To characterize corneal biomechanics in post-small-incision lenticule extraction (SMILE), post-laser-assisted subepithelial keratomileusis (LASEK), and normal eyes using Brillouin microscopy.
MethodsThis study included myopic patients who underwent corneal refractive surgery (SMILE or LASEK) at least 1 month prior to ensure corneal stability. A total of 177 eyes (79 post-SMILE, 24 post-LASEK, and 74 untreated normal eyes) from 177 patients were evaluated using Pentacam HR and Brillouin microscopy for morphological and biomechanical assessment, respectively. Among them, 30 eyes (20 post-SMILE and 10 post-LASEK) from 30 participants underwent both pre- and post-operative Brillouin and Pentacam examinations, enabling within-subject comparisons. Corneal biomechanics were assessed using Brillouin modulus (BM), where lower values indicate weaker biomechanical properties.
ResultsNo significant differences were observed in Central BM, Mean BM, or Max BM among the groups. Compared with the normal eyes, Min BM was significantly lower in the post-SMILE and post-LASEK groups (P = 0.004 and 0.002, respectively) and Max–Min BM significantly increased after SMILE and LASEK (both P < 0.001). In post-SMILE corneas, standardized deviation BM was significantly higher than in normal corneas (P < 0.001). Within-subjects comparisons (pre- vs post-operation) further confirmed above results. Multiple linear regression analysis revealed a negative correlation between Central BM and post-operative corneal thickness in post-SMILE corneas (coefficient = –0.016, P = 0.025). In the post-LASEK group, Max-Min BM showed a positive correlation with mean corneal curvature (coefficient = 0.031, P = 0.001).
ConclusionSMILE and LASEK can induce localized changes in corneal biomechanics, as observed by Brillouin microscopy, while maintaining overall corneal biomechanics.
With the increasing prevalence of myopia in recent years, refractive surgeries have become popular for myopia correction.1,2 However, the occurrence of iatrogenic ectasia following refractive surgery underscores the importance of pre-operative prediction and long-term ectasia risk monitoring.3 Small-incision lenticule extraction (SMILE) and laser-assisted subepithelial keratomileusis (LASEK) are stromal flap-free procedures, showing a lower association with corneal ectasia compared to laser in situ keratomileusis (LASIK).4 This reduced risk may be attributed to factors such as the absence of a corneal flap and considerations like residual stromal bed thickness.5 Consequently, eyes post-SMILE and post-LASEK serve as valuable models for comprehending the impact of tissue removal on the cornea.
Corneal biomechanical properties, crucial for predicting keratoectasia pre- and post-refractive surgery, are traditionally assessed using devices like the ocular response analyzer (ORA) and corneal visualization Scheimpflug technology (Corvis ST).6,7 While these studies consistently demonstrate overall biomechanical weakening post-operatively, there remains ongoing debate regarding which surgical techniques (e.g., flap-based vs. cap-based procedures) better preserve corneal integrity. This controversy stems from fundamental differences in how these techniques modify corneal structure—particularly regarding anterior stromal ablation depth versus preservation of the stronger anterior lamellae.8–10 However, these deformation-based methods are susceptible to confounding factors such as central corneal thickness (CCT) and intraocular pressure (IOP), complicating their interpretation in post-surgical or thin corneas when comparing with normal and pre-operative results.5,9,11 The newly developed Corvis ST biomechanical index-laser vision correction (CBI-LVC) metric by Corvis ST, associated with post-LVC, lacks validated indices for diagnosing post-LVC ectasia at various stages, and its stability requires further long-term research.11
In contrast, Brillouin microscopy offers a transformative approach as a non-contact, all-optical technique that probes intrinsic biomechanical properties via the Brillouin frequency shift and modulus (BM).12 By quantifying the cornea’s longitudinal modulus at the molecular level—a property dictated by collagen organization and hydration—it is less affected by CCT and IOP.13–16,33 This technique has found application in evaluating corneal diseases and assessing of outcomes in corneal refractive and cross-linking surgeries, with capability to distinguishes depth-dependent variations within different corneal layers, from the epithelium to the endothelial layer.17,18 While showing promise in these areas, its capacity to identify post-operative biomechanical changes in refractive surgery, especially those with subtle effects, and the factors influencing such changes, remains unknown.19–22 Consequently, Brillouin microscopy is suitable for both pre- and post-operative measurements of corneal biomechanical properties.
This study’s primary objective is to discern differences in corneal biomechanics between normal corneas and those that underwent flapless corneal refractive surgeries, specifically SMILE and LASEK, using Brillouin microscopy. This study aims to contribute to a more comprehensive understanding of the disparities in corneal biomechanics between normal corneas and those that have undergone specific corneal refractive surgeries.
