Antimicrobial, cytotoxicity and clinical performance of a new soft contact lens multi-purpose solution (COMPLETE® RevitaLens) based on polyquaternium-1 and alexidine dihydrochloride (NuMPS) was evaluated.
MethodsAntimicrobial efficacy was assessed according to ISO 14729 for both biocidal and regimen performance against bacteria and fungi. Acanthamoeba efficacy was tested along with ability to retain antimicrobial activity on partial evaporation. In vitro cytotoxicity of NuMPS and OPTI-FREE® RepleniSH® MPS (MPS-3) was assessed based on ISO 10993-5 and United States Pharmacopeia (USP) methods. In addition, a 3 month, double-masked, parallel group clinical trial comparing safety and acceptability with respect to MPS-3 was conducted with 4 silicone hydrogel (SiHy) and FDA Group IV lens types.
ResultsNuMPS showed broad antimicrobial efficacy, including Acanthamoeba, giving a 3–4 log10 reduction in viability after 6 hours contact time. NuMPS also passed ISO 14729 regimens with SiHy and etafilcon lenses for bacteria, fungi and also Acanthamoeba. The cytotoxicity of NuMPS was equivalent or better compared to MPS-3. In the clinical trial, there was no statistically significant between-group difference in corneal staining (p > 0.05). Patients using MPS-3 had more adverse events than patients using NuMPS: 11.8 % (11/93) versus 2.8 % (5/177), respectively, (p < 0.05). There were no differences noted in cleanliness or wearing comfort (p > 0.05).
ConclusionTaken together, the results of these studies indicate that the NuMPS is a novel and effective soft contact lens care solution.
Se evaluó el rendimiento clínico, antimicrobiano y citotoxicidad de una nueva solución multiusos para lentes de contacto blandas (COMPLETE®RevitaLens) basada en policuaternio-1 y diclorhidrato de alexidina (NuMPS).
MétodosSe evaluó la eficacia antimicrobiana en concordancia con ISO 14729, tanto en términos de rendimiento biocida como en regimenes de higiene contra bacterias y hongos. Se comprobó la eficacia contra Acanthamoeba, así como su habilidad para mantener la actividad antimicrobiana ante una evaporización parcial. Se evaluó la citotoxicidad in vitro de NuMPS y de OPTI-FREE® RepleniSH® MPS (MPS-3). Además, se llevó a cabo un ensayo clínico simultáneo, a doble ciego y de 3 meses de duración que comparó la seguridad y la aceptabilidad de MPS-3 para 4 lentes de hidrogel de silicona (SiHy) y lentes del grupo IV de la FDA.
ResultadosLa solución NuMPS mostró una eficacia antimicrobiana amplia, incluso contra Acanthamoeba, y produjo una reducción logarítmica de 3–4 (log10) en la viabilidad tras 6 horas de contacto. Dicha solución también superó ISO 14729 en regímenes con SiHy y lentes etafilcon para bacterias, hongos y Acanthamoeba. La citotoxicidad de NuMPS fue equivalente o mejor que la de MPS-3. En el ensayo clínico, no se obtuvieron diferencias estadísticamente significativas entre los grupos en cuanto a tinción corneal (p > 0,05). Los pacientes que utilizaban MPS-3 presentaron más fenómenos adversos que los que usaron NuMPS: 11,8 % (11/93) frente a 2,8 % (5/177), respectivamente (p < 0,05). No se observaron diferencias en higiene ni en comodidad durante su uso (p > 0,05).
ConclusionesEn su conjunto, los resultados de estos estudios indican que la NuMPS es una nueva solución eficaz para el cuidado de lentes de contacto blandas.
Multi-purpose solutions (MPS) represent the majority of systems used for the care of soft contact lenses.1 They comprise a single solution for the rinsing, disinfection and storage of lenses and are typically composed of a preservative, buffer system and other agents to aid lens comfort and cleaning.2 The ability of these components to achieve sufficient antimicrobial efficacy is fundamental to safe contact lens use.
Microbial keratitis is a rare but significant risk among soft contact lens wearers with a reported incidence of 4 cases per 10,000 users per year.3 In keratitis, due to the free-living amoebae Acanthamoeba, almost 90 % of cases occur in contact lens wearers and the reported incidence varies from 1–2 cases per million in the USA or 17–20 in the UK.4 Accordingly, the use of MPS with effective disinfectant properties, in conjunction with good compliance to lens care hygiene, reduces the incidence of microbial keratitis through the prevention or inhibition of potentially pathogenic organism growth on the lens surface and within the lens storage case.5–9 The majority of MPS utilize the preservative polyhexamethylene biguanide (PHMB) but others use the quaternary ammonium compound polyquaternium-1 (PQ-1) alone or in combination with the amido amine myristamidopropyl dimethylamine (MAPD).2 According to international standards, including ISO 14729 and FDA 510(k) guidance, contact lens solutions are required to exhibit antimicrobial efficacy against select reference strains of common bacterial and fungal ocular pathogens.10,11 However, the rigor of such testing has been questioned following the association of two MPS with a significant rise in cases of keratitis due to the filamentous mould Fusarium and to Acanthamoeba.12–15
The purpose of this review is to evaluate the antimicrobial, cytotoxicity and clinical performance of a new MPS (NuMPS: COMPLETE® RevitaLens) based on the novel dual disinfection combination of PQ-1 and the bis-biguanide alexidine.
