A novel combination device for objective and subjective refraction was assessed regarding repeatability, reproducibility, validity, vision quality, and timing for experienced and untrained examiners.
MethodsSix examiners (each three trained optometrists and untrained examiners) examined 33 healthy participants for objective and subjective refraction. The ZEISS VISUCORE 500 (VC) objective and subjective refraction combination device with an algorithm-based guided and regular custom mode was compared to a conventional control procedure (wavefront aberrometer and phoropter).
ResultsFor the objective measurement of spherical equivalent refractive error (SE), VC shows good repeatability (≤ ±0.29 D) and reproducibility (≤ ±0.28 D) across all examiners versus control (±0.34 D and ≤ ±0.30 D, respectively), measured only by trained examiners. VC measures slightly negative objective refractions for SE with a mean difference (MD) and 95% limits of agreement (LoA) of -0.07 ± 1.24 D analyzed for a trained examiner. Subjective refraction for VC guided and custom mode showed good repeatability (≤ ±0.64 D) and reproducibility (≤ ±0.59 D) regarding SE for the examiner level. The results were similar to control (±0.54 D and ±0.51 D, respectively) with MD and LoA of +0.12 ± 0.67 D and +0.14 ± 0.66 D for VC guided and custom for trained examiner level. No significant differences were observed in best-corrected visual acuity across the three refraction workflows (all p > 0.05). The VC guided procedure was the most time-efficient, requiring less than 5 min of chair time.
ConclusionsThe tested combination refraction device provides reliable and efficient objective and subjective refraction measurements across trained and untrained examiners.
Accurate and efficient refraction is fundamental for prescribing corrective lenses and assessing visual function. Conventional refraction procedures, including objective and subjective methods, require training, time, and specialized equipment. Objective refraction, commonly performed with autorefractors or wavefront aberrometers, provides a starting point by estimating refractive error without patient input.1,2 However, the final prescription accuracy depends on the subjective refraction, where the patient's response fine-tunes the correction.3 While subjective refraction remains the gold standard, it is examiner-dependent, time-consuming, and requires professional training to follow a decades-old standardized process for determining monocular and binocular sphero-cylindrical refractive errors.2,3
Standard subjective refraction presents several challenges. From a human resource perspective, an experienced optometrist is required to perform the subjective refraction procedure and to obtain reliable results that qualify for a lens prescription. This process is also space-consuming, typically requiring a room with a ≥ 5 meter refraction lane along with several hardware components such as a chair, phoropter/trial lenses, projection screen, and a separate autorefractor for objective refraction. Furthermore, subjective refraction workflows are time-consuming, usually taking around 8 minutes to complete.4 These factors contribute to bottlenecks in highly frequented optician stores or practices, especially as the demand for prescription lenses is increasing due to an aging population and increasing prevalence of refractive errors.5–7 Consequently, there is a growing need for more time-, training- and space-efficient refraction workflows and setups that can facilitate these challenges.
The ZEISS VISUCORE 500 (VC) is a novel instrument integrating objective and subjective refraction in one system. It fuses the hardware needed for objective and subjective refraction procedures on one table, taking up less space than a conventional multi-device setup. Its algorithm-based guided subjective refraction mode assists untrained examiners by providing step-by-step guidance, including question prompts, answer interpretation, and subsequent lens adjustment. An additional custom subjective refraction mode provides full manual control, allowing experienced optometrists to conduct refraction without assistance.
Therefore, this study investigates the efficiency and examiner dependency of the novel VISUCORE 500 by assessing repeatability, reproducibility, visual acuity, and test duration of objective and subjective refraction for trained and untrained examiners. The results are compared against conventional procedures, to determine whether the novel approach can provide a reliable and time-efficient alternative for refraction methods in optician stores and clinical practices.
Material & methodsStudy participantsIn total, both eyes of 33 participants (24 females, 9 males) were measured. Exclusion criteria were as follows: self-reported ocular pathology, previous ocular surgery, rigid gas permeable contact lens wear within the last 4 weeks, and soft contact lens wear within the last 24 h prior to measurements. The prospective study was approved by the Ethics Committee of the University of Tübingen and conducted at the Institute for Ophthalmic Research, Tübingen. Signed informed consent was obtained from all study participants before measurements were taken.
