To investigate the effect of benzalkonium chloride (BAK)-preserved latanoprost and bimatoprost, polyquad (PQ)-preserved travoprost, and preservative-free (PF) latanoprost and tafluprost, all prostaglandin analogues (PGAs), on human conjunctival goblet cell (GC) survival. Furthermore, to investigate the effect of BAK-preserved and PF latanoprost on the cytokine secretion from GC.
MethodsPrimary human conjunctival GCs were cultivated from donor tissue. Lactate dehydrogenase (LDH) and tetrazolium dye colorimetric (MTT) assays were used for the assessment of GC survival. A cytometric bead array was employed for measuring secretion of interleukin (IL)-6 and IL-8 from GC.
ResultsBAK-preserved latanoprost and bimatoprost reduced cell survival by 28% (p = 0.0133) and 20% (p = 0.0208), respectively, in the LDH assay compared to a negative control. BAK-preserved latanoprost reduced cell proliferation by 54% (p = 0.003), BAK-preserved bimatoprost by 45% (p = 0.006), PQ-preserved travoprost by 16% (p = 0.0041), and PF latanoprost by 19% (p = 0.0001), in the MTT assay compared to a negative control. Only PF tafluprost did not affect the GCs in either assay. BAK-preserved latanoprost caused an increase in the secretion of pro-inflammatory IL-6 and IL-8 (p = 0.0001 and p = 0.0019, respectively) compared to a negative control, which PF latanoprost did not.
ConclusionBAK-preserved PGA eye drops were more cytotoxic to GCs than PQ-preserved and PF PGA eye drops. BAK-preserved latanoprost induced an inflammatory response in GC. Treatment with PF and PQ-preserved PGA eye drops could mean better tolerability and adherence in glaucoma patients compared to treatment with BAK-preserved PGA eye drops.
Prostaglandin analogue (PGA) eye drops are the first choice in the treatment of glaucoma due to a good intraocular pressure (IOP)-lowering efficacy, relatively mild topical adverse events and no systemic adverse events.1 Furthermore, PGA eye drops only require instillation once daily. Topical adverse events have been reported to include hyperemia, itching, burning, foreign body sensation, and blurred vision.2
PGA eye drops include latanoprost, travoprost, bimatoprost and tafluprost. The inactive forms of the PGAs are different, but when administered to the ocular surface, they are all metabolized to active prostaglandins F2α.3 PGA eye drops decrease resistance in the extracellular matrix of the conventional and unconventional outflow pathways through activation of matrix metalloproteinase (MMP) and relaxation of the ciliary body, which increases the outflow and reduces the IOP.3
PGA eye drops are available with and without preservatives. The most used preservative is benzalkonium chloride (BAK). The use of BAK is of major concern as IOP-lowering eye drops preserved with BAK have been reported to cause decreased tolerability and adherence compared to preservative-free (PF) PGA eye drops.45 Treatment with BAK-preserved eye drops is associated with the presence of dry eye disease (DED).67 The latter is characterized by a loss of tear film homeostasis. Tear film instability, hyperosmolality, ocular surface damage, and inflammation are key components in the development and maintenance of DED.8 DED is present in 60% of glaucoma patients,6 while only 5–30% of the elderly population experience symptoms of DED.9
In this context, goblet cells (GCs) are of major importance. GCs are secretory cells located at the mucosal surfaces, including the conjunctiva.10 GCs constitute a small part of the conjunctiva but are responsible for the secretion of soluble mucins of which MUC5AC is the most important. MUC5AC, densely packed in secretory granule, rapidly expands when released in the correct electrolyte and water composition.10 As part of the tear film MUC5AC contributes to the maintenance of a healthy ocular surface and lubrication upon blinking. Besides soluble mucins, transmembrane mucins including MUC1, MUC4 and MUC16 form a glycocalyx between the corneal epithelial cells and the tear film providing a pathogen barrier and increased lubrication.11
Thus, any loss of GCs can cause tear film instability. BAK-preserved PGA eye drops were previously identified as cytotoxic to cultured human conjunctival GCs.1213 BAK was further reported to increase inflammation identified through increased anterior chamber flare in patients treated with BAK-preserved eye drops compared to PF eye drops.14,15 BAK may thus contribute to the DED cascade through tear film instability and inflammation.