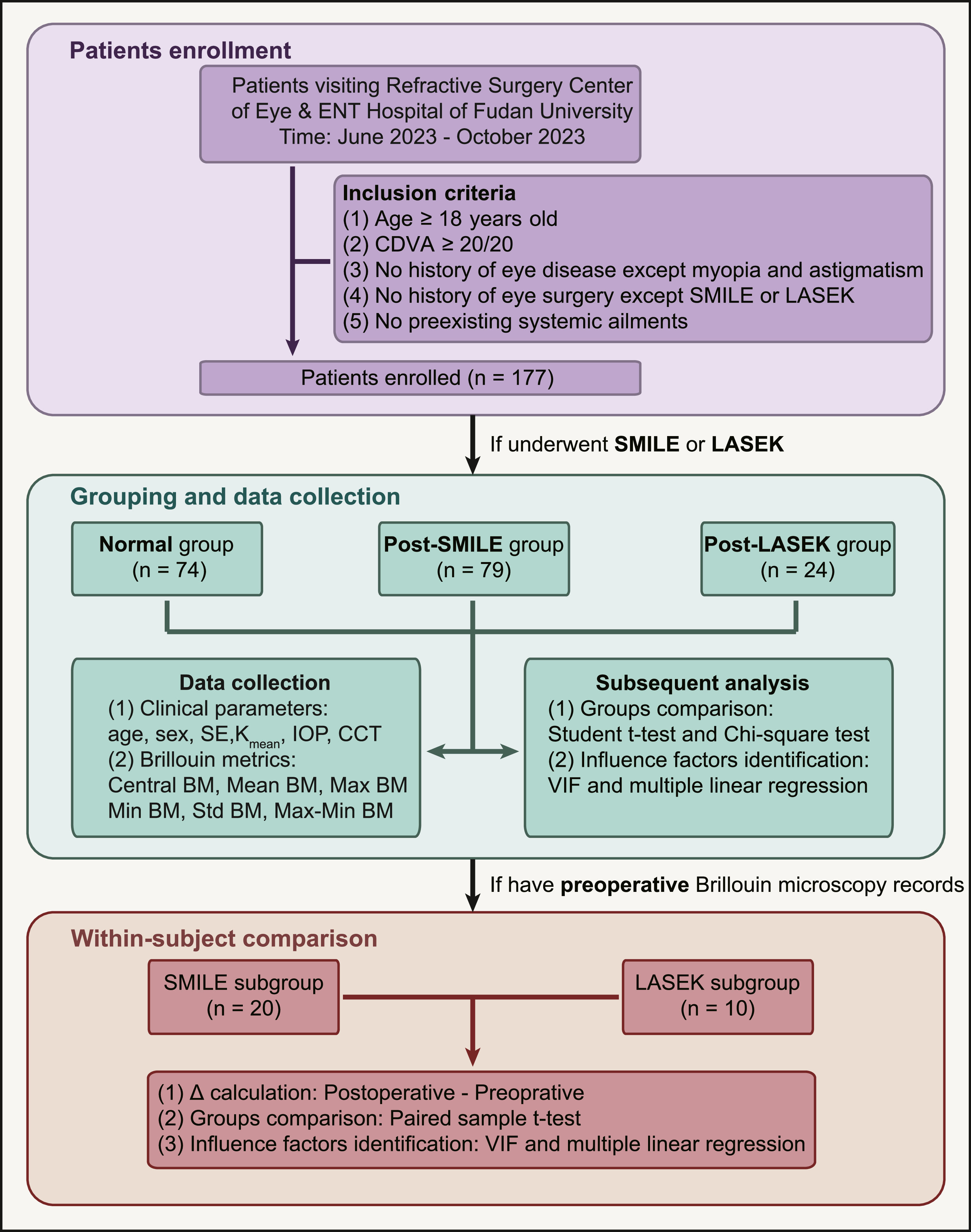
Materials and methodsParticipants and ethical approvalThis study was approved by the Ethics Committee of the Eye & ENT Hospital of Fudan University (No. 2020530) and adhered to the tenets of the Declaration of Helsinki. All individuals provided written consent after receiving comprehensive information about the study's objectives. Our recruitment involved the consecutive inclusion of patients who sought consultation and underwent either SMILE or LASEK between June 2023 and October 2023 at the Refractive Surgery Center of the Department of Ophthalmology, Eye & ENT Hospital of Fudan University. Inclusion criteria comprised age >18 years and a corrected distance visual acuity (CDVA) of 20/20 or better. Those who had experienced ocular trauma other than laser vision correction or any preexisting systemic ailments were not considered suitable for inclusion in the study. Patients who did not undergo refractive surgery and excluded the risks of keratoconus comprised the normal group, while those who underwent SMILE and LASEK were assigned to the post-SMILE and post-LASEK groups, respectively. Only data from the right eye were analyzed. The study design is illustrated in Fig. 1.
Study design flowchart. This flowchart delineates the step-by-step study design process, encompassing participant recruitment, grouping, data collection, and subsequent analyses. Arrows indicate the sequential progression of tasks, facilitating a clear understanding of the study's meticulous methodology. CDVA, corrected distance visual acuity; SMILE, small-incision lenticule extraction; LASEK, laser-assisted subepithelial keratomileusis; SE, spherical equivalent; Kmean, mean keratometry; IOP, intraocular pressure; CCT, central corneal thickness, BM, Brillouin modulus; VIF, variance inflation factor; Δ, delta.
All surgical procedures were performed by the same surgeon (JZ). SMILE procedures were performed using a VisuMax femtosecond laser system (Carl Zeiss Meditec, Jena, Germany) with a 130 nJ pulse energy. The cap thickness was configured to 120 μm, with a 7.5 mm diameter, and a 2 mm side cut. The superior and inferior surfaces of the lenticule were separated from the anterior stroma, and subsequent extraction was performed through a side cut.
LASEK procedures were performed using a Mel 90 excimer laser system (Carl Zeiss Meditec, Jena, Germany). Corneal epithelial trephines (Model 52,503 B; 66 Vision Tech Co., Ltd., Suzhou, China) with an 8.5 mm inner diameter, along with a 20 % ethanol-aqueous solution, were utilized to create an epithelial flap. A 250 kHz excimer laser with a pulse energy of 150 nJ was employed to ablate the corneal stroma. The epithelial flap was repositioned following excimer laser treatment.
MeasurementsClinical metricsFor each patient evaluated, age, sex, manifest refraction sphere and cylinder, manifest refraction spherical equivalent (SE), and CDVA were recorded to assess the comparability of these characteristics between the groups. The sphere and cylinder values of the participants, both pre- and post-operatively, were determined through autorefraction and manifest refraction. The SE was calculated using the following formula: spherical equivalent = spherical + 1/2 cylinder. Tomographic and topographic parameters (Pentacam HR; Oculus Optikgeräte, Wetzlar, Germany) included in the study were the mean front keratometry (Kmean) in the central 3 mm and central corneal thickness (CCT). On the same day the Brillouin microscopy examination was conducted. IOP was measured using a Canon Full Auto Tonometer TX-F (Canon Inc., Tokyo, Japan).
