To evaluate the indications and efficacy of high gas permeable scleral contact lenses (GP-SCL).
MethodsA total database of 97 consecutive patients (140 eyes) fitted with GP-SCL between January 2003 and December 2008, was retrospectively analyzed to determine the benefits of fitting scleral lenses. All lenses were fitted by preformed technique and were non-fenestrated. Patients included keratoconus – 88 eyes (63 %); corneal irregularities after penetrating keratoplasty – 39 eyes (28 %); various ocular surface disorders: Stevens-Johnson syndrome (SJS), graft versus host disease (GVHD) and exposure keratopathy – 6 eyes (4 %); post refractive surgery keratoectasia – 4 eyes (3 %), and high refractive error – 3 eyes (2 %).
ResultsMean follow up was 27.5 months (range 1–71), mean wearing time in successful wearers group was 12.2 hours per day (range 10–16), mean wearing time in group of wearers who dropped out using GP-SCL, was 5.8 hours (range 3–8). Keratoconus patients achieved median best corrected visual acuity (BCVA) of 20/32, 84 % of patients achieved BCVA of 20/40 or more. The post keratoplasty group achieved median BCVA of 20/25, 92 % of patients achieved BCVA of 20/40 or better. In the other groups, median BCVA was as follows: ocular surface disorders – 20/50, keratoectasia – 20/30, high refractive error – 20/32. Positive fluid-venting was highly associated with successful GP-SCL wearing. Twenty patients (21 %) failed to wear GP-SCL.
ConclusionsGP-SCL's expand the management of various corneal abnormalities. The main indication for GP-SCL is optical correction of an irregular corneal surface, especially keratoconus and corneal transplant.
Evaluar las indicaciones y eficacia de las lentes de contacto esclerales de alta permeabilidad al gas (LCE-PG).
MétodosSe analizó de forma retrospectiva una base de datos con un total de 97 pacientes consecutivos (140 ojos) que fueron prescritos LCE-PG entre enero 2003 y diciembre 2008, para determinar los beneficios de llevar lentes esclerales. Todas las lentes fueron adaptadas mediante técnica preformada y no estaban fenestras. Entre los pacientes, había 88 (63 %) ojos queratoconos; 39 ojos (28 %) con irregularidades corneales después de queratoplastia penetrante; diversos trastornos superficiales oculares: síndrome de Stevens-Johnson (SSJ), enfermedad injerto contra huésped (GVHD, en inglés) y 6 ojos (4 %) con queratopatía por exposición; 4 ojos (3 %) con queratectasia tras cirugía refractiva, y 3 ojos (2 %) con elevado defecto refractivo.
ResultadosEl seguimiento medio fue de 27,5 meses (intervalo 1–71), el tiempo medio de uso en el grupo que se adaptó con éxito a las LCE-PG fue de 12,2 horas por día (intervalo 10–16), la media de uso en el grupo que las abandonó fue de 5,8 horas (intervalo 3–8). Los pacientes con queratocono lograron una mediana de mejor agudeza visual corregida (BCVA, en inglés) de 20/32, el 84 % de los pacientes lograron una BCVA de 20/40 o superior. El grupo de posqueratoplastia logró una BCVA mediana de 20/25, el 92 % de los pacientes lograron una BCVA de 20/40 o superior. En los otros grupos, la BCVA mediana fue la siguiente: trastornos oculares superficiales: 20/50; queratectasia: 20/30, defecto refractario alto: 20/32. La ventilación de fluido positiva estaba altamente asociada con una adaptación exitosa a las LCE-PG. Veinte pacientes (21 %) no lograron usar las LCE-PG.
ConclusionesLas LCE-PG amplían las posibilidades de tratamiento de diversas anormalidades corneales. La indicación principal para las LCE-PG es la corrección óptica de una superficie corneal irregular, especialmente en queratocono y trasplante corneal.
Thousands of people all over the world are considered legally blind or have very poor vision due to keratoconus, high or irregular astigmatism and other forms of corneal irregularities or dystrophies.
