The aim of the present meta-analysis is to compare the efficacy of cyclopentolate and tropicamide in controlling accommodation during refraction.
MethodsA comprehensive literature search was performed in PubMed, Scopus, Science direct and Ovid databases by the key words: “tropicamide”; “cyclopentolate”; “cycloplegia” and “cycloplegic” from inception to April 2016. Methodological quality of the literature was evaluated according to the Oxford Center for Evidence Based Medicine and modified Newcastle-Ottawa scale. Statistical analyses were performed using Comprehensive Meta-Analysis (version 2; Biostat Inc., USA).
ResultsThe present meta-analysis included six studies (three randomized controlled trials and three case–control studies). Pooled standardized difference in the mean changes in the refractive error was 0.175 D [lower and upper limits: −0.089; 0.438] more plus in the cyclopentolate group compared to the tropicamide group; however, this difference was not statistically significant (p=0.194; Cochrane Q value=171.72 (p<0.05); I2=95.34%). Egger's regression intercept was −5.33 (p=0.170). Considering type of refractive errors; refractive assessment procedure and age group; although cycloplegic effect of cyclopentolate was stronger than tropicamide; however, this effect was only statistically significant in children; hyperopic patients and with retinoscopy.
ConclusionWe suggest that tropicamide may be considered as a viable substitute for cyclopentolate due to its rapid onset of action. Although these results should be used cautiously in infants and in patients with high hyperopia or strabismus when using tropicamide as the sole cycloplegic agent especially in situations that the findings are variable or there is no consistency between the examination results and clinical manifestations of the visual problems.
El objetivo del presente meta-análisis es comparar la eficacia de ciclopentolato y tropicamida a la hora de controlar la acomodación durante la refracción.
MétodosSe realizó una búsqueda amplia en la literatura en las bases de datos de PubMed, Scopus, Science direct y Ovid, utilizando las palabras clave: “tropicamida”, “ciclopentolato”, “cicloplejia” y “ciclopléjico” desde sus inicios a Abril de 2016. La calidad metodológica de la literatura se evaluó con arreglo a Oxford Center for Evidence Based Medicine y la escala Newcastle-Ottawa modificada. Los análisis estadísticos se realizaron utilizando el software Comprehensive Meta-Analysis (versión 2, Biostat Inc., EEUU).
ResultadosEl presente meta-análisis incluyó seis estudios (tres ensayos controlados aleatorizados y tres estudios de casos-control). La diferencia estandarizada combinada de los cambios medios del error refractivo fue de 0,175 D [límites inferior y superior: −0,089, 0,438], más acusada en el grupo ciclopentolato con respecto al grupo tropicamida aunque, sin embargo, esta diferencia no fue estadísticamente significativa (p=0,194, Valor Q de Cochrane=171,72 (p<0,05), I2=95,34%). El valor del intercepto de regresión de Egger fue de −5,33 (p=0,17). Considerando el tipo de errores refractivos, el procedimiento de valoración refractiva y el grupo de edad, aunque el efecto ciclopléjico de ciclopentolato fue más fuerte que el de tropicamida, dicho efecto fue únicamente significativo en niños, pacientes hipermétropes, y con retinoscopia.
ConclusiónSugerimos la consideración de tropicamida como sustituto viable de ciclopentolato, debido a su rápido inicio de acción. Aunque estos resultados deberían utilizarse con precaución en niños y en pacientes con elevada hipermetropía o estrabismo al utilizar tropicamida como único agente ciclopléjico, especialmente en situaciones en las que los hallazgos sean variables, o no exista consistencia entre los resultados del examen y las manifestaciones clínicas de los problemas visuales.