Materials and methodsTest organisms and solutionsFor the microbiological analysis, the following organisms were studied: Pseudomonas aeruginosa (ATCC 9027), Serratia marcescens (ATCC 13880), Staphylococcus aureus (ATCC 6538), Candida albicans (ATCC 10231), Fusarium solani (ATCC 36031) and Acanthamoeba castellanii (ATCC 50370). Test solutions studied are given in Table 1.
Solutions studied
| Code | Commercial name | Composition | Manufacturer |
| MPS-1 | AQuify® Multi-Purpose Solution | Sorbitol, Tromethamine, Pluronic F127, sodium phosphate dihydrogen, Dexpant 5 (Despanthenol), EDTA, PHMB (0.0001 %) | Ciba Vision |
| MPS-2 | ReNu® Multi-Purpose Solution | Hydroxyalkylphosphonate, Poloxamine, PHMB (0.0001 %), boric acid, EDTA, sodium borate, sodium chloride | Bausch & Lomb |
| MPS-3 | OPTI-FREE® RepleniSH® Multi-Purpose Solution | Sodium citrate, boric acid/borate, propylene glycol, TearGlyde™ (Tetronic® 1304 + C-9 ED3A), polyquaternium-1 (0.001 %), myristamidopropyl dimethylamine (0.0005 %) | Alcon Laboratories Inc. |
| MPS-4 | MeniCare Soft (Epica Cold) Multi-Purpose Solution | Macrogolglycerol hydroxysterate 60, EDTA, tonicity agents, propylene glycol, PHMB (0.0001 %) | Menicon |
| MPS-5 | ReNu with MoistureLoc® Multi-Purpose Solution | Boric acid/borate, Alexidine (0.00045 %), Hydranate, polyquarternium-10, Poloxamine, Poloxamer 407 | Bausch & Lomb |
| Perox | Ciba Clear Care® | 3 % H2O2, surfactant and platinum neutralizing disc | Ciba Vision |
| NuMPS | COMPLETE® RevitaLens | Sodium citrate, boric acid/borate, polyquaternium-1 (0.0003 %), alexidine dihydrochloride (0.00016 %), EDTA, Tetronic 904 | Abbott Medical Optics Inc |
For the bacteria and fungi, stand-alone (biocidal) and regimen assays were performed according to ISO 14729.10 Briefly, in the stand-alone procedure the test solutions were challenged with 1 × 106/ml organism and the number of survivors determined by culture viability at 0 and 6 hr using the WASP Spiral Plater and ProtoCOL colony counter system.16 At each time point the test samples were first diluted 1:10 into DifcoW Letheen Broth neutralizing broth before determining the number of surviving organisms as colony forming units (cfu).
In the regimen assay, four sets of the following lenses were tested per organism: Soflens® 38 (polymacon, Bausch & Lomb), Acuvue® 2 (etafilcon A, Vistakon), Acuvue® Advance® (galyfilcon, Vistakon), O2Optix®/AirOptix® (lotrafilcon B, CIBA Vision), PureVision® (balafilcon A, Bausch & Lomb) and Biofinity® (comfilcon A, CooperVision) lenses. Although not required by the protocol, organic soil was included in the organism challenge inoculum and comprised a final concentration of 300–3000 heat killed Saccharomyces cerevisiae (ATCC 9763) in 0.003 % heat-inactivated fetal bovine serum. Briefly, four lenses of each test type were inoculated with approximately 2 × 106 organisms and left to adhere for 5 minutes. Each lens was then subjected to a “No Rub but Rinse” with NuMPS using 5 seconds rinse per side followed by a 6 hour soaking time in 3 ml of the solution in a contact lens storage cases (ABS/polypropylene, Abbott Medical Optics Inc.). At the end of the regimen procedure, each test lens and soaking solution was neutralized in Difco™ Letheen Broth followed by filtration and culture of the lens and membrane to determine the presence of surviving organisms (cfu).
Acanthamoeba trophozoite and cyst biocidal and regimen assays were performed as described previously.5,17,18 For the regimen assay, lenses were inoculated with 4 × 104 trophozoites or cysts per lens and subjected to a “Rub and Rinse” regimen with the NuMPS using 4 seconds rub and 5 seconds rinse per side, followed by a 6 hour soaking time in 3 ml of the solution in a contact lens storage cases (ABS/polypropylene, Abbott Medical Optics Inc.). Remaining viable Acanthamoeba on the lenses and in the soak solution were determined by culture assay as described previously.5
The capacity for the test solutions to induce Acanthamoeba trophozoite encystment during incubation in the solutions was performed according to the method described by Kilvington et al. using Complete® MoisturePlus® MPS as the positive control solution. This MPS formulation has previously been shown to induce significant trophozoite encystment.19
Loss of MPS antimicrobial efficacy following partial evaporation was also studied. The solutions were evaporated under a stream of air to 2× and 4× concentration by weight, challenged with F. solani or A. castellanii trophozoites and the level of organism kill determined after 6 hr contact time.