In this study, the cell survival of human GCs in primary cultures incubated with the following PGA eye drops were examined: Taflotan® (tafluprost, PF), Monoprost® (latanoprost, PF), Xalatan® (latanoprost, BAK-preserved), Lumigan® (bimatoprost, BAK-preserved) and Travatan® (travoprost, polyquad (PQ)-preserved). Furthermore, the effect of BAK-preserved and PF latanoprost eye drops on cytokine secretion from the GCs was compared.
Materials and methodsPGA eye drops usedPGA eye drops included the following ophthalmic solutions: Taflotan® (Santen, Tampere, Finland, 15 µg/mL tafluprost, PF), Monoprost® (Laboratoires Théa, Clermont-Ferrand, France, 50 µg/mL latanoprost, PF), Xalatan® (Pfizer, New York, NY, USA, 50 µg/mL latanoprost, 200 µg/mL BAK), Lumigan® (Allergan, Madison, NJ, USA, 300 µg/mL bimatoprost, 200 µg/mL BAK), and Travatan® (Novartis Europharm Limited, Dublin, Ireland, 30 µg/mL travoprost, 10 µg/mL PQ).
Cultivation of human conjunctival GCThe Danish National Committee on Health Research (H-17,007,902) and the Norwegian Regional Committees for Medical and Health Research Ethics (REK: 2013/803) approved the cultivation of GCs from human conjunctival tissue. GCs were cultivated for 14 days and incubated for three to five days after replating as previously described.16 The viability assays and cytokine measurements were performed on these first passage GCs. No data from donors was achieved as cell survival and cytokine release were calculated relative to a negative control from the same donor.
Measurement of GC survivalLactate dehydrogenase (LDH) and tetrazolium dye (MTT) assays were used to determine GC survival after cultures were incubated for 30 min with each eye drop diluted 1:7 in culture medium. As a negative control, GCs incubated with culture medium were included in all assays and cell survival was calculated relative to this control. The analyses were performed as previously described.12,16 Assays were performed on cultures from at least three different donors.
Measurement of cytokines and chemokine secretion from GCCytokine and chemokine measurements were performed on GC cultures incubated 30 min with BAK-preserved and PF latanoprost eye drops diluted 1:7 in culture medium. GC culturess incubated with culture medium were included as a negative control. The array was performed as previously described (11). The supernatants were incubated with a cytokine XL and chemokine cytometric bead array from Becton Dickinson (BD) (BD Biosciences, NJ, USA) following the manufacturer's instructions. The secretion of the following cytokines and chemokines was determined by a BD Accuri™ C6 personal flow cytometer (BD Biosciences, NJ, USA) array: interleukin (IL)‐8 (C‐X‐C Motif Chemokine Ligand (CXCL)‐8), IL‐1b, IL‐6, IL‐10, tumor necrosis factor (TNF), IL‐10p70, C‐C Motif Chemokine Ligand (CCL)‐5/Regulated upon Activation, Normal T‐cell Expressed, and Secreted (RANTES), CXCL9/monokine induced by gamma interferon (MIG), CCL2/monocyte chemo attractant protein (MCP)‐1 and CXCL10/Interferon gamma‐induced protein (IP)‐10. The array was performed on cultures from three different donors. A total of three measurements were performed on each sample.
Statistical analysisThe software program GraphPad Prism version 9.0.0 for Windows (GraphPad Software, San Diego, California USA) was used for statistical analyses and graphics. One significant outlier was discarded from the IL-6 data set based on an 0.05 significance level. Normal distribution of all data sets was confirmed through QQ-plots. One-way analysis of variance (ANOVA) with mixed effect was applied and Dunnett's multiple comparison test was used. A p-value of ≤ 0.05 was considered statistically significant. Cytokine measurements were interpolated on the standard curve generated from the internal standards of the Cytokine XL and Chemokine kits. Statistical analyses were performed on the ratio of measured cytokines from GCs incubated with eye drops to the control. A mixed effect statistical model was applied as it correlates observations within subjects thus reducing the effect of measurement variability. Due to great donor variability in IL-6 control measurements, analyses were performed on the ratio compared to control and not on absolute numbers.