Brillouin microscopy metricsThe Brillouin corneal biomechanical parameters were measured using the Brillouin Optical Scan System (BOSS; Intelon, USA) with a light source of 780 nm. The Brillouin examinations for both pre- and post-operative participants were conducted by the same experienced physician (JC), occurring 30 min after the ocular examination in a dark room with temperature maintained between 26 °C and 28 °C. Post-operative routine assessments were performed at least 4 weeks post-surgery to ensure the recovery, with examinations conducted after 9 am (at least 2 h after wakeup) to mitigate the influence of corneal hydration on BM.23,24 Each measurement using “4 points” mode and setting of 1.5 mm away from the pupil center, required approximately 4 min to complete in the absence of errors, with an interval of 10 s between the examination of each point to allow participants to close their eyes and rehydrate the cornea.25 Participants were instructed to fixate on an indicator light and avoid blinking or moving during the examination. Data with a quality index of "OK" were included in the analysis.
Parameters derived by BOSS included Central, Mean, maximum (Max), minimum (Min), Standard deviation (Std) and Max-Min BM (the detailed explanation was shown in Supplementary Table 1 and our previous work).25 BM, together with its spectrum-related form, Brillouin frequency shift are both positively correlated with biomechanical properties and viscoelasticity of biomaterials (e.g., cornea and crystalline lens). Due to the Brillouin frequency shift varied a lot in researches utilizing discrepant visible and near infrared light source with different incident wavelength, we tend to use Brillouin modulus to represented the biomechanical evaluation of cornea, given by the equation:
Brillouinmodulus,M′=ρλ2νB24n2
Where ρ is the density index of the medium, n is the refractive index of the medium, the λ and θ is the wavelength and incident angle of light source, respectively.
Data analysisStatistical analysis and visualization were conducted using R software (version 4.3.2). Descriptive statistics are presented as mean ± standard deviation. Ocular outcomes across different groups were compared using Student’s t-test and chi-square test. Analysis of variance (ANOVA) with Welch’s correction (non-parametric method) were employed for each evaluated Brillouin metric to assess population mean differences among the normal, post-SMILE, and post-LASEK groups. In cases of unequal group sizes or non-homogeneous variances, Welch’s ANOVA was applied to ensure robustness. For patients with pre-operative Brillouin microscopy records, we introduced delta (Δ) values as the post-operative minus pre-operative values, forming self-compare subgroups to discern the Brillouin modulus changes due to surgery. To identify potential influencing factors for Brillouin variables, we initially standardized each variable and eliminated covariates using the variance inflation factor (VIF) method, considering a VIF > 10 as indicative of significant multicollinearity with the remaining variables, before executing multiple linear regression analysis.26 For within-subject comparisons, a paired sample t-test was used to ascertain changes in BM following SMILE and LASEK. Statistical significance was set at P < 0.05.
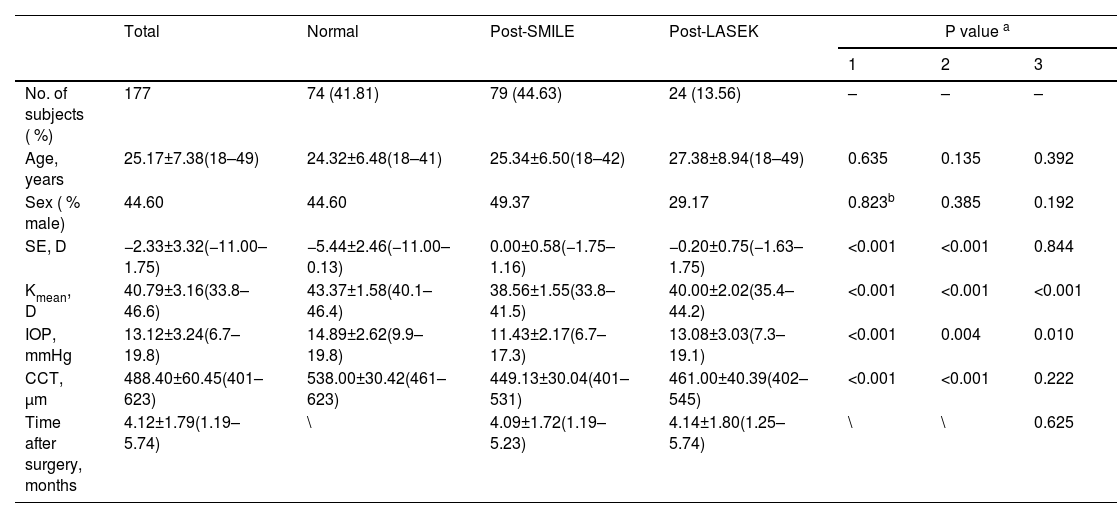
ResultsPatient demographics and clinical metrics comparisonsA total of 177 eyes from 177 individuals were included in the analysis, including 79 who underwent SMILE, 24 who underwent LASEK, and 74 who did not undergo any ocular or refractive surgeries. Table 1 provides an overview of the basic patient demographics. No significant differences were observed in age and gender distribution across the groups, with a mean age of 25.17 ± 7.38 years and 44.60 % male subjects in the overall cohort. In the normal group, the mean values for SE, Kmean, IOP and CCT were –5.44 ± 2.46 D, 43.37 ± 1.58 D, 14.89 ± 2.62 mmHg and 538.00 ± 30.42 μm, respectively. The SE values at 4 weeks post-operatively in the post-SMILE and post-LASEK groups were 0.00 ± 0.58 D and –0.20 ± 0.75 D, respectively, with no significant difference (P = 0.844). Significant differences were observed among the three groups in Kmean (all P < 0.001). The Kmean values in the post-SMILE and post-LASEK groups were 38.56 ± 1.55 D and 40.00 ± 2.02 D, respectively, indicating that corneas after SMILE were flatter than after LASEK (P < 0.001). IOP in both post-SMILE and post-LASEK groups was significantly lower than that in normal corneas (P < 0.001 and P = 0.004, respectively), likely because of decreased corneal thickness. The CCT values were 449.13 ± 30.04 μm and 461.00 ± 40.39 μm in post-SMILE and post-LASEK groups, respectively, with no significant difference between the two groups (P = 0.222).
