To evaluate the efficacy of weekly unilateral application of 1 % atropine on reducing anisometropia in Chinese children with low myopia.
MethodsIn this retrospective cohort study, anisometropic children aged 6–12 years receiving atropine in the more myopic eye for at least 3 months were included in atropine group (n = 150), and baseline matched counterparts without atropine use were included in control group (n = 50). Changes in spherical equivalent refraction (SER), axial length (AL) and other biometric parameters between each visits were analyzed.
ResultsAt 3 months, anisometropia of atropine group reduced by 0.737 ± 0.030 D (P < 0.001), with SER increasing by 0.528 ± 0.024 D and AL decreasing by 0.118 ± 0.008 mm in the atropine-treated eyes. The changes of SER and AL of the contralateral eyes between the two groups showed no significant difference (P = 0.489 and 0.107, respectively). Initial SER in the atropine-treated eyes was positively associated with the change of SER in those eyes (P = 0.042) and negatively associated with the change of anisometropia in atropine group (P = 0.048). With continuous atropine application for 9 months, anisometropia reduced by 1.212 ± 0.153 D (P < 0.001).
ConclusionWeekly unilateral 1 % atropine application in the more myopic eye was effective for reducing anisometropia in children with low myopia and would not alter myopia progression in the contralateral eye. Children with lower initial myopia in the treated eye showed more alleviation of myopia in that eye and more reduction of anisometropia.
Anisometropia represents a unique condition of ocular development, where the two eyes with an identical genetic background develop asymmetrically to produce a significant difference in refractive errors. Anisometropia is typically defined as a between-eye difference in the spherical equivalent refraction (SER) of ≥ 1.00 diopter (D).1 The prevalence of anisometropia is about 1.27–9.4 % in schoolchildren,2–4 and increases with age.2 Anisometropia plays an important role in the establishment of binocular vision during childhood. If the anisometropia is great enough and left uncorrected in children, it may impair binocular visual function and lead to the development of amblyopia.5 Besides, anisometropia can cause aniseikonia, spectacle intolerance, visual fatigue, reduced stereopsis, and subnormal binocular function.6
The structural basis of anisometropia is the interocular difference in axial length (AL).7 It has been reported that in anisometropic children, the eye with longer AL elongated faster than the other eye.8 This explains why the magnitude of anisometropia increases with the progression of myopia.7 Therefore, if the eye with longer AL was left untreated, anisometropia may grow larger. These findings emphasize the importance of retarding the axial elongation in the more myopic eye in anisometropic children.
At present, atropine and orthokeratology have been proven to be effective in myopia control.9–11 In addition, atropine8,12 and orthokeratology13-15 have been investigated for treating myopic anisometropia. A prospective study of 13 children suggested that monocular usage of 1 % atropine eye drops every 3 days for 7 to 16 months was effective for reducing anisometropia in children by shortening the AL of the treated eyes.12 A retrospective study of 20 children suggested that monocular usage of 0.125 % atropine eye drops daily for 1 year was effective at reducing the interocular difference of AL in eyes with axial anisometropia.8 However, the sample sizes of these studies were small and none of these studies have clarified the factors that are associated with the effect of unilateral 1 % atropine on anisometropia control. Whether continuous unilateral atropine application could bring continuous reduction of anisometropia and when the principal efficacy may occur have not been investigated yet. How long the reduction of anisometropia could persist after atropine cessation has not been reported. Therefore, the current study was conducted to investigate the effects of weekly monocular usage of 1 % atropine on controlling anisometropia in Chinese children and efforts were made to answer the abovementioned questions. In a series of studies of atropine for the treatment of myopia, the AL remained essentially unchanged (−0.02 ± 0.35 mm) after 2 years of 1 % atropine eye drops application,11 while the AL increased slightly (0.41 ± 0.32 mm) after 2 years of 0.01 % atropine eye drops application,10 which indicates that atropine eye drops of higher concentration has stronger effect on retarding the axial elongation. Besides, it has been demonstrated that decreasing the frequency of 1 % atropine to once a week was associated with fewer side effects compared with daily use,16,17 and weekly use of 1 % atropine was relatively well tolerated with temporary photophobia and near blurred vision.18 Therefore, our study enrolled subjects with weekly 1 % atropine eye gel application.
MethodsParticipantsThis retrospective cohort study was conducted at Shenzhen Eye Hospital, China, from March 2022 to August 2024 consecutively, designed to investigate the efficacy of weekly unilateral application of 1 % atropine eye gel in diminishing childhood anisometropia. The study was approved by the Institutional Review Board of Shenzhen Eye Hospital (Approval No 2023KYPJ009). All procedures were conducted in accordance with the tenets of the Declaration of Helsinki. The medical records and examination results of 1694 myopic children aged 6–12 years with weekly unilateral 1 % atropine (Sinqi Pharmaceutical Co., Ltd., Shenyang, China) use in the more myopic eyes during the study period were reviewed, and only 150 of them met the inclusion and exclusion criteria. Baseline matched controls during the same period were selected. In order to evaluate the duration of its sustained effects after cessation, atropine was suspended suddenly without tapering. While a gradual tapering approach might minimize rebound effects, it would prolong the treatment period and complicate our assessment of the intervention's direct outcomes.
Inclusion and exclusion criteriaInclusion criteria: (1) age: 6 to 12 years; (2) refraction: low myopia with SER > −3.00 D in the more myopic eyes, and either hyperopia or emmetropia or low myopia with SER > −2.00 D in the contralateral eyes as measured by cycloplegic refraction; anisometropia of SER ≥ 1.00 D as measured by cycloplegic refraction; and astigmatism ≤ 1.50 D as measured by cycloplegic refraction; (3) best corrected visual acuity: equal to or better than logMAR 0 in each eye; (4) intraocular pressure: ≤ 21 mmHg in each eye; (5) refraction, AL, anterior chamber depth (ACD), lens thickness (LT), and corneal power (K) were measured with cycloplegia at baseline and 3 months; (6) follow-up visit: at least 3 months of follow-up with 1-month follow-up window.
Exclusion criteria: (1) other ocular diseases except for refractive error (eg. amblyopia, strabismus, cataract, glaucoma, fundus diseases, ptosis, etc.); (2) previous treatment with atropine in either eye; (3) previous or current use of orthokeratology lenses, peripheral defocus lenses, or low-level red-light therapy; (4) systemic or developmental problems that might affect refractive development; (5) inconsistent usage of the atropine eye gel (discontinuation of atropine for more than once per month).