The concept of optically neutralizing the cornea with a lens which enclosed a liquid reservoir over the corneal front surface was proposed in 1508 by Leonardo da Vinci. 1 Almost 400 years later Fick 2 designed the earliest practical contact lenses, which were scleral lenses. His idea was to neutralize the optical effects of the corneal distortion for patients with irregular astigmatism by using afocal scleral glass shells.
Modern scleral lenses are indicated for several ocular conditions such as keratoconus and other primary corneal ectasia, post corneal transplant when residual high refractive error exists or the surface remains highly irregular, serious dry eye conditions and neurotrophic keratitis, and can nearly always be fitted successfully. 3–5 These lenses offer two unique advantages.
Firstly, they are supported by the sclera and therefore can be fitted on eyes with markedly irregular corneas. This is particularly important for patients with corneal irregularity, when vision cannot be improved with rigid corneal contact lenses, either because of the patients’ poor tolerance level or inability to fit such a lens.
Secondly, there is the constant aqueous reservoir between the lens and the cornea. This reservoir neutralizes most of the irregular astigmatism and protects the corneal surface from the desiccating effects of exposure to air and the friction of blinking. This is especially important in cases of non-healing corneal epithelial defects and other ocular surface diseases that cannot be resolved with other currently available therapeutic modalities.
Until the last decade of the past century, these advantages could not be fully realized because of chronic corneal hypoxia attributed to low gaseous permeability of polymethyl methacrylate (PMMA) and other materials from which the lenses were made. 6–8
The development of rigid gas-permeable plastics greatly reduced the hypoxic complications associated with daily wear of corneal lenses and added a new dimension to the potential of scleral lenses for the visual rehabilitation of patients with markedly irregular corneas and treatment of ocular surface disorders. In 1983 Ezekiel 9 described the use of preformed, fenestrated, silicone/acrylate gas permeable scleral contact lenses in fitting patients with keratoconus, severe myopia, aphakia, and corneal scarring. Since then, new techniques in the manufacture of rigid gas-permeable scleral lenses have been developed. 10–12
In this article, we will report our experience in fitting patients with sealed non-fenestrated GP-SCL and assess the vision rehabilitation and therapeutic benefits in the management of various corneal and ocular surface diseases.
MethodsClinical data from the records of 97 consecutive patients (140 eyes), fitted with GP-SCL between January 2003 and December 2008, were retrospectively analyzed to determine the indications for lens fitting and visual outcomes.
Patients were referred to the contact lens clinic in Hadassah University Hospital, due to severe corneal irregularities or corneal surface disorders resistant to other therapeutic options. Patients referred for vision rehabilitation were unable to wear corneal rigid gas permeable (RGP) lenses or fitting of such lenses was contraindicated. Ocular pathologies included: keratoconus, keratoglobus and pellucid marginal degeneration (KC), corneal irregularity after penetrating keratoplasty (PKP), severe exposure keratopathy, Stevens-Johnson syndrome (SJS), graft versus host disease (GVHD) or other ocular surface disorders (OSD), corneal ectasia after refractive surgery and high refractive errors (HRE).
GP-SCL featuresThe lenses were lathed by City Crown Aspheric 7 CNC lathe (Microlens Ltd. Tel Aviv). Two highly gas permeable polymers were used: TYRO-97 (Lagado Corp.) having a permeability Dk value of 97 × 10—11 cm 2 mL O2/sec mL mm Hg (International Organization for Standardization [ISO]/Fatt) and Optimum Extreme (Contamac corp.) Dk value of 125 × 10—11 cm 2 mL O2/sec mL mm Hg (ISO/Fatt) units.