Demographic and ocular parameters of participants.
| Total | Normal | Post-SMILE | Post-LASEK | P value a | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| No. of subjects ( %) | 177 | 74 (41.81) | 79 (44.63) | 24 (13.56) | – | – | – |
| Age, years | 25.17±7.38(18–49) | 24.32±6.48(18–41) | 25.34±6.50(18–42) | 27.38±8.94(18–49) | 0.635 | 0.135 | 0.392 |
| Sex ( % male) | 44.60 | 44.60 | 49.37 | 29.17 | 0.823b | 0.385 | 0.192 |
| SE, D | −2.33±3.32(−11.00–1.75) | −5.44±2.46(−11.00–0.13) | 0.00±0.58(−1.75–1.16) | −0.20±0.75(−1.63–1.75) | <0.001 | <0.001 | 0.844 |
| Kmean, D | 40.79±3.16(33.8–46.6) | 43.37±1.58(40.1–46.4) | 38.56±1.55(33.8–41.5) | 40.00±2.02(35.4–44.2) | <0.001 | <0.001 | <0.001 |
| IOP, mmHg | 13.12±3.24(6.7–19.8) | 14.89±2.62(9.9–19.8) | 11.43±2.17(6.7–17.3) | 13.08±3.03(7.3–19.1) | <0.001 | 0.004 | 0.010 |
| CCT, μm | 488.40±60.45(401–623) | 538.00±30.42(461–623) | 449.13±30.04(401–531) | 461.00±40.39(402–545) | <0.001 | <0.001 | 0.222 |
| Time after surgery, months | 4.12±1.79(1.19–5.74) | \ | 4.09±1.72(1.19–5.23) | 4.14±1.80(1.25–5.74) | \ | \ | 0.625 |
Data were expressed as mean ± standard deviation (range). SE, spherical equivalent; D, diopters; Kmean, mean keratometry; IOP, intraocular pressure; CCT, central corneal thickness. Statistically significant P values are bolded.
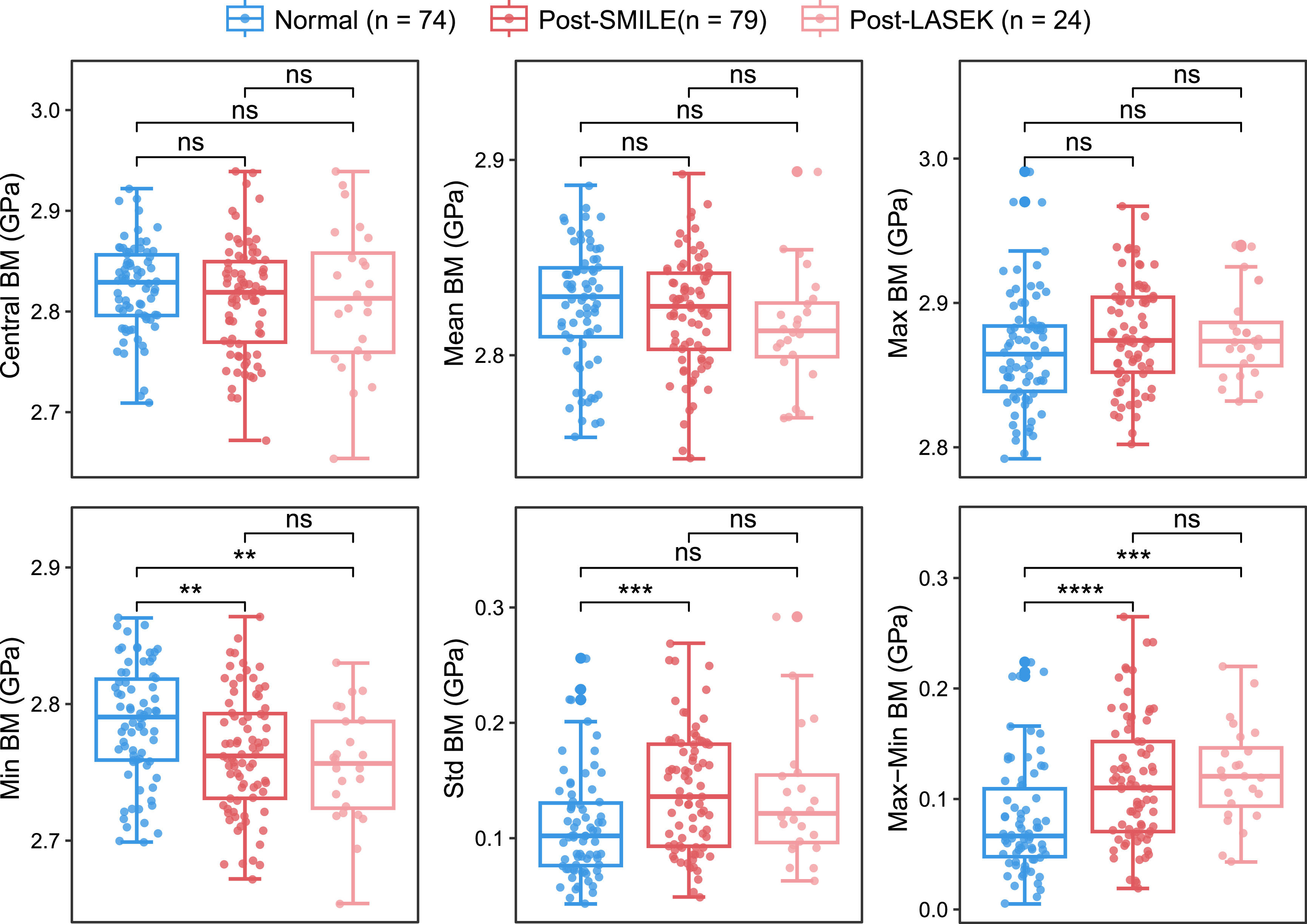
Comparisons of Brillouin metrics among post-SMILE, post-LASEK, and normal eyes are shown in Fig. 2. No significant differences were observed in Central, Mean, or Max BM among the three groups. However, Min BM was significantly lower in the post-SMILE and post-LASEK groups than in normal eyes (P = 0.004 and P = 0.002, respectively), with no significant difference between the two post-operative groups (P = 0.459). Correspondingly, the Max–Min BM significantly increased after both SMILE and LASEK (both P < 0.001) compared with normal eyes, with no significant difference in the extent of increase between the two groups (P = 0.380). Additionally, in post-SMILE corneas, the Std BM was significantly higher than in normal corneas (P < 0.001), while post-LASEK corneas did not exhibit this trend (P = 0.181).