MeasurementsDuring each follow-up visit, distance visual acuity and ocular alignment were examined. Refractive error was measured by cycloplegic refraction, which was obtained 30 min after administration of three cycles of 0.5 % tropicamide along with 0.5 % phenylephrine hydrochloride (Sinqi Pharmaceutical Co., Ltd., Shenyang, China). This cycloplegic regime has been reported to be adequate for cycloplegia as compared to 1 % cyclopentolate.19,20 Cycloplegia was routinely checked by evaluating the degree of pupil dilation and disappearance of light reflection. Cycloplegic autorefraction was taken using a Nidek ARK1 autorefractor (Nidek Co., Ltd., Aichi, Japan) with an average of five consecutive measurements. Autorefraction value was used as a base for subjective refraction. Cycloplegic subjective refraction was conducted by experienced optometrists. Difference of no >0.25 D was allowed between autorefraction and subjective refraction. These two methods of refraction were both conducted in this study as a double check to ensure the accuracy of refraction results. SER was calculated as spherical power plus half of the cylinder power. AL, ACD, LT, and K were measured using an IOL-Master 700 (Carl Zeiss Meditec AG, Jena, Germany) after cycloplegia. The pupil size recorded by IOL-Master 700 could verify the cycloplegia effect.
Statistical analysisThe baseline characteristics were described as proportion or mean ± standard deviation. Between group differences of categorical data were analyzed by the χ2 test. Between group and within group differences of continuous data were assessed using the Student t-test or the Mann-Whitney U test. Within group differences in ocular parameters between the baseline and the 3 months follow-up visit were analyzed using the paired t-test or the Wilcoxon signed ranks test for related samples. Within group differences in ocular parameters for repeated measurements of longitudinal data were analyzed using the generalized estimating equations. Multiple linear regression was performed using the SER change or anisometropia change as the dependent variable to assess its possible association with age, sex, and other ocular parameters. Statistical analysis was performed using SPSS version 26 (IBM, Armonk, NY, USA). A 2-tailed P value of <0.05 was considered statistically significant.