Use of hyper-Dk/t RGP lens materials (> 100 Dk/t) have been shown to supply enough oxygen to the cornea in all day lens wearers, which does not compromise corneal health in terms of increased corneal swelling. 13
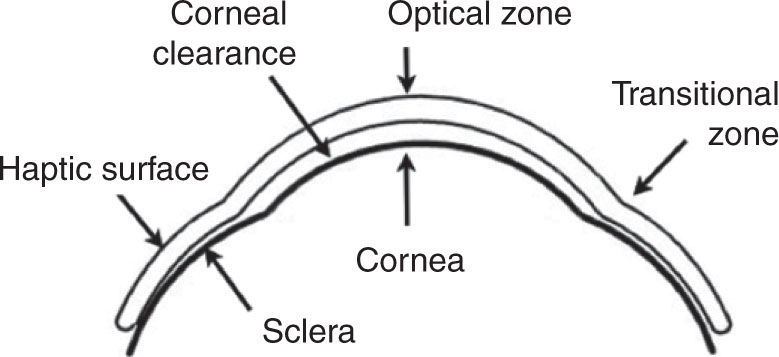
Each sleral lens was preformed, non-fenestrated and sealed, with an overall diameter of 18.5 mm, designed to barely clear the highest point of the cornea and to provide the most uniform thickness profile of the fluid accumulated between the lens and the cornea. By maintaining the minimal thickness of this reservoir, we optimized the oxygen transmissibility of the lens-cornea system and minimized the degradation of vision that occurs if excessive tear debris accumulate inside this reservoir during lens wear. The thickness of this profile ranged between one third of corneal thickness and 300–400 μm. The curvature of the central back surface of each lens (optic zone) was chosen to maintain a shallow, but definite clearance of the cornea and limbus, thus the lens was entirely supported by the sclera. Corneal clearance was evaluated by slit lamp examination to establish the optimal sagittal height of each lens.
In an average lens, the internal optic zone of 12.5 mm was a single curve surrounded by a 1.5–2.0 mm wide transitional zone vaulting the limbus and merging seamlessly into the posterior haptic surface (Figure 1). The haptic parameters of individual lenses were determined by observing the vascular compression patterns of the bulbar conjunctiva, using a series of diagnostic lenses of different haptic designs aimed at minimizing lens induced compression of conjunctival blood vessels and enabling unobstructed blood circulation. The average center thickness of the lenses was 0.35 mm.
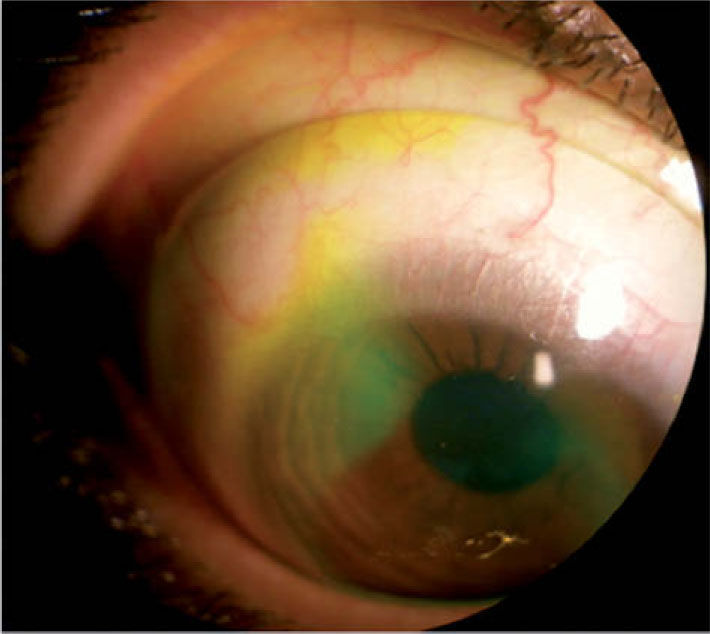
Fluorescein fluid-venting testAfter the lens had been placed on the eye with sterile saline and had been in position for at least 2 hours, fluorescein dye was installed. After 5 minutes, a slit lamp examination with cobalt blue filter or optic section was performed. 4,5,14 In cases when fluorescein diffused under the lens haptic and reached the pre-corneal liquid chamber independently or by gentle palpation, the test was considered positive (Figure 2) if no fluorescence was seen - the test was considered negative.
Indications for success/failureWe considered the fitting of GP-SCL to be successful when wearing time of 10 hours a day or more, with no lens adhesion on removal, was reached. Partial success was defined as ability to wear the lenses between 6 to 10 hours a day. Wearing of GP-SCL less then 6 hours a day was considered to be a failure.