Refractive assessment especially in patients younger than 40 years is usually impressed by the accommodative spasm and possibly a myopic shift in the results. Spasm of accommodation become evident as pesudomyopia or latent hyperopia. Therefore, cycloplegic refraction using cycloplegic drugs is an effective way to reduce fluctuation in accommodation or spasm of ciliary muscle.1–3
The drug of choice should have a rapid onset, appropriate cyclopegic effect and minimum side effects. Although, atropine is the gold standard for its cycloplegic effects, however, its prolonged blur vision, slow onset and long recovery time have limited its application to very specific conditions.4 Moreover, it can be associated with serious side effects including allergic contact dermatitis of the lids, allergic conjunctivitis, keratitis, and increase in intraocular pressure.5 Atropine may be used with caution due to possibility of converting intermittent esotropia to constant esotropia.6 Two of the most commonly used agents include tropicamide and cyclopentolate. The major differences between them are duration of the onset, maximum efficacy, recovery time and probable side effects.5
Cyclopentolate is a synthetic antimuscarinic cycloplegic agent and is available in 0.5% and 1% solutions. It is widely accepted drug of first choice for patients of all ages which provides excellent short-term cycloplegia. Its cycloplegic effects begins at 25–75min after administration of the drop and recovery appears through 6–24h later.6 Ocular side effects may include irritation, lacrimation, allergic blepharoconjunctivitis, conjunctival hyperemia, and increase in intraocular pressure. Some of its sstemic side effects are drowsiness, ataxia, disorientation, incoherent speech, restlessness, and visual hallucinations.4
Tropicamide, as an alternative, is a synthetic analog of tropic acid and is known as a safe agent for cycloplegic refraction. It characterized by a rapid onset and the cycloplegia effect appears 20–30min after administration. Its recovery appears 6h later.7 Ocular side effects include stinging sensation in the eye, corneal irritation, and increase in intraocular pressure. Systemic side effects include dry mucous membranes, flushing, and tachycardia.4
Several studies have been conducted to compare the cycloplegic effect of these two agents and different results have been reported.8–13 Because of inconsistencies in the previous reported results, this meta-analysis was conducted to summarize the results regarding the comparison of the cycloplegic effect, in terms of refractive outcome, between cyclopentolate and tropicamide and provide the final conclusion.
Material and methodsSearch strategy and inclusion criteriaThe PRISMA guidelines for performing the current meta-analysis was followed. A comprehensive literature search of PubMed, Scopus, Science direct and Ovid databases was carried out to find the relevant peer-reviewed publications investigated the effects of cyclopentolate and tropicamide cycloplegic agents with regard to the changes in the mean spherical equivalent for each drop. To find the relevant studies, a search algorithm based on the combination of the terms: “tropicamide”, “cyclopentolate”, “cycloplegia” and “cycloplegic” was used. The search was performed from inception to September 2016. In addition, references list of relevant studies were reviewed for possible missing citations. The search was performed by two authors independently (NY & AE).
Inclusion criteriaParticipants of the included studies had no history of cardiovascular or neurological diseases, no history of ocular pathology, no history of allergy to drops, taking no ocular or systemic medicines, and no history of amblyopia or strabismus.
Quality assessmentQuality scoring of included studies were carried out using the Newcastle – Ottawa quality assessment scale for case control studies (Table 1) and Oxford Center for Evidence-Based Medicine (Table 2) for randomized controlled trials studies.
Quality assessment for case–control studies.
| Study | Case selection | Assessment of the outcome |
|---|---|---|
| Pi12 | 1. The actual age when examining was between 6 and 15 years. 2. Children were not diagnosed with systemic cardiovascular disease or neurological diseases. | Retinoscope |
| Lin11 | 1. Either gender 2. Age 7–13 years 3. Refractive errors between −1.5D to −6.0D 4. Astigmatism less than −1.5D 5. Free of ocular and systemic disease 6. No contraindication for pupillary dilation 7. Taking no ocular or systemic medications | Auto refractometer and retinoscope |
| Egashira9 | 1. Non-strabismic children 2. Non-amblyopic children 3. Hyperopic children with a mean refractive error of +1.48±1.10D 4. 6–12 years old children | Retinoscope and auto refractometer |
Quality assessment for randomized controlled trial studies.