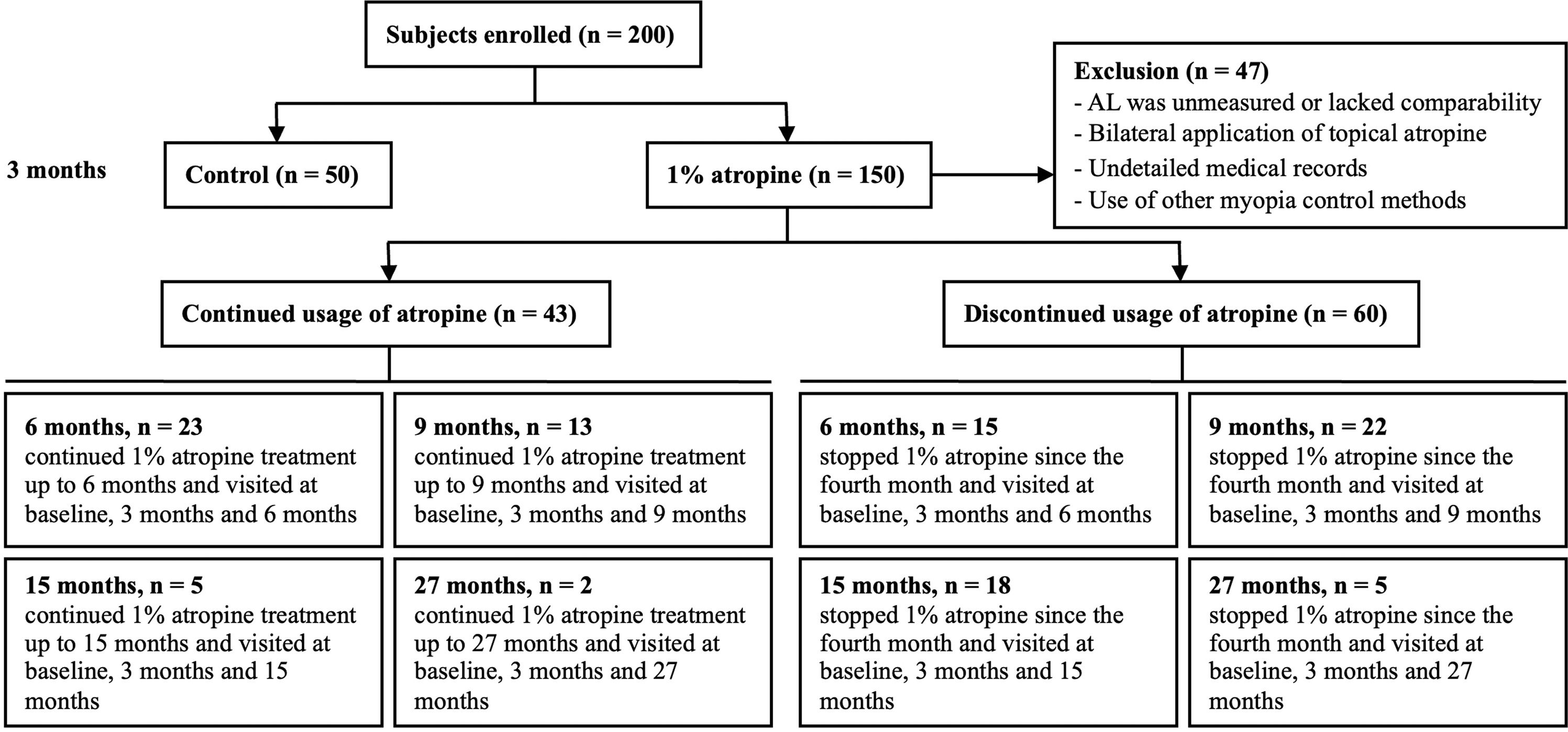
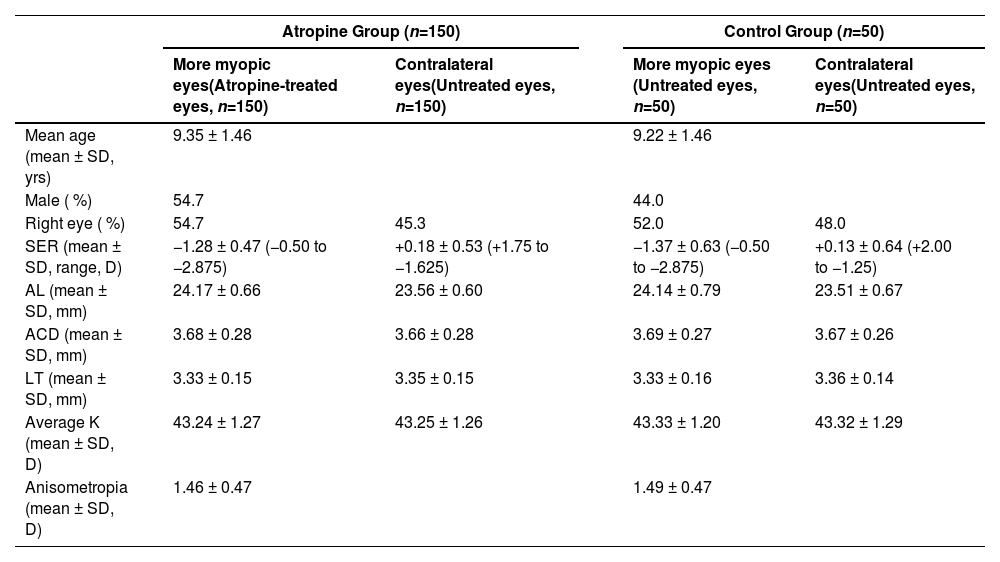
ResultsPatient characteristicsA total of 200 subjects who met the inclusion and exclusion criteria were enrolled, with 150 subjects in atropine group and 50 subjects in control group. The basic characteristics, including demographics, SER, AL, ACD, LT, average K, and anisometropia, showed no significant difference between the two groups (Table 1). In addition, the percentage of unilateral myopia (the contralateral eyes with emmetropia or hyperopia) between the atropine group and the control group at the baseline showed no significant difference (76 % vs. 68 %, P = 0.264). Among the 150 children in the atropine group, 70 eyes (46.7 %) achieved anisometropia ≤ 0.5D after 3 months of treatment. Forty-seven participants were excluded from further follow-up analysis due to the following reasons: (1) AL was not measured or measured by a different brand instrument at later follow-up visits; (2) both eyes were prescribed with topical atropine at later follow-up visits; (3) the medical records concerning atropine eye gel application were not detailed enough to evaluate the compliance of the subjects; (4) the subjects turned to use other myopia control methods such as orthokeratology lenses, peripheral defocus lenses, or low-level red-light therapy. A total of 103 children completed at least 6 months of follow-up. Among these, 43 patients (41.7 %) continued using atropine for >6 months, while 60 patients (58.3 %) discontinued atropine after 3 months of use. The reasons for discontinuation were: (1) anisometropia ≤ 0.50D (32 patients); (2) photophobia occurred for the first 2–3 days after atropine administration, requiring sunglasses for symptom relief under sunlight (28 patients). Among these 150 participants, only 28 (18.7 %) discontinued atropine use due to intolerance to its photophobic side effects. Of the 43 children who continued atropine treatment, 16 had already achieved anisometropia ≤ 0.5D at the 3-month mark. However, these patients and their parents opted to continue treatment to consolidate the therapeutic effect and potentially further reduce the anisometropia. A patient enrollment flowchart was shown in Fig. 1. The best-corrected distance visual acuity of all subjects throughout the study was equal to or better than logMAR 0. No patients exhibited increased phoria or developed strabismus after atropine use.