ResultsA total of 97 patients (140 eyes) included 29 females (30 %) and 68 males (70 %), the patients’ ages ranged between 16–77 years. Mean follow up was 27.5 months (range 1–71).
IndicationsThe primary clinical indications for GP-SCL could be categorized in three main categories: vision improvement (134 eyes), management of severe ocular surface disease and corneal protection (6 eyes), and multiple indications that include both vision improvement and corneal protection (10 eyes).
The most common diagnoses for use of GP-SCL was keratoconus (KC) – 88 eyes (63 %), followed by extreme corneal irregularities or high astigmatism after penetrating keratoplasty (PKP) – 39 eyes (28 %). Other indications included various ocular surface disorders (OSD) – 6 eyes (4 %): 2 eyes with corneal stem cell disorder (Steven-Johnson syndrome), 2 eyes with severe dry eye syndrome due to GVHD, 1 eye was treated for severe exposure keratopathy and 1 eye had post herpetic keratitis.
Four eyes (3 %) were fitted with GP-SCL for post refractive surgery keratoectasia (2 eyes PRK, 2 eyes LASIK); 3 eyes (2 %) fitted due to high refractive errors: 2 eyes were aphakic and 1 eye had high myopia with spherical equivalent of —16.00 diopter.
EfficacyEighty eight of the 134 eyes that were fitted primarily to improve vision had keratoconus. Another 39 eyes were fitted due to inadequate spectacle-corrected vision after successful penetrating keratoplasty. Some of those eyes had undergone multiple unsuccessful surgical procedures for reducing the iatrogenic astigmatism. In most of these cases, the scleral lens provided useful corrected vision, regardless of the amount or type of corneal astigmatism present.
Median visual acuity (VA) with GP-SCL was 20/32 (Snellen fraction) and ranged from 20/400 to 20/20. The median best spectacle corrected VA without a contact lens was 20/70 (range 20/800–20/30). There was a significant increase in VA with scleral lenses compared to the best spectacle corrected VA (P < 0.001, signed rank test).
Improvement in best-corrected visual acuity (BCVA), defined as a gain of two or more decimal acuity lines, was observed in 125 eyes (93 %) out of the 134 eyes treated for vision improvement.
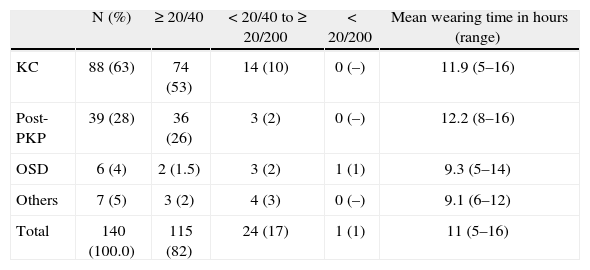
Visual outcomes varied according to the preexisting ocular status. The best visual results were obtained in the post PKP group, which achieved 20/40 or more in 92 % of cases with median BCVA of 20/25. Eyes in the keratoconus group achieved 20/40 or more in 84 % of cases with median BCVA of 20/32. In other groups, median BCVA was as follows: OSD 20/50, keratoectasia 20/30, HRE 20/32 (Table 1).
Best visual acuity and average wearing time achieved with GP-SCL fitted for various conditions and range
| N (%) | ≥ 20/40 | < 20/40 to ≥ 20/200 | < 20/200 | Mean wearing time in hours (range) | |
| KC | 88 (63) | 74 (53) | 14 (10) | 0 (–) | 11.9 (5–16) |
| Post-PKP | 39 (28) | 36 (26) | 3 (2) | 0 (–) | 12.2 (8–16) |
| OSD | 6 (4) | 2 (1.5) | 3 (2) | 1 (1) | 9.3 (5–14) |
| Others | 7 (5) | 3 (2) | 4 (3) | 0 (–) | 9.1 (6–12) |
| Total | 140 (100.0) | 115 (82) | 24 (17) | 1 (1) | 11 (5–16) |
KC: keratoconus; OSD: ocular surface disease; PKP: penetrating keratoplasty.