| Study | Randomization method | Blinding | Similarity of the groups | Aside from the allocated treatment, were groups treated equally? | Lost to follow up? | Objective measures? |
|---|---|---|---|---|---|---|
| Hofmeister10 | By using a random number table | Examiners and patients were blinded to randomization | Yes | Yes | 2/30 | Yes, by automated phoropter |
| Twelker8 | The bottles containing both cycloplegic agents were masked and the drop that was administered at the first visit was randomly assigned | The examiner was masked to all prior Non-cycloplegic and cycloplegic retinoscopy findings. | Yes | Yes | NA | Yes, by retinoscopy |
| Rajan13 | Not mentioned | NA | Yes | Yes | NA | Yes, by retinoscopy |
For each study, difference in mean of the changes in the spherical equivalent for the cyclopentolate and tropicamide groups was used as the main effect size. To pool the effect sizes across studies, a random effects model was used. Heterogeneity was evaluated by the Cochrane Q test (the significance level was considered to be 0.05.), and I2 index.14 Publication bias was evaluated graphically by funnel plots and statistically by Egger's regression intercept method.15 Sub-group analysis was performed for exploring the effect of underlying refractive error, age of the patients, and measurement device on the pooled effect sizes. All statistical analyses were performed using Comprehensive Meta-Analysis (version 2, Biostat Inc., USA).
ResultsPRISMA flow chart (PRISMA 2009 flow diagram) of the study is shown in Fig. 1. Overall six studies were included in the present meta-analysis. Three studies had randomized controlled trial (RCT) design and three studies were case–control. The details of these studies are illustrated in Table 3.
Characteristic of included articles.
| Study | Study design | Region | Sample (n) | Age | RE | Device | Agents | Tropicamide (mean diff.±SD) | Cyclopentolate (mean diff.±SD) | Mean diff. (mean±SD) between agents | Cycloplegic agents dosage/interval time |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hofmeister et al. 10 | RCT | USA | Adult (30) | 21–50 y | M | Auto/Phora | Trop 1% – Cyclop 1% | – | – | OD: 0.054±0.214 OS: 0.054±0.253 | Trop: 2 drops/5min Cyclop: 2 drops/5min |
| Lin et al. 11 | CC | China | Children (37) | 7–13 y | M/Astig | Auto | Trop 1% – Cyclop 1% | OU: −0.36±−0.23 | OU: −0.28±−0.35 | – | Trop: 2 drops/5min Cyclop: 2 drops/5min |
| Egashira et al.9 | CC | USA | Children (20) | 6–12 y | H | Auto/Ret | Trop 1% – Cyclop 1% | – | – | Ret: 0.11±0.32 Sub. R: 0.06±0.34 Auto R: 0.14±0.30 | Trop: 2 drops/5min Cyclop: 2 drops/5min |
| Twelker et al.8 | RCT | USA | Infants (29) | 4–7 m | H/Astig | Ret | Trop 1% – Cyclop 1% | OU: 0.89±0.66 | OU: 1.04±0.94 | OU: 0.07±0.80 | Trop: 2 drops/5min Cyclop: 2 drops/5min |
| Pi et al.12 | CC | China | Children (1907) | 6–15 y | H/M/E | Ret | Trop 0.5% – Cyclop 1% | OD: 0.19±1.18 OS: 0.25±1.10 | OD: 0.5±1.23 OS: 0.57±1.16 | OD: 0.31 OS: 0.32 | Trop: 3 drops/10min Cyclop: 3 drops/10min |
| Rajan et al.13 | RCT | India | Children (25) | 6–15 y | H | Ret | Trop 1% – Cyclop 0.5% | – | – | OU: 0.54±0.18 | Trop: 3 drops/5min Cyclop: 2 drops/5min |
RCT: randomized controlled trial; CC: case–control; RE: refractive error; Astig: astigmatism; M: myopia; H: hyperopia; E: emmetropia; Phora: phoropter; Ret: retinoscopy; Auto R: auto-refraction; Sub. R: subjective refraction; Trop: tropicamide; Cyclop: cyclopentolate; OD: right eye; OS: left eye; OU: both eyes; mean diff: mean difference between mean of agents.
The pooled difference in mean of refractive change was 0.175D (95%CI: −0.089, 0.438) higher in the cyclopentolate group as compared to the tropicamide group (p=0.194) cycloplegic refraction results with cyclopentolate was more plus than tropicamide. Overall, cyclopentolate had a stronger but no statistically significant cycloplegic effect. Eggers regression intercept was −5.33, p=0.170.