Baseline characteristics of the atropine group and the control group.
ACD: anterior chamber depth, AL: axial length, K: corneal power, LT: lens thickness, SER: spherical equivalent refraction.
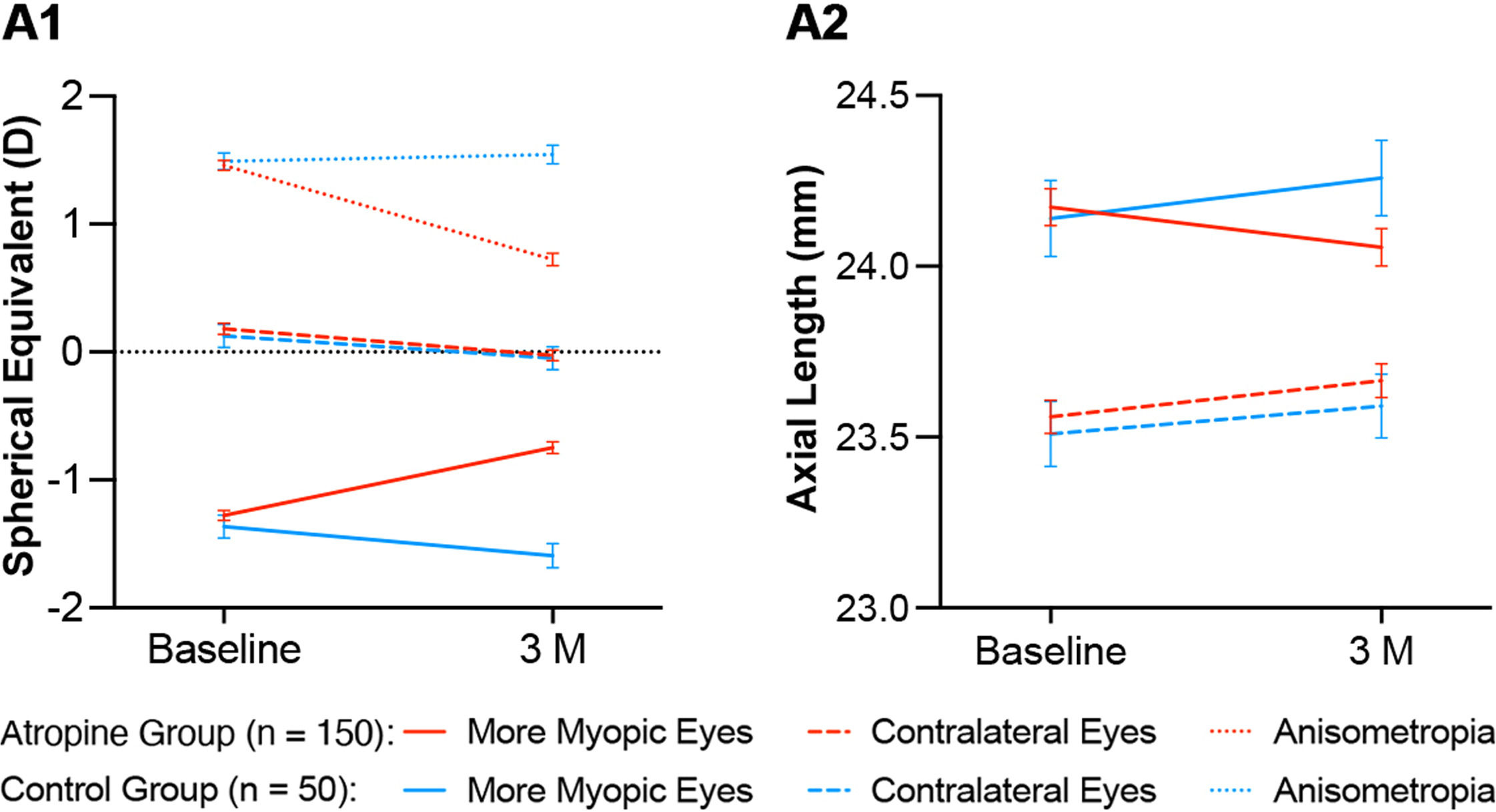
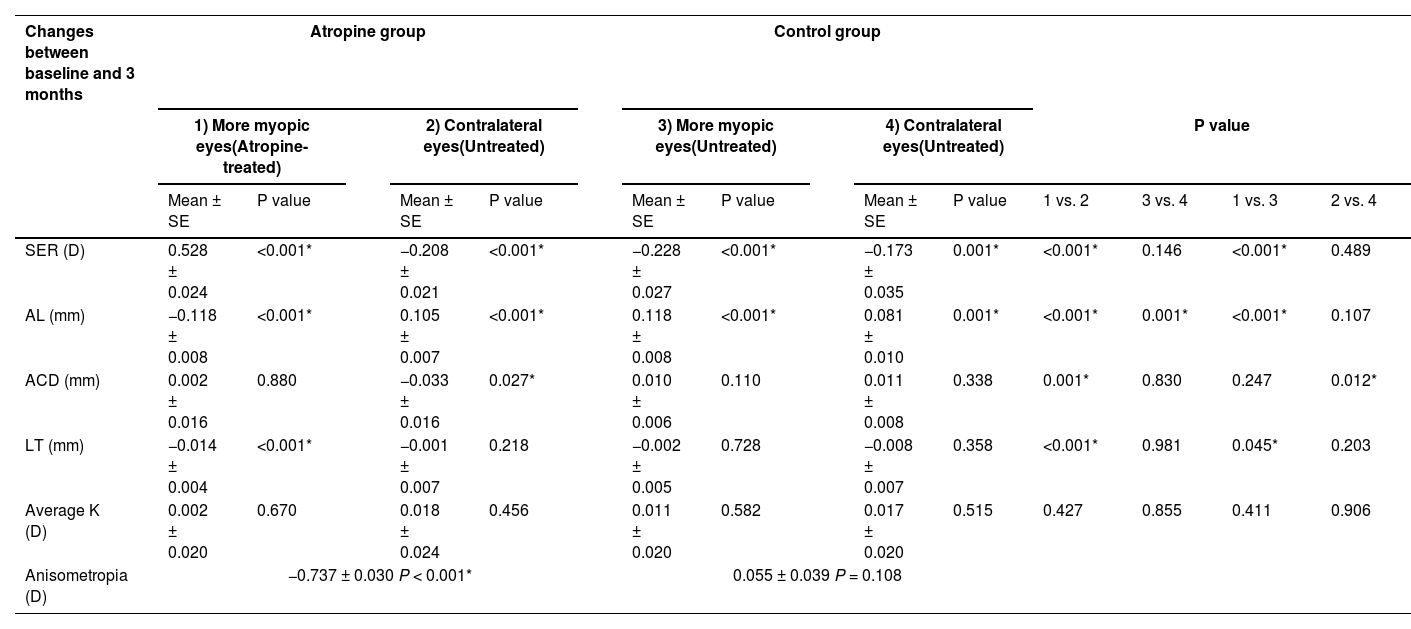
In the atropine group, anisometropia decreased by 0.737 ± 0.030 D (P < 0.001), with a hyperopic shift of 0.528 ± 0.024 D (P < 0.001) in the more myopic eyes (atropine-treated eyes) and a myopic shift of −0.208 ± 0.021 D (P < 0.001) in the contralateral eyes at 3 months. Concomitantly, the AL in the atropine-treated eyes decreased by 0.118 ± 0.008 mm (P < 0.001), while the AL in the contralateral eyes increased by 0.105 ± 0.007 mm (P < 0.001) at 3 months. In addition, the LT of the atropine-treated eyes and the ACD of the contralateral eyes of the atropine group decreased by 0.014 ± 0.004 mm (P < 0.001) and 0.033 ± 0.016 mm (P = 0.027) respectively at 3 months. In the control group, the myopia progression of the more myopic eyes and the contralateral eyes showed no significant difference between baseline and 3 months (−0.228 ± 0.027 D vs. −0.173 ± 0.035 D, P = 0.146), and the anisometropia remained unchanged during this period (0.055 ± 0.039, P = 0.108). However, in the control group, the AL of the more myopic eyes increased more than that of the contralateral eyes at 3 months (0.118 ± 0.008 mm vs. 0.081 ± 0.010 mm, P = 0.001).
A comparison of the atropine group and the control group was made at 3 months. The changes in SER, AL and LT of the more myopic eyes between the two groups were of significant differences (P < 0.001, P < 0.001, and P = 0.045, respectively). The changes of SER and AL of the contralateral eyes between the two groups showed no significant difference (P = 0.489 and 0.107, respectively), while the change of ACD of the contralateral eyes between the two groups was of significant difference (P = 0.012). These results have been summarized in Table 2 and Fig. 2.
Within and between group analysis of changes in ophthalmic parameters between baseline and 3 months.
ACD: anterior chamber depth, AL: axial length, K: corneal power, LT: lens thickness, SER: spherical equivalent refraction.