Comparisons of corneal biomechanics between post-SMILE, post-LASEK, and normal eyes with regard to Brillouin modulus. This figure illustrates the comparison of corneal biomechanics among eyes following small-incision lenticule extraction (SMILE), laser-assisted subepithelial keratomileusis (LASEK), and normal eyes, focusing on the Brillouin modulus. Statistical significance is denoted by asterisks: **P < 0.01, ***P < 0.001, and ****P < 0.0001. Not significant comparisons are marked as "ns.".
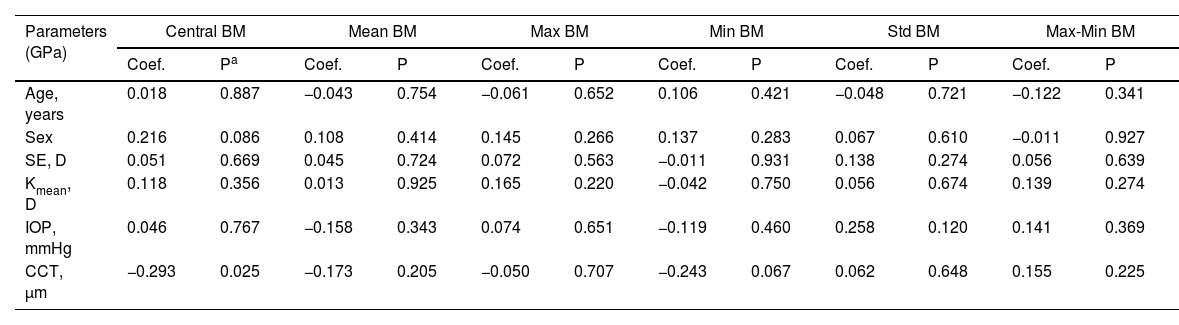
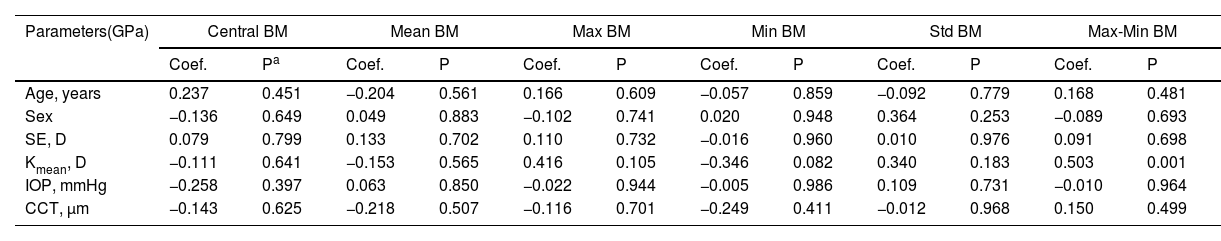
In the post-SMILE group, only Central BM was negatively correlated with post-operative CCT (coefficient=−0.016, P = 0.025) in Table 2. Conversely, in the post-LASEK group, only the Max-Min BM showed a positive correlation with Kmean (coefficient=0.031, P = 0.001) in Table 3.
Correlation between gender, age, CCT, SE, IOP and corneal curvature and Brillouin modulus in post-SMILE corneas.
| Parameters (GPa) | Central BM | Mean BM | Max BM | Min BM | Std BM | Max-Min BM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | Pa | Coef. | P | Coef. | P | Coef. | P | Coef. | P | Coef. | P | |
| Age, years | 0.018 | 0.887 | −0.043 | 0.754 | −0.061 | 0.652 | 0.106 | 0.421 | −0.048 | 0.721 | −0.122 | 0.341 |
| Sex | 0.216 | 0.086 | 0.108 | 0.414 | 0.145 | 0.266 | 0.137 | 0.283 | 0.067 | 0.610 | −0.011 | 0.927 |
| SE, D | 0.051 | 0.669 | 0.045 | 0.724 | 0.072 | 0.563 | −0.011 | 0.931 | 0.138 | 0.274 | 0.056 | 0.639 |
| Kmean, D | 0.118 | 0.356 | 0.013 | 0.925 | 0.165 | 0.220 | −0.042 | 0.750 | 0.056 | 0.674 | 0.139 | 0.274 |
| IOP, mmHg | 0.046 | 0.767 | −0.158 | 0.343 | 0.074 | 0.651 | −0.119 | 0.460 | 0.258 | 0.120 | 0.141 | 0.369 |
| CCT, μm | −0.293 | 0.025 | −0.173 | 0.205 | −0.050 | 0.707 | −0.243 | 0.067 | 0.062 | 0.648 | 0.155 | 0.225 |
Coef., coefficient. Statistically significant P values are bolded.
