equally effective as the conventional protocol for preoperative pupillary dilatation with reduced side effects in the patient's blood pressure.
Author's contributionPB conceived of the study, participated in its design and coordination, and wrote the manuscript. SS carried out the patient's randomization and data collection. PA carried out the mixture preparation and stability test. All authors read and approved the final manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Ms. Parichat Damthongsuk for the statistical analysis.