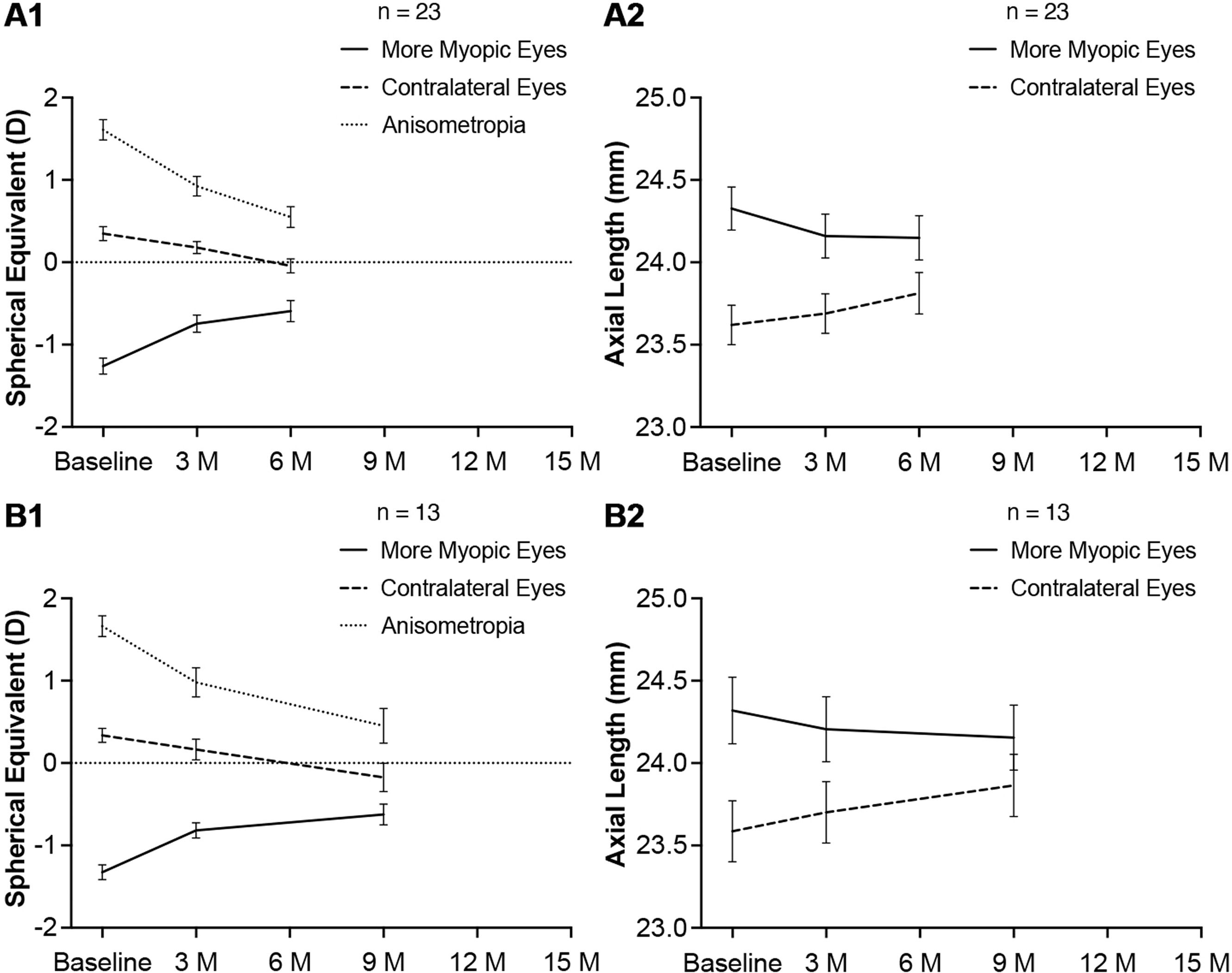
Twenty three subjects continued 1 % atropine eye gel treatment up to 6 months and visited at baseline, 3 months and 6 months. Anisometropia decreased by 0.685 ± 0.048 D (P < 0.001) and 1.060 ± 0.058 D (P < 0.001) at 3 months and 6 months respectively. The reduction of anisometropia between 3 months and 6 months was statistically significant (0.375 ± 0.063 D, P < 0.001). In the atropine-treated eyes, the hyperopic shift was 0.516 ± 0.060 D (P < 0.001) at 3 months. From 3 months to 6 months, the hyperopic shift was small but statistically significant (0.152 ± 0.054 D, P = 0.005). There was a pronounced reduction in AL at 3 months (−0.167 ± 0.016 mm, P < 0.001). From 3 months to 6 months, the reduction of AL was not statistically significant (−0.011 ± 0.011 mm, P = 0.357). In the untreated eyes, there was a steady myopia progression from baseline to 6 months, as demonstrated by the changes in SER and AL. These results have been illustrated in Fig. 3 A1, A2.
Thirteen subjects continued 1 % atropine eye gel treatment up to 9 months and visited at baseline, 3 months and 9 months. Anisometropia decreased by 0.683 ± 0.099 D (P < 0.001) and 1.212 ± 0.153 D (P < 0.001) at 3 months and 9 months respectively. The reduction of anisometropia between 3 months and 9 months was statistically significant (0.529 ± 0.102 D, P < 0.001). In the atropine-treated eyes, the hyperopic shift was 0.510 ± 0.067 (P < 0.001) at 3 months. From 3 months to 9 months, the hyperopic shift was small but statistically significant (0.192 ± 0.086 D, P = 0.025). There was a pronounced reduction in AL at 3 months (−0.114 ± 0.025 mm, P < 0.001). From 3 months to 9 months, the reduction of AL was small but statistically significant (−0.051 ± 0.023 mm, P = 0.026). In the untreated eyes, there was a steady myopia progression from baseline to 9 months, as demonstrated by the changes in SER and AL. These results have been illustrated in Fig. 3 B1, B2.
Five subjects and two subjects who continued 1 % atropine eye gel treatment up to 15 months and 27 months respectively were not evaluated because of the limited sample sizes.