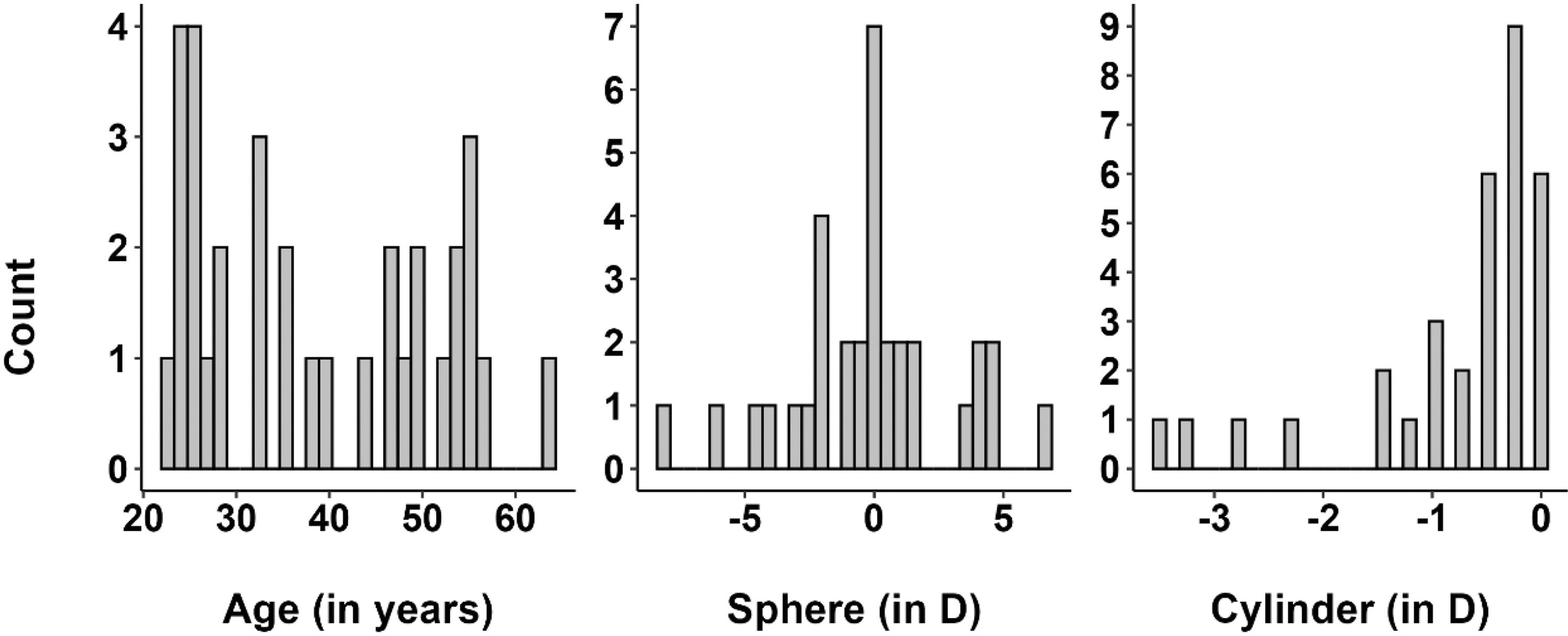
The median age of the study participants was 36 years, with an age range of 22 to 63 years (Fig. 1a). The median subjective spherical refractive error of the right eyes of the sample was 0.00 D (range +6.75 to −8.00 D, Fig. 1b), and the median cylinder power was −0.50 D (range 0.00 to –3.5 D; Fig. 1c), measured with a phoropter by a trained optometrist.