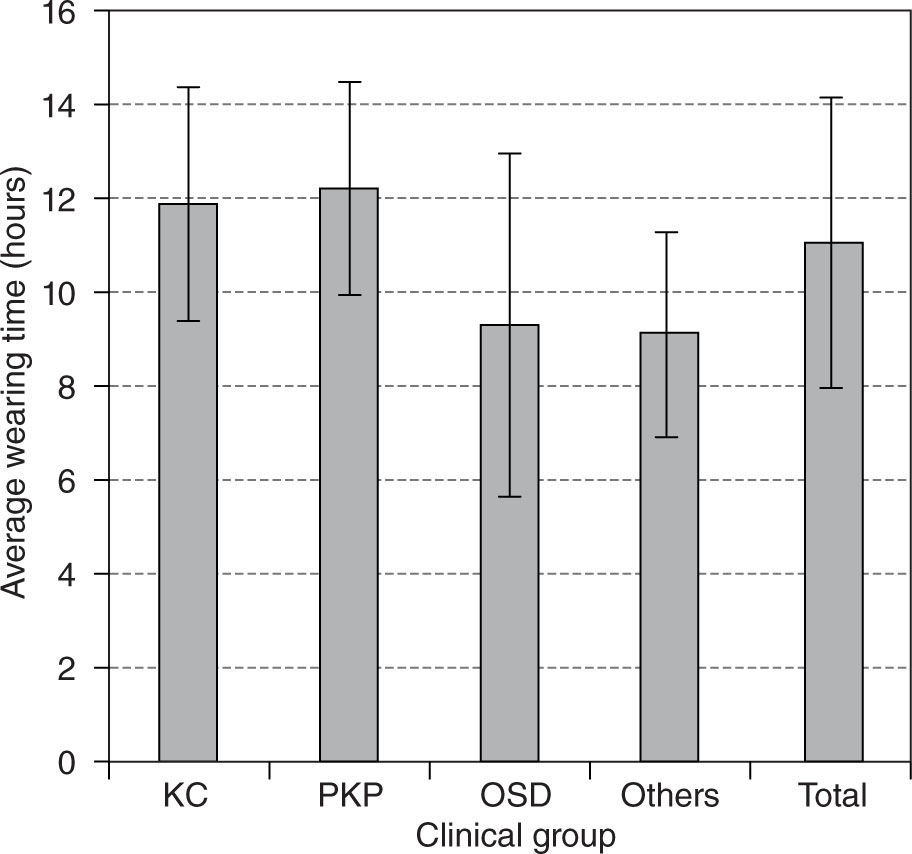
During the period of study 102 eyes (73 %) were successfully fitted, as defined by success criteria, i.e. able to wear the lenses for more than 10 hours a day, and 15 eyes (11 %) achieved partial success with GP-SCL. Mean wearing time of successful wearers was 12.2 hours a day (range 10–16). The mean wearing time among all fitted patients was 11 hours a day (ranged 5–16). Wearing times of the treated groups are shown in Figure 3.
Seventy eight eyes (77 %) in the successful wearers group had positive fluorescein dye test (Table 2). Fluorescein dye penetration under the lens haptic, i.e. positive fluid-venting was highly associated with successful GP-SCL wearing, (P = 0.0007, chi-square test)
Twenty patients (21 %) failed to wear GP-SCL. Mean wearing time in this group was 5.8 hours a day (range 3–8) and mean drop-out period was 5.1 month. Seven successfully fitted patients (7 %) dropped-out wearing GP-SCL due to further penetrating keratoplasty procedure (mean follow up 16.8 month).
DiscussionThis retrospective review shows that medical applications of GP-SCL include both visual and therapeutic uses for a range of complicated corneal and ocular surface conditions for which treatment by other methods is either unsuitable or less effective.
Diagnoses may be categorized into four main groups: primary or induced keratoectasia, post penetrating keratoplasty, ocular surface diseases and high refractive errors. In the current study, patients with keratoconus and other forms of irregular corneal surface formed the largest group (63 %), followed by post penetrating keratoplasty iatrogenic astigmatism group (28 %), and ocular surface disorders group (4 %).
Vision improvement was the primary indication for GP-SCL in nearly 95 % of the fitted eyes, and was achieved in most instances by neutralizing the corneal irregularities or correcting existing refractive errors.