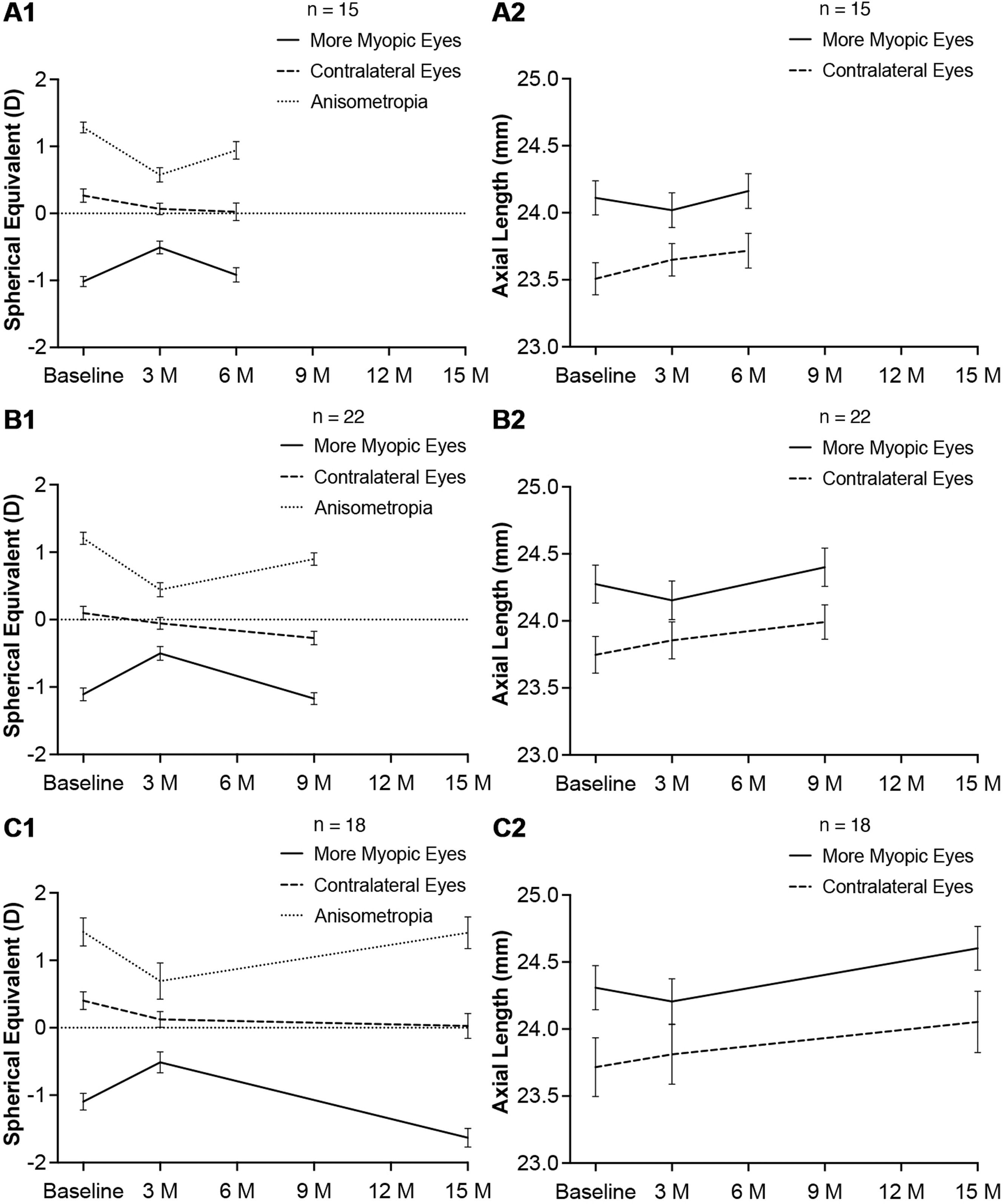
Changes in SER and AL after ceasing topical atropineFifteen subjects stopped 1 % atropine eye gel application since the fourth month and visited at baseline, 3 months and 6 months. The reduction of anisometropia was 0.708 ± 0.082 D (P < 0.001) at 3 months. After ceasing topical atropine for 3 months, the anisometropia increased by 0.367 ± 0.100 D (P = 0.003), though the reduction of anisometropia was still statistically significant as compared to the baseline (0.342 ± 0.142 D, P = 0.016). In the atropine-treated eyes, the hyperopic shift was 0.508 ± 0.066 D (P < 0.001) and the reduction of AL was 0.092 ± 0.026 mm (P < 0.001) at 3 months, and there were marked decrease of SER and increase of AL (−0.408 ± 0.079 D, P < 0.001 and 0.143 ± 0.018 mm, P < 0.001, respectively) when atropine was stopped for 3 months. The SER and AL in atropine-treated eyes showed no significant differences between baseline and 6 months (−1.017 ± 0.076 D vs. −0.917 ± 0.106 D, P = 0.207 and 24.111 ± 0.128 mm vs. 24.163 ± 0.130 mm, P = 0.085, respectively). When topical atropine was stopped for 3 months, myopia progressed more in the previously atropine-treated eyes than in the contralateral eyes (SER: −0.408 ± 0.079 D vs. −0.042 ± 0.087 D, P = 0.003 and AL: 0.143 ± 0.018 mm vs. 0.067 ± 0.025 mm, P = 0.009). These results have been illustrated in Fig. 4 A1, A2.
Twenty two subjects stopped 1 % atropine eye gel application since the fourth month and visited at baseline, 3 months and 9 months. The reduction of anisometropia was 0.761 ± 0.072 D (P < 0.001) at 3 months. After ceasing topical atropine for 6 months, the reduction of anisometropia was still statistically significant (0.307 ± 0.090 D, P = 0.001). In the atropine-treated eyes, the hyperopic shift was 0.608 ± 0.058 D (P < 0.001) and the reduction of AL was 0.121 ± 0.021 mm (P < 0.001) at 3 months. When atropine was stopped for 6 months, there were marked decrease of SER and increase of AL (−0.670 ± 0.078 D, P < 0.001 and 0.248 ± 0.032 mm, P < 0.001, respectively). The SER in atropine-treated eyes showed no significant differences between baseline and 9 months (−1.018 ± 0.095 D vs. −1.170 ± 0.087 D, P = 0.322). However, the AL in atropine-treated eyes elongated by 0.126 ± 0.027 mm (P < 0.001) at 9 months as compared to the baseline. When topical atropine was stopped for 6 months, myopia progressed more in the previously atropine-treated eyes than in the contralateral eyes (SER: −0.670 ± 0.078 D vs. −0.216 ± 0.088 D, P = 0.001 and AL: 0.248 ± 0.032 mm vs. 0.137 ± 0.030 mm, P = 0.001). These results have been illustrated in Fig. 4 B1, B2.