Instrumentation and setupObjective refraction accuracy was assessed by comparing the novel binocular open-field device (ZEISS VISUCORE 500 "VC", Carl Zeiss Vision GmbH, Germany) with a conventional monocular closed-field device (ZEISS i.Profiler+, Carl Zeiss Vision GmbH, Germany). Both devices use Hartmann-Shack wavefront aberrometry. Objective refraction was performed in a darkened room to allow for a maximum pupil size. Subjective refraction was performed with room lights at 260 lux, adhering to DIN EN ISO 8596 standards.8,9 All measurements were performed without cycloplegia, to reflect real-world clinical conditions. Subjective refraction of the VC was evaluated using a built-in phoropter and a complementary display positioned 1 meter from the participant. This setup utilized internal mirror projections to simulate an optical path distance of 5 meters (see Fig. 2a). The subjective refraction workflows of the VC are based on the objective measurements of the VC as starting values. The results were compared to those obtained from a conventional phoropter (ZEISS VISUPHOR 500, Carl Zeiss Vision GmbH, Germany) paired with a calibrated display (ZEISS VISUSCREEN 500, Carl Zeiss Vision GmbH, Germany) that was placed 4.80 meters from the participant. The starting values of the conventional subjective refraction procedure were based on the conventional monocular closed-field wavefront aberrometer. For both devices and refraction procedures, the right eyes were always measured first.
Fig. 2a) Setup of the VISUCORE 500 with its main refraction components: (a) binocular open field refractor and phoropter unit with folding near display (currently closed on the image) and (b) display unit containing mirrors at 1 m distance from the phoropter imitating a far vision distance of 5 m. The total setup requires about 1.5 m² of space. Fig. 2b) Algorithm outline for the VISUCORE 500 guided subjective refraction procedure, on which the VISUCORE 500 custom and conventional procedures were based.
For the combination of objective and subjective refraction, a headrest and a detachable chin rest were used for the VC, allowing head stabilization during objective refraction and free speech during subjective refraction. All devices were controlled by a tablet (iPad Air 2, Apple Inc., USA) and an integrated application (ZEISS VISUCONSULT 500, v3.5.1., Carl Zeiss Vision GmbH, Germany).
Subjective refraction methodsThe VC subjective refraction included two test modes, a guided mode (VC guided) and a custom mode (VC custom), whereby objective VC values served as a baseline for the subjective investigation.
The VC guided mode is an algorithm-based assisted method for examiners with little to no subjective refraction training and experience. Therefore, it provides detailed instructions on the control interface for the examiner to conduct the refraction, like the questions to be asked to the participant (e.g. “Please read the displayed letters.” or “Does it get blurry with the next lens?”). The examiner then has to indicate the participant’s answer on the control interface (e.g. the number of correctly read letters or “Yes”/“No”). Based on the given answer as input, the built-in algorithm automatically determines the next refraction steps, e.g. lenses/letters to be shown and questions to be asked with predefined answer options, as described in Fig. 2b.
In case of low visual acuity (≥ 0.3 logMAR), large interocular differences (≥ 0.2 logMAR) or large deviations from the objective values (sph/cyl ≥ 1.00 D, axis ≥ 15°), the guided mode stops the ongoing subjective refraction process. It informs the examiner that the refraction process needs to be restarted. In the case of two cancellations in the VC guided mode, the respective examiners stopped this specific workflow in the current study. A workflow was considered successful if the subjective refraction process was completed without any cancellations.
In contrast to the VC guided mode, the VC custom mode is intended for experienced examiners without support during the refraction process. The refraction flow, stimuli and increments for sphere, cylinder, and axis were set to be the same as during the VC guided mode: First, monocular visual acuity, best sphere, cylinder axis, and power via the split cross-cylinder, and the best sphere was measured for each eye. Afterward, the binocular balance test and best-sphere testing were performed.
The standard subjective and VC custom refraction procedure included the same steps and minimum increments for sphere, cylinder, axis, and addition as the VC guided mode.
Study protocol and examinersEach participant was measured by three expert examiners who were trained optometrists (“trained”) and three untrained examiners who were not optometrists, with no prior experience in performing subjective refraction and only basic general knowledge about human vision (“untrained”). Measurements were split into separate visits to avoid participant fatigue and loss of attention. All sessions were scheduled at the same time of day to minimize the influence of diurnal visual fluctuations. At the beginning of each session, a 10-minute washout phase without near work was included to minimize the influence of prior visual tasks.