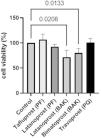
ResultsGC survivalCell survival was significantly lower in cultured GCs incubated with BAK-preserved latanoprost (72%, SD 14.0, p = 0.0133) and BAK-preserved bimatoprost (80%, SD 8.6, p = 0.0208) compared to the control according to the LDH assay (Fig. 1). PF tafluprost (107%, SD 11.0, p = 0.4443), PF latanoprost (93%, SD 5.0, p = 0.2146) and PQ-preserved travoprost (101%, SD 8.5, p = 0.9988) did not affect cell survival.
Relative cell survival of primary cultured human conjunctival goblet cells after 30 min incubation with prostaglandin analogue eye drops assessed by lactate dehydrogenase assays. Results are shown as mean cell survival relative to 100% survival in the control group. The bars indicate the standard deviation. Only p-values < 0.05 are shown. BAK = benzalkonium chloride, PF = preservative-free, PQ = polyquad.
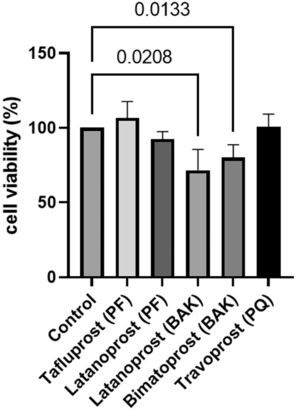
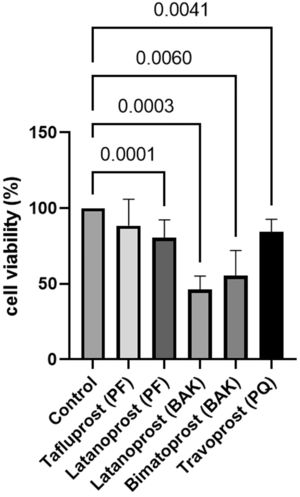
Cell survival was significantly lower in cultured GCs incubated with BAK-preserved latanoprost (46%, SD 9.0, p = 0.003), BAK-preserved bimatoprost (55%, SD 16.6, p = 0.006), PQ-preserved travoprost (84%, SD 8.5, p = 0.0041) and PF latanoprost (81%, SD 11.7, p = 0.0001) compared to the control according to the MTT assay (Fig. 2). Only PF tafluprost (88%, SD 17.5, p = 0.3301) did not affect cell survival.
Relative cell survival of primary cultured human conjunctival goblet cells after 30 min incubation with prostaglandin analogue eye drops assessed by tetrazolium dye colorimetric assays. Results are shown as mean cell survival relative to 100% survival in the control group. The bars indicate the standard deviation. Only p-values < 0.05 are shown. BAK = benzalkonium chloride, PF = preservative-free, PQ = polyquad.
Secretion of the pro-inflammatory IL-6 and IL-8 was detected. Expression of the remaining cytokines included in the analyses was not detectable.
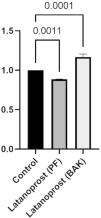
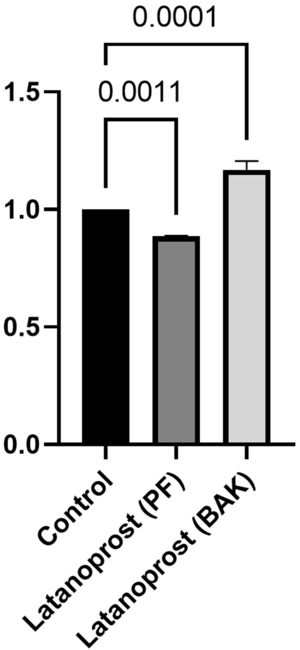
IL-6 secretion was significantly higher in cultured GCs incubated with BAK-preserved latanoprost (641 pg/mL, SD 235) compared to the control (554 pg/mL, SD 206, p = 0.0001), while the secretion was significantly lower for GCs incubated with PF latanoprost (504 pg/mL, SD 169) compared to the control (p = 0.0011) (Fig. 3).