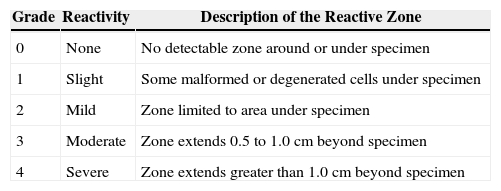
Cytotoxicity studiesIn vitro cytotoxicity potential of NuMPS and MPS-3 (OPTI-FREE® RepleniSH® Multi-Purpose Solution) was evaluated according to USP < 87 > and ISO 10993-5.11,20,21 Five soft contact lens types were studied: Soflens® 38 (polymacon, Bausch & Lomb), Acuvue Advance® (galyfilcon A, Vistakon), O2Optix® (lotrafilcon B, CIBA Vision), PureVision® (balafilcon A, Bausch & Lomb) and Biofinity® (comfilcon A, CooperVision) lenses. Briefly, the lenses were soaked in 100 ml of the test solutions, in triplicate, for four days. Confluent monolayers of L929 mouse fibroblasts were then exposed to treated contact lenses for 24 hours and scored for reactivity according to USP Direct Contact Test criteria (Table 2).21 Polypropylene pellets and latex rubber served as negative and positive controls, respectively.
USP in vitro cytotoxicity direct contact test criteria
| Grade | Reactivity | Description of the Reactive Zone |
| 0 | None | No detectable zone around or under specimen |
| 1 | Slight | Some malformed or degenerated cells under specimen |
| 2 | Mild | Zone limited to area under specimen |
| 3 | Moderate | Zone extends 0.5 to 1.0 cm beyond specimen |
| 4 | Severe | Zone extends greater than 1.0 cm beyond specimen |
A three-month, double-masked, parallel group clinical trial comparing the safety and acceptability (comfort and lens cleanliness) of the NuMPS (test) to MPS-3 (control) was conducted with four types of silicone hydrogel contact lenses: Acuvue® Advance® (galyfilcon A, Vistakon), PureVision® (balafilcon A, Bausch & Lomb), O2Optix® (lotrafilcon B, CIBA Vision), Biofinity® (comfilcon A, CooperVision) and a FDA Group IV material (e.g., Acuvue® 2, [etafilcon A Vistakon]). Subjects were enrolled in a 2:1 ratio (test vs. control) for each lens material. Of the 270 subjects enrolled, 177 were assigned to the test regimen and 93 to the control. In the test group the subjects used a “Rub and Rinse” regimen in their lens care whereas for the control a “No Rub but Rinse” regimen was used. Safety was primarily assessed through slit-lamp observations and the occurrence of adverse events (e.g. keratitis, hypersensitivity or visual acuity loss).22 All slit lamp evaluation findings were graded on a 0 to 4 scale, where 0 = None, 1 = Trace, 2 = Mild, 3 = Moderate, and 4 = Severe. Findings were categorized as: corneal edema, corneal neovascularization, corneal staining, bulbar hyperemia, palpebral conjunctiva and other findings. Acceptability was assessed via lens cleanliness (lens deposition as described below) and lens wearing comfort rated on a scale from 0–10 (0 = intolerable and 10 = cannot feel lens) compared to baseline assessments.
Protein and lipid deposit assessment of worn lensesLenses worn in a daily mode using either the test (NuMPS) or control multipurpose solution (MPS-3: see above section, Clinical investigation) were assessed at the end of a wear period (2 wks or 30 days, depending on lens type): n > 30/15 for test/control lenses for protein (left lens) or lipid (right lens) analysis for each lens type tested in the clinical investigation (see above section). All involved parties (including the analyst) were masked regarding control and test lenses. Protein analysis was by a modified Lowry technique utilizing acetonitrile:water (1:1) containing 0.1 % trifluoroacetic acid to extract protein from lenses.23 Lipid analysis involved extracting lipids from lenses with toluene:isopropanol (4:1), followed by deposition of the extracted lipids on a Teflon circular membrane (6 mm diameter) in a well of a 96-well plate (glass) by air evaporation.24,25 Mid-infrared transmission analysis of the Teflon membrane with deposited lipids, based upon functional groups specific to lipid types, was done using a Nicolet Avatar-370 Fourier Transform Infrared Spectrometer. Glyceryl tristearate (99 %, Aldrich, USA) was used to prepare standards for the analysis.
Statistical analysisCochran-Mantel-Haenszel test was used to compare difference in overall corneal staining between the two regimens and Fisher's exact test for adverse events. General Linear Model analysis was used to analyze acceptability measures for overall cleanliness and lens wearing comfort. Analysis of protein and lipid deposition on lenses was performed using Wilcoxon rank-sum test.