Visual outcomes varied according to the preexisting ocular status. The best visual results were obtained in the post keratoplasty group, which achieved 20/40 or better in 92 % of cases. These patients were fitted with GP-SCL to correct post PKP corneal astigmatism or corneal irregularities, induced by transplant misalignment.
The patients suffering from keratoconus had severe forms of the disease leading to failure with corneal contact lenses or were unable to tolerate wearing this type of lenses. They were fitted with GP-SCL as the last alternative before surgery. Visual outcomes obtained in this group showed significant improvement of visual acuity, 20/40 or better in 84 % of patients.
Keratoconus patients are commonly adolescents, and may require corneal surgery during their lifetime. The risks associated with the keratoplasty procedure, which include infection, rejection, and severe astigmatism, may be greater than those attributed to GP-SCL wear. 15
Comparison with previous studiesVarious evaluation methods of GP-SCL performance demonstrate different results, thus it is difficult to make direct comparisons between studies. Since the first successful application of gas-permeable scleral lenses by Ezekiel 9 in 1983, several studies have been published. The study designs varied in size, diagnoses, fitting methods, scleral lens types and materials. However, as in our study the most common indication was keratoconus, followed by post keratoplasty. 5,16–18 The large retrospective studies of Pullum and Buckley 16 and Rosenthal and Croteau 4 on 530 and 538 patients, respectively, included a host of clinical indications but keratoconus and post penetrating keratoplasty still comprised the two largest groups. However in a study carried out in the early nineties, Tan et al. 19,20 reported keratoconus as the most common condition requiring scleral lenses followed by aphakia and then post keratoplasty. Lenses used varied greatly in size from 15 mm to 23mm. 4,5,18,21 Schornack and Patel 21 used a scleral lenses with an overall diameter of 18.2 mm, which is similar to the lenses used in our study (18.5 mm). They suggested that lenses that are supported by the sclera and clear both the cornea and limbus could be defined as ‘scleral’ lenses irrespective of the actual overall diameter.
The characteristics of our patients and our results were similar to those reported by other studies 5,18 regarding visual rehabilitation. Our study confirmed the best acuity results achieved in patients with irregular or severe astigmatic topography. 5,16–18,20 Studies that included indications other than visual correction, treatment of ocular surface disease for example, showed less marked improvement in VA. The reason of lower visual results is primarily therapeutic indication for scleral lens fitting i.e. corneal protection, tear conservation, or pain relief.
Success rateDevelopment of scleral lenses with a smoother continuous back surface that follows more precisely the underlying scleral surface has made prolonged, comfortable scleral lens wear possible. Penetration of liquids under the lens in situ, i.e. positive fluid-venting was found to be associated with successful fit and increased wearing time.
Moreover, the combination of high oxygen permeability and favorable tear exchange allowed by the lens back geometry may diminish the incidence of complications such as corneal edema or lens adhesion.
Failure rates during the period of our study were 21 %. This number concurs with the results of other investigations. In a retrospective study, Tan et al. 20 found that 71 % of the eyes could continue wearing the scleral lens, Pullum and Buckley 16 described that 22 % of their cases failed a scleral trial or stopped wearing their scleral lenses completely. Failure rate of 10.4 % was reported by Segal et al.,5 while Schornack and Patel 21 reported that 12 out of 32 patients (38 %) decided not to pursue scleral lens wear after initial evaluation.
In conclusion, modern scleral contact lenses can be used successfully for visual rehabilitation and management of a wide range of corneal disorders that have not responded adequately to other treatments. The main indication for GP-SCL is optical correction of an irregular corneal surface, especially due to keratoconus and post corneal transplant. In the keratoconus group, GP-SCL serves as an alternative to surgery. In the post-PKP group GP-SCL provided an alternative to refractive surgery or repeated corneal transplantation. Scleral lenses were found beneficial in several forms of ocular surface diseases. Therapeutic benefits of GP-SCL enhance the management of ocular surface diseases when medical treatments have failed and surgery is undesirable.
Conflict of interestThe authors have no proprietary, financial or commercial interest in any material or method mentioned in this study.