Ratio of secretion of interleukin (IL)−6 from primary cultured human conjunctival goblet cells after 30 min incubation with benzalkonium chloride (BAK) and preservative-free (PF) latanoprost eye drops compared to a negative control assessed by a cytometric bead array. Bars indicate standard deviation. The brackets show the p-value. Only p-values < 0.05 are shown.
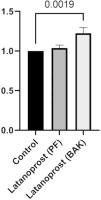
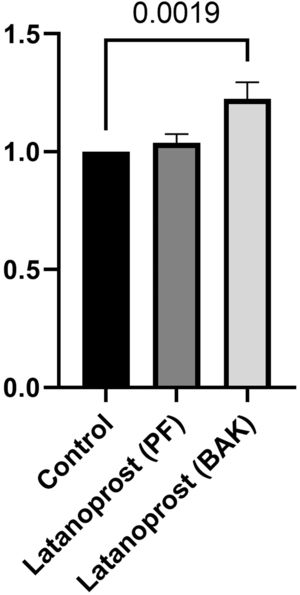
IL-8 secretion was significantly higher in cultured GCs incubated with BAK-preserved latanoprost (1523 pg/mL, SD 179) compared to the control (1254 pg/mL, SD 100, p = 0.0019). No difference between the control and GCs incubated with PF latanoprost was identified (1295 pg/mL, SD 59, p = 0.555) (Fig. 4).
Ratio of secretion of interleukin (IL)−8 from primary cultured human conjunctival goblet cells after 30 min incubation with benzalkonium chloride (BAK) and preservative-free (PF) latanoprost eye drops compared to a negative control assessed by a cytometric bead array. Bars indicate standard deviation. The brackets show the p-value. Only p-values < 0.05 are shown.
IOP-lowering prostaglandin analogue eye drops preserved with BAK, Polyquad or PF were evaluated regarding the effect on cell survival of primary cultured human GCs. Furthermore, pro-inflammatory cytokines IL-6 and IL-8 secretion from GCs incubated with PF or BAK-preserved latanoprost was evaluated.
BAK-preserved latanoprost and bimatoprost eye drops reduced GC survival significantly in both the LDH assay and MTT assay. PQ-preserved travoprost and PF latanoprost also reduced GC survival according to the MTT assay, but less than BAK-preserved latanoprost and bimatoprost. Only PF tafluprost was not cytotoxic in either assay. BAK is hypothesized to be cytotoxic by inhibiting complex I in mitochondria, thereby inhibiting ATP and O2 consumption.17 MTT assays measure the mitochondrial effect rather than cell death. This could explain the dramatic effect of BAK-preserved eye drops in the MTT assay. The generally lower cell survival measured in the MTT assay could be due to decreased or blocked proliferation or stress identified in the MTT assay which, ultimately, did not lead to irreversible cell death and hence was not measured in the LDH assay. PQ-preserved travoprost was previously found not to be cytotoxic in human GC cultures evaluated by LDH assay after 120 min incubation,12 different when compared to 30 min in the present study. Reduced cell survival was, however, evident in the present study after only 30 min in the MTT assay supporting this theory of mitochondrial dysfunction. BAK-preserved PGA eye drops are also more cytotoxic to conjunctival and meibomian epithelial cells compared to PF and PQ-preserved PGA eye drops.18–21 These findings suggest that BAK not only damages the GCs but the entire ocular surface.