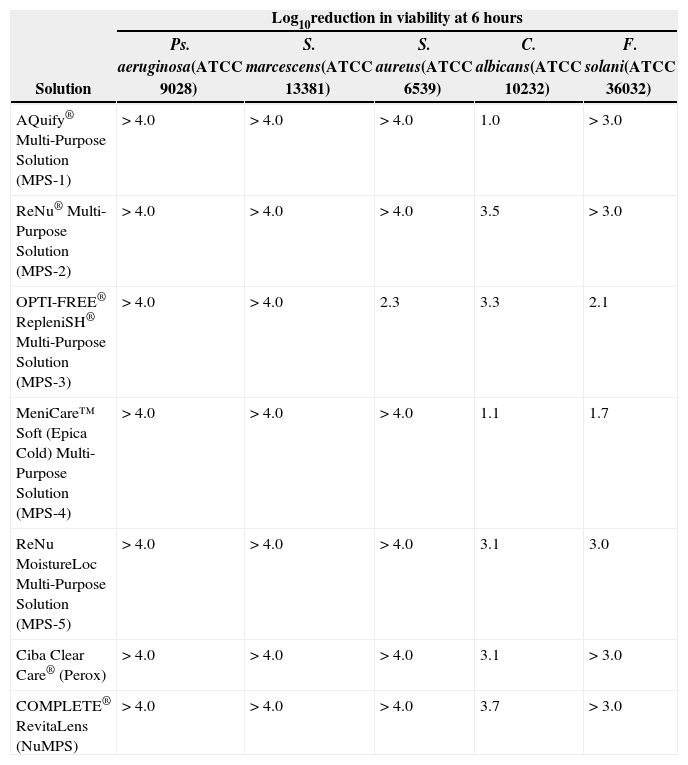
ResultsMicrobiologyThe log10 reduction in bacterial and fungal viability 6 hours exposure time to the test solutions is shown in Table 3. With the exception of MPS-3, which gave a 2.3 log10 kill for S. aureus, all commercial MPS and Perox gave > 4.0 log10 reduction in bacterial viability. A greater range in efficacy was observed with the fungi. For C. albicans, MPS-1 and MPS-4 gave 1.0–1.1 log10 kill respectively and > 3.0 log10 for the other solutions, including Perox. For F. solani, a 1.7–2.1 log10 reduction was obtained for MPS-4 and MPS-3, respectively, and > 3.0 log10 for all other commercial solutions. NuMPS showed > 4.0 and > 3.0 log10 reduction for all bacteria and fungi at 6 hours, respectively.
Log10 reduction in bacterial and fungal viability after 6 hours exposure to test solutions
| Solution | Log10reduction in viability at 6 hours | ||||
| Ps. aeruginosa(ATCC 9028) | S. marcescens(ATCC 13381) | S. aureus(ATCC 6539) | C. albicans(ATCC 10232) | F. solani(ATCC 36032) | |
| AQuify® Multi-Purpose Solution (MPS-1) | > 4.0 | > 4.0 | > 4.0 | 1.0 | > 3.0 |
| ReNu® Multi-Purpose Solution (MPS-2) | > 4.0 | > 4.0 | > 4.0 | 3.5 | > 3.0 |
| OPTI-FREE® RepleniSH® Multi-Purpose Solution (MPS-3) | > 4.0 | > 4.0 | 2.3 | 3.3 | 2.1 |
| MeniCare™ Soft (Epica Cold) Multi-Purpose Solution (MPS-4) | > 4.0 | > 4.0 | > 4.0 | 1.1 | 1.7 |
| ReNu MoistureLoc Multi-Purpose Solution (MPS-5) | > 4.0 | > 4.0 | > 4.0 | 3.1 | 3.0 |
| Ciba Clear Care® (Perox) | > 4.0 | > 4.0 | > 4.0 | 3.1 | > 3.0 |
| COMPLETE® RevitaLens (NuMPS) | > 4.0 | > 4.0 | > 4.0 | 3.7 | > 3.0 |
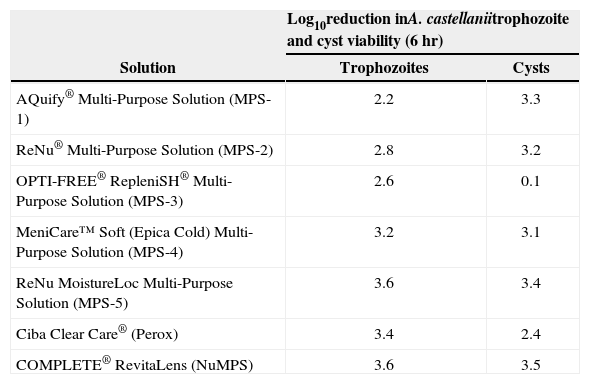
The results for the Acanthamoeba biocidal studies are shown in Table 4. All the solutions showed activity against trophozoites, ranging from 2.2–3.6 log10 kill after 6 hours. The results for the cysts were more variable and ranged from 0.1–2.4 log10 for MPS-3 and Perox, respectively, and > 3.0 log10 kill for the others.
Log10 reduction in A. castellanii trophozoite and cyst viability after 6 hours exposure to test solutions
| Solution | Log10reduction inA. castellaniitrophozoite and cyst viability (6 hr) | |
| Trophozoites | Cysts | |
| AQuify® Multi-Purpose Solution (MPS-1) | 2.2 | 3.3 |
| ReNu® Multi-Purpose Solution (MPS-2) | 2.8 | 3.2 |
| OPTI-FREE® RepleniSH® Multi-Purpose Solution (MPS-3) | 2.6 | 0.1 |
| MeniCare™ Soft (Epica Cold) Multi-Purpose Solution (MPS-4) | 3.2 | 3.1 |
| ReNu MoistureLoc Multi-Purpose Solution (MPS-5) | 3.6 | 3.4 |
| Ciba Clear Care® (Perox) | 3.4 | 2.4 |
| COMPLETE® RevitaLens (NuMPS) | 3.6 | 3.5 |
With the regimen testing, the NuMPS gave < 10 cfu surviving bacteria and fungi on the lenses or in the soaking solution under No Rub but Rinse conditions and < 10 viable Acanthanmoeba trophozoites or cysts when Rub and Rinse was used.