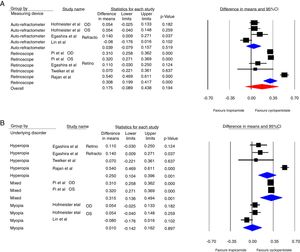
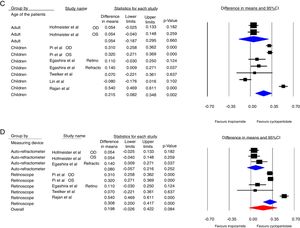
Fig. 2 shows the forest plots of the main analysis. Fig. 2A illustrated that difference between two drugs was more evident with retinoscopy (p<0.001, 95%CI: 0.199, 0.417) compared to the auto-refractor (p=0.519, 95%CI: −0.079, 0.157). The overall results have not been changed, even after excluding the study by Lin et al.11 which evaluated the outcome of the eye drops in every 15min (Fig. 2D). The Lin et al. study resulted in a mean pooled difference −0.08 (95%CI: −0.176, 0.016) in refractive error (p=0.102).11
Fig. 2B shows that in the patients with myopia, cyclopentolate efficacy was not significantly different from tropicamide (p=0.897, 95%CI: −0.142, 0.162) compared to the hyperopia (p=0.001, 95%CI: 0.104, 0.396) and mixed refraction (p=0.001, 95%CI: 0.136, 0.494) group.
Fig. 2C shows that there was no significant difference between the two drops in adults (p=0.660, 95%CI: 0.187, 0.295), unlike children (p=0.002, 95%CI: 0.082, 0.348).
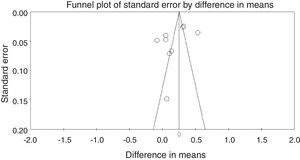
Funnel plot of the meta-analysis is shown in Fig. 3. Subgroup analyses of the study are shown in Table 4.
Subgroup analysis based on the applied device, type of refractive error and sample.
| Variables | Applied device | Type of refractive error | Sample | ||||
|---|---|---|---|---|---|---|---|
| Retinoscopy | Auto-refract meter | Myopia | Hyperopia | Mixed | Adults | Children | |
| Pooled standardized difference in means [lower and upper limits] p-value (reference) | OD: 0.310 [0.258 and 0.362] p<0.001(12) | OD: 0.054 [−0.025 and 0.133] p=0.182(10) | OD: 0.054 [−0.025 and 0.133] p=0.182(10) | Ret: 0.110 [−0.030 and 0.250] p=0.124(9) | OD: 0.310 [0.258 and 0.362] p<0.001(12) | OD: 0.054 [−0.025 and 0.133] p=0.182(10) | OD: 0.310 [0.258 and 0.362] p<0.001(12) |
| OS: 0.320 [0.271 and 0.369] p<0.001(12) | OS: 0.054 [−0.040 and 0.148] p=0.259(10) | OS: 0.054 [−0.040 and 0.148] p=0.259(10) | Auto: 0.140 [0.009 and 0.271] p=0.037(9) | OS: 0.320 [0.271 and 0.369] p<0.001(12) | OS: 0.054 [−0.040 and 0.148] p=0.259(10) | OS: 0.320 [0.271 and 0.369] p<0.001(12) | |
| 0.110 [−0.030 and 0.250] p=0.124(9) | 0.140 [0.009 and 0.271] p=0.037(9) | −0.080 [−0.176 and 0.016] p=0.102(11) | 0.070 [−0.221 and 0.361] p=0.637(8) | Ret: 0.110 [−0.030 and 0.250] p=0.124(9) | |||
| 0.070 [−0.221 and 0.361] p=0.637(8) | −0.080 [−0.176 and 0.016] p=0.102(11) | 0.540 [0.469 and 0.611] p<0.001(13) | Auto: 0.140 [0.009 and 0.271] p=0.037(9) | ||||
| 0.540 [0.469 and 0.611] p<0.001(13) | 0.070 [−0.221 and 0.361] p=0.637(8) | ||||||
| −0.080 [−0.176 and 0.016] p=0.102(11) | |||||||
| 0.540 [0.469 and 0.611] p<0.001(13) | |||||||
Overall, this study showed that cyclopentolate produces an insignificant mean refractive change of 0.175D more plus than tropicamide. However, subgroup analysis displayed higher and statistically significant dioptric changes using cyclopentolate compared to tropicamide by retinoscopy versus autorefraction (0.31 vs. 0.04D) and in children versus adults (0.22 vs. 0.05D) and in hyperopic and mixed versus myopic refractive errors (0.25 and 0.31 vs. 0.01D). Although these changes are statistically significant but the means or even upper limits of the 95%CI are not clinically significant based on the proposed criterion by Guha et al., who considered a dioptric difference more than 0.50 in sphere or cylinder as a significant clinical change.16
Cycloplegic refraction is a procedure to determine refractive status while the ciliary muscles are temporarily paralyzed with cycloplegic agents. Inadequate cycloplegic effect may shift results in an under-plus or over-minus direction. In addition to sufficient effect and no adverse reactions, time is an important factor of an ideal cycloplegic agent. Therefore, an ideal agent should have rapid onset of effect and fast recovery of accommodation.17 Cycloplegic maximum effect occurs between 45 and 90min for cyclopentolate 1% and 30–70min for tropicamide 1% following the final eye drop administrated.14 It has been shown that there is a strong correlation between the exact time waiting to complete examination and patient satisfaction levels so that minimizing the waiting time increases the satisfaction levels significantly.18