In the current study, in contrast to PF latanoprost, BAK-preserved latanoprost caused significantly increased secretion of IL-6 and IL-8. IL-6 and IL-8 are pro-inflammatory cytokines.22,23 IL-6 is known to be involved in the pathology of DED24 and low levels of IL-6 in the aqueous humor has been associated with a better outcome after trabeculectomy.25 IL-8 has a prominent pro-angiogenic effect, as IL-8 increases endothelial proliferation, inhibits apoptosis, and increases the expression of MMP.26 Thus, any increase in IL-6 and IL-8 poses a risk to the homeostasis of the ocular surface. In patients treated with BAK-preserved PGA eye drops, the concentrations of multiple cytokines (including IL-6 and IL-8) in the tear film increased compared to patients treated with PF PGA eye drops.27,28 When switching from BAK-preserved latanoprost to PF tafluprost, a decrease in tear cytokine secretion was observed, suggesting that there is a recovery potential when switching to PF eye drops.29 The absence of inflammatory response when applying PF latanoprost suggests that PF is favorable to BAK-preservation in terms of minimizing stress responses at the ocular surface and risk of developing DED. As the major difference between the eye drops is the presence of BAK, BAK is likely the cause of this increase. PF latanoprost appeared to decrease the IL-6 secretion. This is, however, likely due to a small population size and large intra- and inter-donor variations rather than a potentially immunosuppressant effect.
The study was performed on cultured GCs, and cells may act differently in vitro compared to in vivo. The cells were subjected to a single, constant 30 min exposure to PGA eye drops. In patients, the concentration of drugs and preservatives at the ocular surface is not constant but will quickly decrease. On the other hand, the administration of eye drops will be repeated every day for many years. The present study does, however, give valuable insight into the effect of BAK on the local environment of the ocular surface and its potentially damaging role in the development of DED.
Adherence to treatment is generally poor in glaucoma patients partly due to side effects.30 As mentioned, preservation with BAK has been associated with more severe DED.6 Preservation with BAK is problematic, as it may contribute to decreased disease control and preventable blindness. Evidence suggests that BAK even reaches deeper structures of the eye than the ocular surface. In rabbits treated with BAK-preserved eye drops, BAK was identified at the trabecular meshwork (TM) and the optic nerve.31,32 In cultured TM cells, BAK caused increased IL-6 and IL-8 levels.33 BAK may, thus, cause inflammation and damage to the TM. This will increase resistance, decrease outflow, and increase IOP. Potential cytotoxic damage to the optic nerve will cause contradictory damage to the very same tissue, that the treatment is intended to preserve, thus risking progression of the disease and potential blindness. Altogether, BAK may not only increase the risk of topical adverse events and thus decrease adherence to drug prescriptions but may also prevent treatment from lowering the IOP and increase the retinal damage. Alternative preservatives and PF options are readily available thus use of BAK seems unnecessary.
ConclusionWe conclude that BAK-preserved PGA eye drops are more cytotoxic to human cultured GCs compared to PQ-preserved and PF PGA eye drops. Furthermore, BAK-preserved eye drops appear to have a pro-inflammatory effect on cultured GCs through increased secretion of IL-6 and IL-8. The use of PGA eye drops is lifelong, and the preservation with BAK can cause long-term damage to the ocular surface. Overall, based on the current knowledge and this study, PF or PQ-preserved PGA treatments should be prioritized compared to BAK-preserved PGA to reduce topical adverse events in patients with glaucoma.
Ethical approval statementThe study has been approved by the Danish National Committee on Health Research (H-17,007,902) and the Norwegian Regional Committees for Medical and Health Research Ethics (REK: 2013/803).
Contribution statementConceptualization of the study was performed by MK, SH, DD, TU, and GP. Data curation was conducted by AH, JF, PMH, and GBL. Formal analysis was performed by AH, GBL, MK, and GP. Writing of the original draft was performed by AH and MK. All authors revised and & edited the final manuscript.
Special thanks to laboratory technician Charlotte Taul.
The study was funded by The Danish Eye Research Foundation, Fight for Sight Denmark, The Synoptik Foundation, and the Bagenkop Nielsens Eye-Foundation.