No evidence for trophozoite encystment (0 %–5 %) was observed during 24 hr incubation in any of the test or NuMPS solutions. The control encystment solution of Complete® MoisturePlus® MPS gave 30 % encystment.
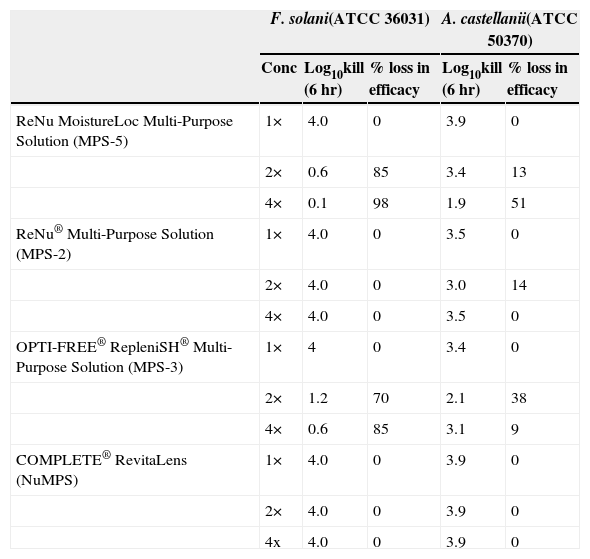
The efficacy of test solutions against F. solani and A. castellanii trophozoites after evaporation to 2× and 4× concentration and a 6 hr disinfection time is shown in Table 5. For F. solani, MPS-5 and MPS-3 lost 85 %–98 % and 70 %–85 % of activity at 2× and 4× concentration, respectively. No loss in efficacy on concentration was found with MPS-2 or NuMPS. For A. castellanii trophozoites, MPS-5 showed a 13 %–51 % reduction in efficacy and MPS-3 a 38 %–9 % loss at 2× concentration and 4×, respectively. MPS-2 gave a 14 % loss in activity at 2× concentration and 0 % at 4×. No loss in trophozoite efficacy on concentration was found with NuMPS.
Effect of evaporation on F. solani and A. castellanii trophozoite disinfection
| F. solani(ATCC 36031) | A. castellanii(ATCC 50370) | ||||
| Conc | Log10kill (6 hr) | % loss in efficacy | Log10kill (6 hr) | % loss in efficacy | |
| ReNu MoistureLoc Multi-Purpose Solution (MPS-5) | 1× | 4.0 | 0 | 3.9 | 0 |
| 2× | 0.6 | 85 | 3.4 | 13 | |
| 4× | 0.1 | 98 | 1.9 | 51 | |
| ReNu® Multi-Purpose Solution (MPS-2) | 1× | 4.0 | 0 | 3.5 | 0 |
| 2× | 4.0 | 0 | 3.0 | 14 | |
| 4× | 4.0 | 0 | 3.5 | 0 | |
| OPTI-FREE® RepleniSH® Multi-Purpose Solution (MPS-3) | 1× | 4 | 0 | 3.4 | 0 |
| 2× | 1.2 | 70 | 2.1 | 38 | |
| 4× | 0.6 | 85 | 3.1 | 9 | |
| COMPLETE® RevitaLens (NuMPS) | 1× | 4.0 | 0 | 3.9 | 0 |
| 2× | 4.0 | 0 | 3.9 | 0 | |
| 4x | 4.0 | 0 | 3.9 | 0 | |
In vitro cytotoxicity results are described in Table 6. Here NuMPS showed grade 1 (slight) with PureVision® and grade 2 (mild) with Acuvue Advance® and O2Optix® lenses, compared to MPS-3 which exhibited a grade 3 (moderate) for these SiHy lenses. Both solutions scored a grade 2 for Soflens® 38 lenses and grade 3 for Biofinity® lenses.
In vitro cytotoxicity scores for NuMPS and MPS-3 according to USP direct contact test criteria
| Cytotoxicity scores | |||||
| PureVision® | Acuvue® Advance® | O2Optix® | Soflens®38 | Biofinity® | |
| NuMPS | 1 | 2 | 2 | 2 | 3 |
| MPS-3 | 3 | 3 | 3 | 2 | 3 |
| Latex rubber (positive control) | 4 | 4 | 4 | 4 | 4 |
| Polypropylene pellets (negative control) | 0 | 0 | 0 | 0 | 0 |
The overall results indicated no significant difference in corneal staining between the two regimens. Corneal staining incidence at Day 90 was 18 % trace and 2.9 % mild with NuMPS; and 11.9 % trace, 3.6 % mild, and 1.2 % moderate with MPS-3 (p > 0.05). However, the incidence of adverse events in control subjects was significantly greater than the test subjects with 11.8 % (11/93) versus 2.8 % (5/177), respectively (p < 0.05). These adverse events were not specific to any one lens type, but were more often inflammatory (keratitis, conjunctivitis) or hypersensitivity responses (solution sensitivity, redness, tearing, etc.) with no cases of confirmed microbial infection. With regard to acceptability measures, there was no significant difference between the two regimens among all lenses tested in terms of overall cleanliness and lens wearing comfort, with lens wearing comfort mean ratings in the test and control groups of 8.7 and 8.6, respectively (p > 0.05).