Correlation between gender, age, CCT, SE, IOP and corneal curvature and Brillouin modulus in post-LASEK corneas.
| Parameters(GPa) | Central BM | Mean BM | Max BM | Min BM | Std BM | Max-Min BM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | Pa | Coef. | P | Coef. | P | Coef. | P | Coef. | P | Coef. | P | |
| Age, years | 0.237 | 0.451 | −0.204 | 0.561 | 0.166 | 0.609 | −0.057 | 0.859 | −0.092 | 0.779 | 0.168 | 0.481 |
| Sex | −0.136 | 0.649 | 0.049 | 0.883 | −0.102 | 0.741 | 0.020 | 0.948 | 0.364 | 0.253 | −0.089 | 0.693 |
| SE, D | 0.079 | 0.799 | 0.133 | 0.702 | 0.110 | 0.732 | −0.016 | 0.960 | 0.010 | 0.976 | 0.091 | 0.698 |
| Kmean, D | −0.111 | 0.641 | −0.153 | 0.565 | 0.416 | 0.105 | −0.346 | 0.082 | 0.340 | 0.183 | 0.503 | 0.001 |
| IOP, mmHg | −0.258 | 0.397 | 0.063 | 0.850 | −0.022 | 0.944 | −0.005 | 0.986 | 0.109 | 0.731 | −0.010 | 0.964 |
| CCT, μm | −0.143 | 0.625 | −0.218 | 0.507 | −0.116 | 0.701 | −0.249 | 0.411 | −0.012 | 0.968 | 0.150 | 0.499 |
Coef., coefficient. Statistically significant P values are bolded.
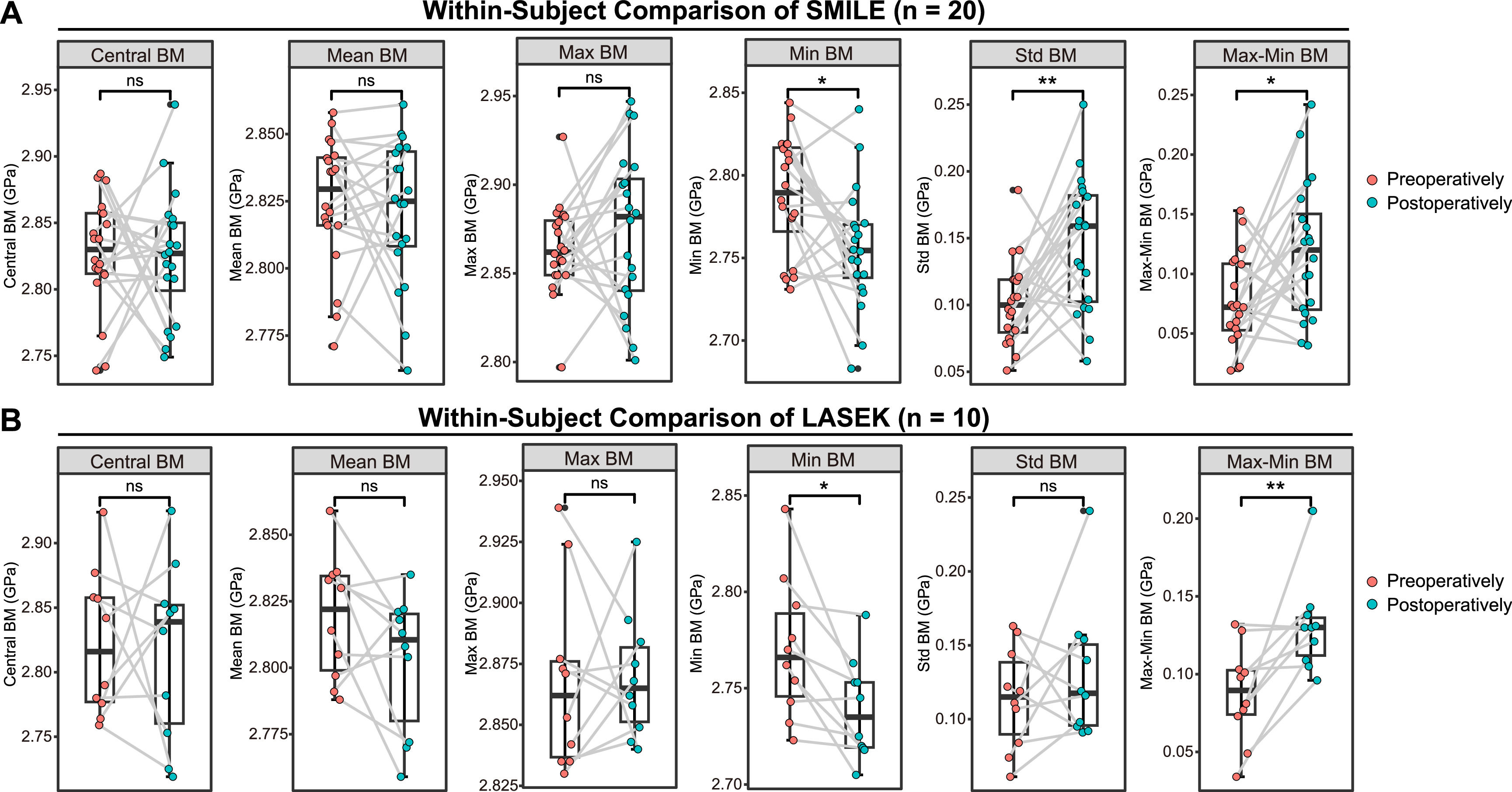
The demographic and ocular parameters of these subgroups are presented in Supplementary Table 2 encompassing 20 individuals who underwent SMILE and 10 who underwent LASEK. The within-subject comparison results for SMILE and LASEK are depicted in Fig. 3. Both SMILE and LASEK led to a decrease in Min BM (P = 0.012 and P = 0.038, respectively) and an increase in Max-Min BM (P = 0.018 and P = 0.007, respectively). Additionally, SMILE, but not LASEK, increased the Std BM (P = 0.004 and P = 0.710, respectively).