Residual accommodation is another parameter in choosing an appropriate cycloplegic drug. Previously it was believed that tropicamide provides an unacceptable cycloplegic effect especially in children. This view was based on the subjective accommodative assessment to determine the amount of residual accommodation for example using the push-up test to measure the accommodative amplitude. Further investigations using the objective methods illustrated that the residual accommodation was not higher than the accepted level (less than 1D).19,20
Measuring device can also influence the achieved results. More cycloplegic effect obtained by cyclopentolate compared to tropicamide by retinoscopy technique may be attributed to better stability of the accommodative system especially when looking to a non-accommodative fixation target used at distance. This issue is very important in refractive assessment of infants, young children and also in uncooperative subjects who performing autorefraction is very difficult.
The findings of many comparative studies of the cycloplegic effects of tropicamide and cyclopentolate in children9,14,19,21 and adults10 are in agreed with the results of the present meta-analysis.
Hofmeister et al. reported no statistically significant difference between these two agents (mean difference 0.05±0.25D) in the myopic adults (age range 21–50 years).10 Egashira et al. concluded that although the inhibitory effect of tropicamide on the accommodative system is weaker compared to other agents, it may be used as an effective cycloplegic drug in children with low to moderate levels of hyperopia.9
The current results are also in concordance with Pi et al.12 but in contrast with the findings of Egashira et al. who reported insignificant difference using cycloplegic retinoscopy between the two agents and statistically but no clinically considerable change toward more hyperopia with cyclopentolate using autorefraction.9 Sometimes, cycloplegic disparity of these two agents using retinoscopy may be linked to differences in the peak time and recovery speed of their effects.14 The relatively long-lasting maximum cycloplegic effect of cyclopentolate compared to tropicamide makes it a more suitable agent for cycloplegic retinoscopy in children and especially poor cooperative children who need longer duration of retinoscopy. On the other hand, Twelker and Mutti8 comparing tropicamide 1%, and cyclopentolate 1% in infants noted a statistically significant but not a clinically significant finding of 0.14D toward more hyperopia with cyclopentolate using autorefraction15 that in in line with the present study.
A stronger cycloplegic effect has been observed using cyclopentolate in children consistent with the findings of Pi et al.12 and Owens et al.21 This finding can be attributed to differences in the accommodative capacity in the two age groups.12,21 Higher disparity seen in the subjects with mixed and hyperopic refractive errors (0.250 and 0.315D, respectively) was suggested that cyclopentolate has more efficacy and inhibitory ability in those refractive conditions than in myopia (0.010D) which is in line with the findings of Pi et al.12
In conclusion, although cycloplegic effect of cyclopentolate was statistically stronger when its effect was evaluated by the type of refractive errors, refractive assessment procedure and age group, however, this effect was not clinically significant in children, hyperopic patients and by using retinoscopy. One important point to note is that although retinoscopy is a more reliable objective verification method, however, results may vary based on several factors such as cooperation of the patient and skill of the clinician. According to the above mentioned findings and from the standpoint of rapid onset cycloplegic effect and shorter duration of peak effect, tropicamide can be considered as a viable substitute for cyclopentolate. In infants and patients with high hyperopia or strabismus, use caution when using tropicamide as the sole cycloplegic agent, especially in situations that the findings are variable or there is no consistency between the examination results and clinical manifestations of visual problems.
Conflicts of interestThe authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.