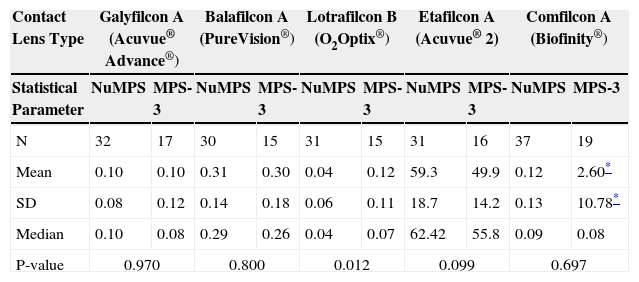
Protein and lipid deposit assessment of worn lensesThe conventional etafilcon A (Acuvue 2®) worn lenses were found to contain much higher levels of protein than any of the four silicone hydrogel types, with average protein levels of 50-60 μg/mg of dry lens material (Table 7). Although the average protein for this lens type (FDA Group IV) slightly favored the control solution (MPS-3), it was not statistically significant (p = 0.10). All four silicone hydrogel types showed an average of less than 1 μg protein per mg of dry lens material for lenses used with either the control (MPS-3) or test solution (NuMPS). No statistically significant difference was found between the solutions regarding protein deposition on three of the silicone hydrogel lens types (p = 0.70–0.97). However, lotrafilcon B lenses (O2Optix®) exhibited a significantly lower level of protein deposition with NuMPS (0.04 μg/mg of lens) compared to the control MPS-3 (0.12 μg/mg, p = 0.01).
Protein deposition levels (μg/mg of dry lens material) after continuous daily use (for the recommended maximum time) of NuMPS relative to MPS 3 as assessed by ex-vivo analysis of human worn lenses
| Contact Lens Type | Galyfilcon A (Acuvue® Advance®) | Balafilcon A (PureVision®) | Lotrafilcon B (O2Optix®) | Etafilcon A (Acuvue® 2) | Comfilcon A (Biofinity®) | |||||
| Statistical Parameter | NuMPS | MPS-3 | NuMPS | MPS-3 | NuMPS | MPS-3 | NuMPS | MPS-3 | NuMPS | MPS-3 |
| N | 32 | 17 | 30 | 15 | 31 | 15 | 31 | 16 | 37 | 19 |
| Mean | 0.10 | 0.10 | 0.31 | 0.30 | 0.04 | 0.12 | 59.3 | 49.9 | 0.12 | 2.60* |
| SD | 0.08 | 0.12 | 0.14 | 0.18 | 0.06 | 0.11 | 18.7 | 14.2 | 0.13 | 10.78* |
| Median | 0.10 | 0.08 | 0.29 | 0.26 | 0.04 | 0.07 | 62.42 | 55.8 | 0.09 | 0.08 |
| P-value | 0.970 | 0.800 | 0.012 | 0.099 | 0.697 | |||||
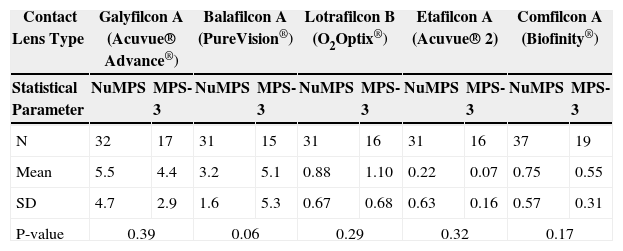
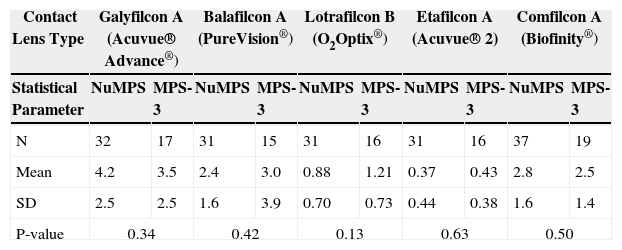
As shown in Tables 8-9, lipid levels were significantly higher for all worn silicone hydrogel lenses (1-5 μg/mg of dry lens material, depending on lens type) than for the conventional etafilcon A (Acuvue 2®) lens type (0.4 μg/mg of lens). Of the silicone hydrogel lenses, galyfilcon A (Acuvue Advance®) showed the highest total lipid deposition (∼4.0 μg/mg) and lotrafilcon B (O2Optix®) exhibited the lowest (∼1.0 μg/mg). There were no statistically significant differences in the amount of lipid deposition between the test and control solutions, although balafilcon A lenses (PureVision®) deposited less ester-type lipids (such as aliphatic glyceride esters) with NuMPS than MPS-3 with data tending toward significance (p = 0.06). Overall, the average amount of total lipid deposition for all lenses was identical for the two products and averaged 2.2 μg of lipid per mg of lens.