Within-subject comparisons of corneal biomechanics in patients undergoing SMILE and LASEK eyes with regard to Brillouin modulus. This figure presents within-subject comparison analysis of corneal biomechanics before and after SMILE in panel (A) and LASEK in panel (B), specifically examining the Brillouin modulus. Statistical significance is indicated by asterisks: *P < 0.05 and **P < 0.01. Not significant comparisons are marked as “ns.”.
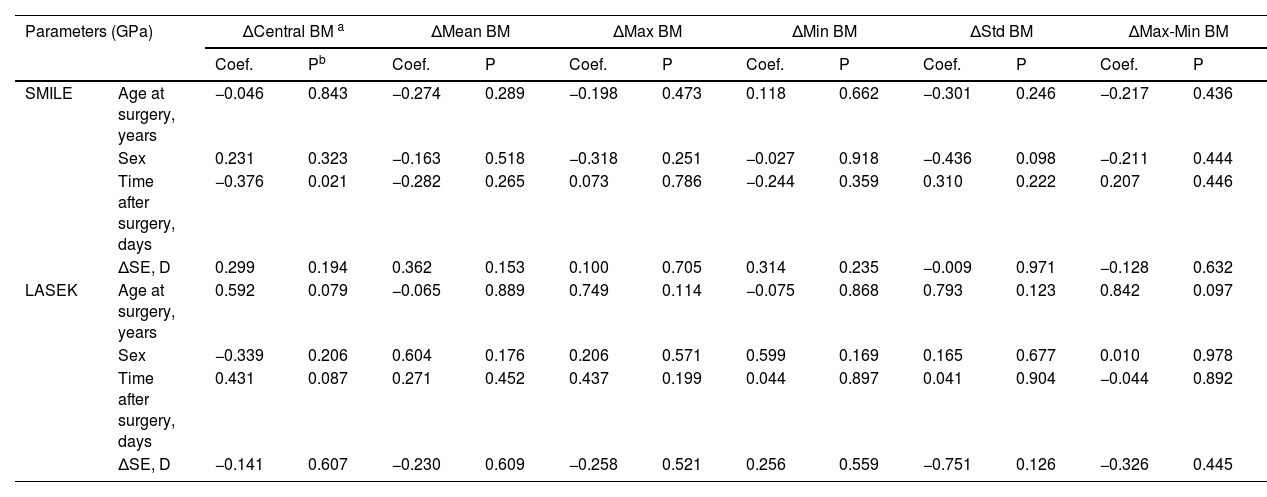
In the analysis of influencing factors for Brillouin metrics in the subgroups, a covariance analysis was initially conducted using Δ values. ΔIOP, ΔCCT, and ΔKmean were excluded from the multiple linear regression analysis due to VIF > 10. The results of the influencing factor analyses are presented in Table 4. For patients undergoing SMILE, a negative correlation was observed between ΔCentral BM and the time after surgery (coefficient=–0.043, P = 0.021). For both SMILE and LASEK within-subject analyses, no other significant influencing factors affecting the Brillouin metrics were identified.
Analysis of factors influencing changes in Brillouin Modulus in within-subject comparison subgroups.
| Parameters (GPa) | ΔCentral BM a | ΔMean BM | ΔMax BM | ΔMin BM | ΔStd BM | ΔMax-Min BM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | Pb | Coef. | P | Coef. | P | Coef. | P | Coef. | P | Coef. | P | ||
| SMILE | Age at surgery, years | −0.046 | 0.843 | −0.274 | 0.289 | −0.198 | 0.473 | 0.118 | 0.662 | −0.301 | 0.246 | −0.217 | 0.436 |
| Sex | 0.231 | 0.323 | −0.163 | 0.518 | −0.318 | 0.251 | −0.027 | 0.918 | −0.436 | 0.098 | −0.211 | 0.444 | |
| Time after surgery, days | −0.376 | 0.021 | −0.282 | 0.265 | 0.073 | 0.786 | −0.244 | 0.359 | 0.310 | 0.222 | 0.207 | 0.446 | |
| ΔSE, D | 0.299 | 0.194 | 0.362 | 0.153 | 0.100 | 0.705 | 0.314 | 0.235 | −0.009 | 0.971 | −0.128 | 0.632 | |
| LASEK | Age at surgery, years | 0.592 | 0.079 | −0.065 | 0.889 | 0.749 | 0.114 | −0.075 | 0.868 | 0.793 | 0.123 | 0.842 | 0.097 |
| Sex | −0.339 | 0.206 | 0.604 | 0.176 | 0.206 | 0.571 | 0.599 | 0.169 | 0.165 | 0.677 | 0.010 | 0.978 | |
| Time after surgery, days | 0.431 | 0.087 | 0.271 | 0.452 | 0.437 | 0.199 | 0.044 | 0.897 | 0.041 | 0.904 | −0.044 | 0.892 | |
| ΔSE, D | −0.141 | 0.607 | −0.230 | 0.609 | −0.258 | 0.521 | 0.256 | 0.559 | −0.751 | 0.126 | −0.326 | 0.445 | |
Statistically significant P values are bolded.
In this study, through cross-sectional and within-subjects comparison analyses, we confirmed that Central, Mean, and Max BM were not significantly influenced by SMILE or LASEK. However, Min BM decreased and Max-Min BM increased after both procedures. These findings led us to hypothesize that while the two stromal flap-free procedures not significantly impact the overall corneal biomechanical characteristics, they do result in a reduction in the stiffness of the cornea's weakest points. This was quite discrepant with previous contact-based researches, which suggested that the entire corneal deformation was more observed after corneal ablation induced by refractive surgeries, as represented by various Corvis ST and ORA metrics.10,27 However, it worth noting that entire corneal thickness and residual stromal bed are predominant determinant of stiffness decreases after surgeries.8 Still, our results align with previous research on subjects undergoing LASIK and photorefractive keratectomy (PRK), which observed a similar increase in Max-Min BM and Std BM, and a decrease in Min BM.19 Therefore, we posit that refractive surgery may induce regional lesions and focal decreases in corneal biomechanical properties while maintaining consistent stiffness across the entire cornea.