Eighteen subjects stopped 1 % atropine eye gel application since the fourth month and visited at baseline, 3 months and 15 months. The reduction of anisometropia was 0.730 ± 0.121 D (P < 0.001) at 3 months. After ceasing topical atropine for 12 months, the anisometropia increased to the initial state (baseline vs. 12 months: 1.420 ± 0.207 D vs. 1.409 ± 0.235 D, P = 0.938). In the atropine-treated eyes, the hyperopic shift was 0.583 ± 0.072 D (P < 0.001) and the reduction of AL was 0.103 ± 0.027 mm (P < 0.001) at 3 months. When atropine was stopped for 12 months, there were marked decrease of SER and increase of AL in previously atropine-treated eyes (−1.118 ± 0.103 D, P < 0.001 and 0.406 ± 0.040 mm, P < 0.001, respectively). As compared to the baseline, myopia progressed remarkably at 15 months (ΔSER: −0.535 ± 0.110 D, P < 0.001 and ΔAL: 0.295 ± 0.045 mm, P < 0.001, respectively). When topical atropine was stopped for 12 months, myopia progressed more in the previously atropine-treated eyes than in the contralateral eyes (SER: −1.118 ± 0.103 D vs. −0.096 ± 0.096 D, P < 0.001 and the AL: 0.406 ± 0.040 mm vs. 0.166 ± 0.048 mm, P = 0.003). These results have been illustrated in Fig. 4 C1, C2.
Five subjects who discontinued atropine use since the fourth month and had a follow-up of 27 months were not evaluated because of the limited sample size.
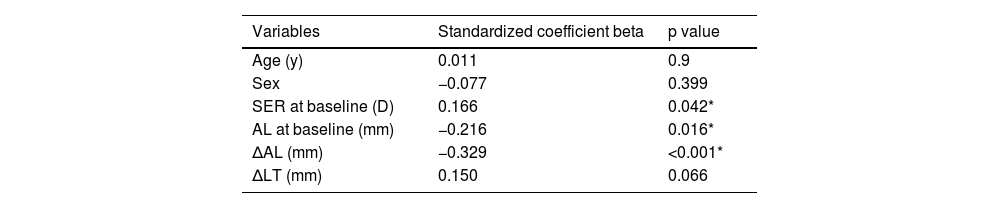
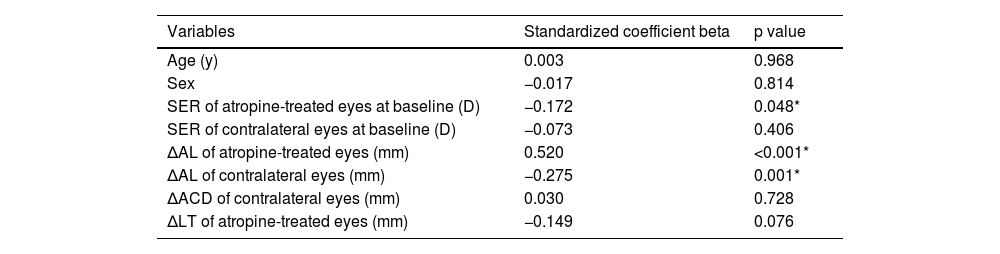
Associated factorsTo determine which factors were associated with the hyperopic shift in the atropine-treated eyes and the reduction of anisometropia, multiple linear regression analyses were performed. The change of SER in the atropine-treated eyes between baseline and 3 months was positively associated with the initial SER (standardized coefficient beta = 0.166, P = 0.042) and negatively associated with the initial AL and the change of AL (standardized coefficient beta = −0.216, P = 0.016 and standardized coefficient beta = −0.329, P < 0.001, respectively, Table 3). The change of anisometropia in the atropine group from baseline to 3 months was negatively associated with the initial SER in the atropine-treated eyes and the change of AL in the contralateral eyes (standardized coefficient beta = −0.172, P = 0.048 and standardized coefficient beta = −0.275, P = 0.001, respectively) and positively associated with the change of AL in the atropine-treated eyes (standardized coefficient beta = 0.520, P < 0.001, Table 4).
Multiple linear regression analysis of associated factors with the change of SER in the atropine-treated eyes from baseline to 3 months (n = 150).
| Variables | Standardized coefficient beta | p value |
|---|---|---|
| Age (y) | 0.011 | 0.9 |
| Sex | −0.077 | 0.399 |
| SER at baseline (D) | 0.166 | 0.042* |
| AL at baseline (mm) | −0.216 | 0.016* |
| ΔAL (mm) | −0.329 | <0.001* |
| ΔLT (mm) | 0.150 | 0.066 |
R2 = 0.170.
AL: axial length, LT: lens thickness, SER: spherical equivalent refraction.
Multiple linear regression analysis of associated factors with the change of anisometropia in the atropine group from baseline to 3 months (n = 150).
R2 = 0.316.
ACD: anterior chamber depth, AL: axial length, LT: lens thickness, SER: spherical equivalent refraction.
The results of our study indicated that unilateral application of 1 % atropine eye gel weekly in the more myopic eyes led to a reduction in anisometropia in children, which was mainly achieved by reducing the AL of the more myopic eyes. The multiple linear regression analysis showed that the reduction of anisometropia in the atropine group was associated with the decrease of AL in the atropine-treated eyes and the increase of AL in the contralateral eyes. The increase of SER in the atropine-treated eyes was associated with the decrease of AL in those eyes. Although the LT in the atropine-treated eyes thinned by 0.014 ± 0.004 mm (P < 0.001), which might lead to the reduction of myopia, the multiple linear regression analysis did not show any association between the change of LT and the change of SER (P = 0.066). The lack of association between these two parameters might be due to the minor change of LT, which might require larger sample size to realize statistical significance in the association between them. A previous study indicated that atropine could alter children’s relative peripheral refraction to become less hyperopic or even myopic, thus controlling axial elongation and myopia progression.21 However, the mechanism of atropine causing relative peripheral myopia remains unclear. It has been demonstrated that atropine could increase subfoveal choroidal thickness in the eyes of myopic children,22 and could alleviate the subfoveal choroidal thinning caused by hyperopic defocus.23,24 Whether atropine increases the choroidal thickness more in subfovea than in perifovea, which may lead to relative peripheral myopia, needs further investigation.
In addition, the multiple linear regression analysis revealed that less initial myopia in the treated eye was associated with greater reduction of myopia in that eye and greater reduction of anisometropia after atropine application for 3 months. This indicated that early application of 1 % atropine in the more myopic eyes of anisometropic children when they just started to develop myopia might be more effective for reducing anisometropia than delayed application. Our finding that atropine was more effective in reducing myopia in lower initial myopia was in line with a recent study, in which children with low myopia experienced better treatment effect of weekly bilateral 1 % atropine than those with moderate myopia during a 2-year period.25 This was different from the observation on the effects of orthokeratology in retarding myopia progression and axial elongation in anisometropic children. Studies have found that orthokeratology was more effective at retarding myopia progression in the more myopic eyes than in the less myopic eyes of myopic anisometropic children.13,15 In unilateral myopic children receiving monocular orthokeratology treatment, children with high anisometropia showed more reduction in AL difference than those with low anisometropia.14 Orthokeratology lenses can induce myopic defocus in the periphery,26 and more severe myopia is corrected by orthokeratology lenses with a flat base curve, more peripheral myopic defocus is induced, thus exerting a greater suppressive effect on AL growth.27 The difference between the efficacy of atropine and orthokeratology on anisometropia control merits future investigation. Atropine might have more flexibility for treating mixed anisometropia with the myopic eyes of too low myopia (SER > −0.75 D) to be corrected by orthokeratology.