Ester-type lipid deposition levels (μg/mg of dry lens material) after continuous daily use of particular lens types (for the maximum recommended time) of NuMPS relative to MPS-3 as assessed by ex-vivo analysis of human worn lenses
| Contact Lens Type | Galyfilcon A (Acuvue® Advance®) | Balafilcon A (PureVision®) | Lotrafilcon B (O2Optix®) | Etafilcon A (Acuvue® 2) | Comfilcon A (Biofinity®) | |||||
| Statistical Parameter | NuMPS | MPS-3 | NuMPS | MPS-3 | NuMPS | MPS-3 | NuMPS | MPS-3 | NuMPS | MPS-3 |
| N | 32 | 17 | 31 | 15 | 31 | 16 | 31 | 16 | 37 | 19 |
| Mean | 5.5 | 4.4 | 3.2 | 5.1 | 0.88 | 1.10 | 0.22 | 0.07 | 0.75 | 0.55 |
| SD | 4.7 | 2.9 | 1.6 | 5.3 | 0.67 | 0.68 | 0.63 | 0.16 | 0.57 | 0.31 |
| P-value | 0.39 | 0.06 | 0.29 | 0.32 | 0.17 | |||||
Total lipid (ester and non-ester types) deposition levels (μg/mg of dry lens material) after continuous daily use of particular lens types (for the maximum recommended time) of NuMPS relative to MPS-3 as assessed by ex-vivo analysis of human worn lenses
| Contact Lens Type | Galyfilcon A (Acuvue® Advance®) | Balafilcon A (PureVision®) | Lotrafilcon B (O2Optix®) | Etafilcon A (Acuvue® 2) | Comfilcon A (Biofinity®) | |||||
| Statistical Parameter | NuMPS | MPS-3 | NuMPS | MPS-3 | NuMPS | MPS-3 | NuMPS | MPS-3 | NuMPS | MPS-3 |
| N | 32 | 17 | 31 | 15 | 31 | 16 | 31 | 16 | 37 | 19 |
| Mean | 4.2 | 3.5 | 2.4 | 3.0 | 0.88 | 1.21 | 0.37 | 0.43 | 2.8 | 2.5 |
| SD | 2.5 | 2.5 | 1.6 | 3.9 | 0.70 | 0.73 | 0.44 | 0.38 | 1.6 | 1.4 |
| P-value | 0.34 | 0.42 | 0.13 | 0.63 | 0.50 | |||||
Microbial keratitis is a rare but significant risk among contact lens wearers and studies have indicated that the risk of infection is around 2 cases per 10,000 soft contact lens wearers per year, rising to 19.5–25.4 cases per 10,000 for conventional hydrogel and silicone hydrogel lenses, respectively, if worn overnight.3 In Acanthamoeba keratitis almost 90 % of cases occur in contact lens wearers and can result in permanent blindness. The reported incidence of Acanthamoeba keratitis varies from 1–2 cases per million in the USA or 17–20 in the UK.4,26 Accordingly, contact lens care solutions have a fundamental role in the destruction or inhibition of potentially pathogenic microbes and the prevention of microbial keratitis.
The antimicrobial efficacy of contact care solutions is typically assessed according to ISO 14729 regulations.10 Using the same bacterial and fungal species and strains as this study, the regulations state that a contact lens solution should display appropriate efficacy by either the Primary Stand Alone (biocidal) or Secondary Regimen tests. In the former, the solution should produce a 3 log10 reduction in bacteria and a 1 log10 for the fungi without a regimen. If this is not possible, then the solution may be eligible for Regimen Qualification providing it was able to demonstrate, at the manufacturer's recommended soaking time, stasis for the fungi and an average of 5 log10 reduction in the three bacteria, with at least 1 log10 occurring for each bacterium. 10 If this condition is met the solution is eligible for the Secondary Regimen assessment. Here, the test evaluated the antimicrobial efficacy of the entire lens care regimen according to the manufacturer's instructions for use (e.g. rubbing, rinsing, soaking). Contact lenses are inoculated with at least 5 log10 of organism and following the Regimen an average of not more than 10 viable organisms should remain on the lens or the soaking solution.10 To date, no requirement has been established for testing against Acanthamoeba.10
With the exception of MPS-3, which produced only a 2.3 log10 with S. aureus (ATCC 6538), all the commercial solutions and NuMPS studied here met the ISO 14729 Primary Stand Alone (biocidal) criteria for bacterial and fungal efficacy at 6 hr contact time. Failure to pass biocidal criteria does not preclude a MPS from the market place providing it passes the secondary regimen requirement.10 In addition, all of the solutions tested including the NuMPS showed biocidal efficacy against A. castellanii trophozoites and, with the exception of MPS-3, the cysts. The NuMPS also met the ISO 14729 Secondary Regimen test for bacteria and fungi using a No Rub but Rinse procedure. For the Acanthamoeba only a Rub and Rinse regimen was used as previous studies have shown that the organism adheres with great affinity to silicone hydrogel lenses.5,27 As such, only the inclusion of a rub step can ensure satisfactory removal of trophozoites or cysts, as was demonstrated here.5 Therefore, it is recommended that a rubbing step always be included in contact lens care regimen procedures to ensure effective removal of microorganisms and to aid lens cleaning.