Our findings collectively illustrate that Brillouin microscopy parameters are largely independent of IOP, are comparable to the patient's preoperative measurements, and can serve as a valuable supplement or standalone tool for monitoring post-operative corneal biomechanical changes. However, attention should be paid to the influence of corneal curvature, CCT, and follow-up time. Overall, the mean BM and maximum BM emerged as the most stable indicators and were unaffected by the other studied factors.
As we have emphasized, Brillouin measurements are less influenced by CCT and IOP due to the fundamental principles of Brillouin light scattering. The underlying Brillouin light scattering process involves interactions between incident photons and thermodynamic phonons within the corneal tissue. This interaction is primarily governed by the local microstructure, particularly the relative composition of extracellular matrix (fluid phase) and protein content (solid phase), as well as the thermodynamic effect.16,28 More hydrated, thicker corneas (e.g., one hour after wakeup) exhibit weaker Brillouin biomechanics (∼25 MHz Brillouin frequency shift, equivalent to 0.03 GPa BM).23 Moreover, increased collagen fibril density (solid phase content) enhances Brillouin biomechanical properties, rather than the entire cornea.13 This approach fundamentally differs from conventional deformation-based techniques, which rely on: the application of external stress (e.g., air-puff or contact), measurement of the resulting corneal strain, and interpretation based on the balance between external forces and intraocular pressure.
To investigate the factors influencing Brillouin metrics in assessing post-operative corneal biomechanical changes and guiding their clinical application, we performed rigorous variable selection and multiple linear regression analyses. In post-SMILE corneas, the Central BM was negatively associated with CCT (P = 0.028). Generally, a thicker cornea is associated with better biomechanical properties (positive correlation). However, in this study, we report a negative correlation, which may be attributed to the differences in pre-operative corneal status. Specifically, we hypothesize that for higher myopic eyes undergoing SMILE surgery, these eyes exhibit both worse biomechanical pre- and post-outcomes due to corneal structure, greater tissue ablation and lower residual CCT post-operatively.29,30 For post-LASEK corneas, where the stromal layer retains integrity, the Max-Min BM showed a positive correlation with Kmean. This indicates that as the post-LASEK cornea becomes steeper (usually with less corneal ablation), the Max-Min BM tends to increase. Considering that LASEK reduces Min BM and that ablation might induce spatial discrepancy within the cornea compared with normal eyes, our results suggest that the steeper the cornea, the more likely it is to decrease Min BM (coefficient=−0.018, P = 0.082) and consequently increase Max-Min BM.
In the within-subjects comparison analysis, ΔCentral BM was negatively related to the time after SMILE surgery. Our findings suggest that the examination time after surgery can influence the Central BM in post-SMILE corneas, which was not further estimated in previous research.19 Our analysis suggests negative correlation between ΔCentral BM and postoperative time in SMILE cases may reflect both hydration and structure. As previously demonstrated, Brillouin measurements are sensitive to corneal hydration states.23 Individual variability in healing responses may account for the scatter in our data, even one month for LASIK patients was enough for recovery.24 Previous research has demonstrated that refractive surgery may influence both the morphology and function of corneal cells, as well as ocular surface status, three months post-operatively.31 Additionally, the stromal bed thickness has been shown to remain stable after one month following SMILE surgery.32 Based on these findings, we hypothesize that this correlation is attributable to both corneal hydration and structural effects.
The limitations of our study should be acknowledged: (1) The lack of baseline refractive error measurements and surgical correction amounts represents an important limitation, as these factors may influence post-operative corneal biomechanics. However, it's noteworthy that Brillouin microscopy measurements are less affected by residual corneal thickness variations. This characteristic permitted our focus on evaluating intrinsic post-operative biomechanical properties. (2) Recent research indicates that PRK has a smaller impact on corneal biomechanics than SMILE, while LASIK, a prominent refractive surgery procedure, was not considered in this study. This suggests the need for further development of corneal biomechanical studies using Brillouin microscopy. (3) The relatively small sample size of LASEK patients might limit the robustness of our conclusions. Nonetheless, the observed statistical significance suggest that the findings are robust and warrant validation in larger, prospective cohorts. A longer follow-up period is advisable to observe the long-term effects of Brillouin metric changes induced by different surgeries. (4) The absence of biomechanical parameters from other instruments necessitates further investigation into the consistency of Brillouin microscopy in measuring post-operative corneal biomechanical parameters. Therefore, future studies should address this issue. (5) Although the gender distribution between the two surgical groups did not differ significantly, the observed difference (49.37 % vs. 29.17 % female) raises the possibility of gender-related selection bias or physiological differences influencing surgical preference or outcomes. Future studies with larger sample sizes are warranted to explore this potential association.
Our study confirmed that SMILE and LASEK may induce a localized reduction in corneal biomechanical properties, as observed by Brillouin microscopy, while maintaining overall corneal biomechanics. When utilizing Brillouin microscopy for post-operative corneal biomechanics in a clinical setting, consideration of corneal tomographic and topographic parameters is vital.
Data availabilityData are available on reasonable request.
Ethics statementsPatient consent for publication
Ethics approvalThe study adhered to the tenets of the Declaration of Helsinki. The protocols were approved by the Ethics Committee of the Fudan University EENT Hospital Review Board (No.2020530).
The authors declare no conflict of interest.
We would like to thank Editage (www.editage.cn) for English language editing.
Yanze Yu and Jian Cao contributed equally to this manuscript.
Funding Sources: This work was supported by National Natural Science Foundation of China (Grant No. 82271119); Shanghai Rising-Star Program (23QA1401000); Healthy Young Talents Project of Shanghai Municipal Health Commission (2022YQ015); Project of Shanghai Science and Technology (Grant No.20410710100, 21Y11909800). The funding agencies had no role in study design, data collection and analysis, interpretation of data, or writing the manuscript.