The myopia progression of the contralateral eyes between the atropine group and the control group was not statistically different. This indicated that 1 % atropine eye gel application in one eye would not alter myopia progression in the contralateral eye. However, atropine application led to a slight decrease of ACD in the contralateral eyes in atropine group, which was statistically significant as compared to that in the contralateral eyes in control group. This might be resulted from the over stress of the contralateral eye in atropine group. The over stressed ciliary body in the contralateral eye in atropine group might not be sufficiently paralyzed by the regular rapid cycloplegic regime, which was reported to be adequate for cycloplegia in normal conditions with balanced bilateral eye use.19,20 It has been demonstrated that accommodation can led to the decrease of ACD.28 However, due to the retrospective nature of this study, it was impossible to measure accommodation and near vision of each eye before and after treatment.
Changes in SER and AL after continuous atropine eye gel application for 6 months and 9 months were investigated. The results indicated that continuous unilateral 1 % atropine eye gel application could bring continuous reduction of anisometropia during the observation periods, with the principal efficacy seen in the first 3 months, which was in line with the findings of a previous study concerning myopia control.10 Changes in SER and AL after ceasing atropine eye gel application for 3 months, 6 months and 12 months were also investigated. It has been reported that the annualized mean SER change for 6 to 16 years Chinese myopes was 0.76 D,29 and the annualized mean axial elongation for 9 years Chinese myopes was 0.41 mm.30 When topical atropine was stopped for 3 months, the myopia progression (−0.408 ± 0.079 D) and axial elongation (0.143 ± 0.018 mm) in the previously atropine-treated eyes were more than a quarter of the previous reported annualized mean SER change and mean axial elongation respectively. This suggested that the previous treated eyes might have an accelerated rate of myopia progression and axial elongation after ceasing atropine in short-term. A prospective study with more consecutive follow-up time points may verify this speculation in the future. After ceasing topical atropine for 6 months, the anisometropia increased, but the reduction of anisometropia was still statistically significant as compared to the baseline. There were marked increases of myopia and AL in previously atropine-treated eyes, with the SER returning to the baseline level and the AL even surpassing the baseline level. After ceasing topical atropine for 12 months, the anisometropia reverted to the initial state and there were increases of myopia and AL in previously atropine-treated eyes as compared to the baseline. These suggested that the reduction of anisometropia could persist for at least 6 months after atropine cessation, indicating minimal rebound effect.
The most common side effects of topical atropine include photophobia and blurring of near vision. Without photochromic glasses, the incidence of photophobia after 1 % atropine application ranged from 62.12 % to 100 %.18,31,32 In our documented patient records, symptoms of photophobia typically resolved within 2–3 days, and wearing photochromic glasses effectively alleviated discomfort. None of these subjects refused treatment because of near vision problems, possibly due to the monocular application and the contralateral eye could accommodate well for clear near vision. Besides, no allergic reactions or discomfort was reported. However, the tolerance rate of 1 % atropine was not formally assessed, because this was a retrospective study and children with inconsistent usage of the atropine eye gel were excluded at the beginning.
The current study has several limitations. First, its retrospective design inherently restricted our ability to standardize treatment protocols and follow-up intervals, resulting in a 31.3 % dropout rate (47/150) at the 6-month follow-up. Attrition primarily occurred due to patients switching to alternative myopia control methods, insufficient medical documentation, or non-comparable AL data. Among retained subjects, irregular visit intervals led to small sample sizes at extended follow-up points, and selection bias might affect the interpretation of these findings. Second, the usefulness of 1 % unilateral atropine on anisometropia control in the eyes with myopia higher than −3.00 D or in children younger than 6 years or older than 12 years was uncertain based on the current study. Therefore, a randomized prospective intervention study with longer follow-up period and broader SER range is warranted. Furthermore, there was 24 % subjects had bilateral myopia in the present study. Various choices of myopia prevention methods have been offered to children with anisometropia and bilateral myopia. However, since the myopia control treatment lack health insurance coverage, some subjects are unwilling to accept them due to cost concerns. These 24 % subjects were those who chose unilateral 1 % atropine application in the more myopic eye with or without bilateral common eye glasses wearing for vision correction. Indeed, in subjects with bilateral myopia, the myopia control of the contralateral eyes should not be neglected, and unilateral atropine application in the more myopic eye plus bilateral peripheral defocus lenses wearing or orthokeratology lens wearing may serve this end. A future study combining the application of unilateral atropine and bilateral peripheral defocus lenses or orthokeratology lens is warranted to determine their comprehensive effects on anisometropia control.
In conclusion, weekly unilateral application of 1 % atropine in the more myopic eye could reduce anisometropia mainly by reducing the AL of that eye and would not alter myopia progression in the contralateral eye in Chinese children with low myopia during a 3-month observation period. Less initial myopia in the treated eye was associated with greater reduction of myopia in that eye and greater reduction of anisometropia after atropine treatment for 3 months. Continuous unilateral atropine application could bring continuous reduction of anisometropia over the observation period of 9 months, with the principal efficacy occurring in the first 3 months. After atropine cessation, the reduction of anisometropia could persist for at least 6 months, indicating minimal rebound effect. Therefore, unilateral application of 1 % atropine might be a good choice for anisometropic children with low myopia who are not willing to accept optical correction. Nonetheless, findings beyond 3 months should be considered preliminary due to the limited sample size, and further randomized clinical trials with longer study periods are required to confirm these findings and to investigate the optimum atropine treatment regime for anisometropia control.
FundingThis work was supported by Shenzhen Science and Technology Program (No. JCYJ20210324134004013) and Shenzhen Special Fund for Sustainable Development Science and Technology (No. KCXFZ20211020170007011).
The authors declare no conflict of interest.
The data that support the findings of this study are available from the corresponding author upon reasonable request.