MPS-5 (ReNu with MoistureLoc®) was withdrawn from the market in 2006 due to its association with a significant rise in Fusarium keratitis cases.12,28 The formulation, containing alexidine (0.00045 %), showed good activity against F. solani in biocidal assays. However, it was subsequently shown to have lost efficacy on film formation through desiccation in the lens case, most probably exacerbated by the presence of polymer PQ10 in the formulation.14,29 The findings presented here extended this observation, showing that even on partial evaporation the solution lost 85–98 % of Fusarium activity. Loss in Acanthamoeba trophozoite efficacy was also observed, with a 51 % reduction in activity at 4× concentration upon evaporation. This finding is similar to that described previously with ReNu with MoistureLoc® in which a 70 %–80 % loss in Fusarium efficacy was obtained with solutions formulated (rather than evaporated, as was done here) to 2× and 4× concentration. MPS-3 containing PQ-1 (0.001 %) and myristamidopropyl dimethylamine (0.0005 %) also showed a significant loss in Fusarium efficacy ranging from 70 %–85 % at 2× and 4× concentration. However, there are no reports associating MPS-3 with an increased risk of Fusarium keratitis, suggesting additional factors besides loss in biocidal efficacy through evaporation may have been involved in the outbreak. In contrast, the NuMPS formulation, containing PQ-1 and alexidine showed no loss in Fusarium or Acanthamoeba efficacy on evaporation to 2 times and 4 times the concentration.
Another contact lens solution recall occurred in 2007 with the withdrawal of Complete® MoisturePlus® after it was linked to a number of Acanthamoeba keratitis cases.13,15 It was subsequently shown that the solution induced significant Acanthamoeba trophozoite encystment due, primarily, to the presence of propylene glycol in the formulation.19,30 In this study, none of the solutions, including the NuMPS, were found to induce trophozoite encystment. The root cause for the association between Complete® MoisturePlus® and Acanthamoeba keratitis remains unclear and, despite the recall, the number of cases of infection related to the use of other contact lens solutions continues to be reported with increased frequency.31
Although the antimicrobial adequacy of contact lens care solutions is currently directed by ISO 14729, recent events surrounding the recall of such products and the findings of this study indicate that greater stringency is required.10,12,19 Studies have also shown Fusarium strain variation in susceptibility to some MPS and, along with C. albicans, are significantly more resistant to disinfection when present as biofilms compared to the planktonic state.32,33 Based on these observations, it is recommended that the standard assessment of antimicrobial efficacy of contact lens care solutions should be extended to include additional clinical strains of the ISO 14729 reference organisms along with Acanthamoeba for biocidal, regimen and encystment testing. Furthermore, the effect of evaporation on the loss of antimicrobial efficacy should also be considered.
Clinical trials with NuMPS are ongoing and will be published in detail elsewhere. In the study described here, corneal staining change from baseline with NuMPS was found to be no greater than that with the control. Moreover, NuMPS was associated with appreciably fewer adverse events, none of which could be attributed to microbial keratitis. The trial also showed that NuMPS was comparable to the control solution with respect to lens cleanliness or wearing comfort.
Results from the present study showed NuMPS to have only slight to moderate cytotoxicity for all lens types studied and these findings were superior or equivalent to the control solution MPS-3. As the use of MPS-3 with some silicone hydrogel lenses has recently been associated with an increased incidence of corneal infiltrative events,34 the clinical implications of the cytotoxicity results from this study warrant further investigation to correlate in vitro biocompatibility to in vivo performance of the various MPS-CL combinations at the ocular surface. Previous in vitro studies have also reported that certain MPS may exert negative effects on cell viability and possibly other changes in corneal function including permeability, suggesting some cytotoxic influences from these MPS at the ocular surface.35,36 Findings from these studies and others have demonstrated good correlation between cytotoxicity observed in human corneal epithelial cells (HCEC) compared to that in L929 cells.37,38
Tear deposit assessment for total protein from worn lenses in the clinical trials showed greater deposition with the Group IV lenses than the silicone hydrogel lenses and that there were no statistically significant difference in the levels between users of NuMPS and the control solution. Lipid levels were significantly higher for all worn silicone hydrogel lenses compared to the group IV lenses. The observation of higher levels of protein deposition on conventional hydrogel compared with silicone hydrogel lenses is consistent with previous reports, as is the converse finding that less lipid binds to silicone hydrogel lenses.39–42
In conclusion, the NuMPS is a novel contact lens care solution based on the dual disinfection system of PQ-1 and alexidine. This solution was shown to provide antimicrobial activity against the pathogenic bacteria and fungi stipulated under ISO 14729 guidance as well as A. castellanii trophozoites and cysts. Unlike ReNu with MoistureLoc® MPS, which also contained alexidine, NuMPS maintained efficacy on partial evaporation against Fusarium and Acanthamoeba. Furthermore, the NuMPS performed very well in both the in vitro cytotoxicity testing and clinical trials.
Conflict of interestThe authors are employed by Abbott Medical Optics Inc. The authors have no proprietary, financial or commercial interest in any material or method mentioned in this study.
The authors would like to thank Anthony Lam, Marina Nikolic, Nancy Brady, Simon Cheung, Adriana Park, Lisa Hoong and Lauren Crawford from the Corneal Research & Development Department for their technical assistance in conducting this study.