Subsequently, in a randomized order, each participant underwent the following subjective procedures (control, VC guided, and VC custom) with the associated objective measurements (control and VC) beforehand. In addition, the duration of each measurement procedure was recorded, including the time needed to send the values from the objective to the subjective workflow. Furthermore, the best-corrected monocular and binocular visual acuity were determined across the workflows for each examiner.
The three trained examiners performed all procedures on each participant. Individual examiner measurements included one examiner repeating all three workflows (control, VC guided, VC custom) twice and another measuring monocular and binocular best-corrected visual acuity with the conventional acuity display, using the final refractions from each subjective workflow.
The three untrained examiners performed the objective and guided subjective refraction followed by visual acuity testing on all participants using the VC device. The time for each successful completion was recorded for each examiner. One untrained examiner always performed the procedure described twice to assess repeatability.
Refraction data analysis and statisticsData were analyzed using R (Version 4.4.1, R Core Team, Austria) and only the data of the right eye were used to avoid interocular correlation effects.10 All objective values were normalized to a vertex distance of 12 mm and recalculated by a pupil diameter of 3 mm, and subjective values to a vertex distance of 12 mm for consistent analysis. All sphero-cylindrical refractions were transformed to power vectors M (spherical equivalent), J0 (orthogonal astigmatism), and J45 (oblique astigmatism).11 Datapoints outside the 1.5-fold interquartile range (IQR) were defined as outliers and removed from the dataset. In total, six datapoints were identified as outliers. Descriptive statistics are given as median and IQR. Repeatability is expressed by the coefficient of repeatability as 1.96x the standard deviation of differences between repeated measures, and the 95 % limits of agreement, representing the range within 95 % of the two repeated measurements are expected to lie.12 Data for repeatability was obtained by one trained and one untrained examiner performing each workflow twice. Reproducibility, expressed by the coefficient of reproducibility, was analyzed separately across trained and untrained examiners using a cumulative distribution function.13 Agreement between devices and workflows was calculated using Bland-Altman analysis.12 The same trained examiner who initially measured the participants obtained the data to assess agreement. Further statistical analysis was conducted to investigate if there were any differences in visual acuity or timing between examiners and workflows. Normal distribution was tested via the Lilliefors test. In the case of two comparison groups, the paired t-test (parametric distribution) or paired Wilcoxon-test (non-parametric distribution) were applied. ANOVA (parametric distribution) or Kruskal-Wallis test (non-parametric distribution) was used to compare more than two groups. Alpha levels were set to 0.05, and results were considered significant if p < 0.05.
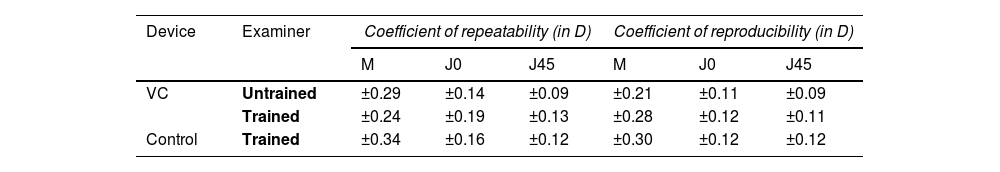
ResultsRepeatability and reproducibility of objective refractionTable 1 gives an overview of the repeatability and reproducibility of the power vectors M, J0, and J45 for both devices and examiner groups. The coefficient of repeatability for M was slightly lower using the VC (±0.29 D and ±0.24 D for the untrained and trained examiner, respectively) compared to the control device (±0.34 D for the trained group). Better results were reported for the cylindrical powers J0 and J45 reporting values between ±0.09 D and ±0.19 D across the examiners and devices.
The VC shows a slightly lower coefficient of reproducibility for M (±0.21 D and ±0.28 D for untrained and trained examiners) compared to the control device (±0.30 D, only trained examiners). Again, J0 and J45 reported better and similar reproducibility than M, ranging from ±0.09 D to ±0.12 D across both devices and examiner levels.
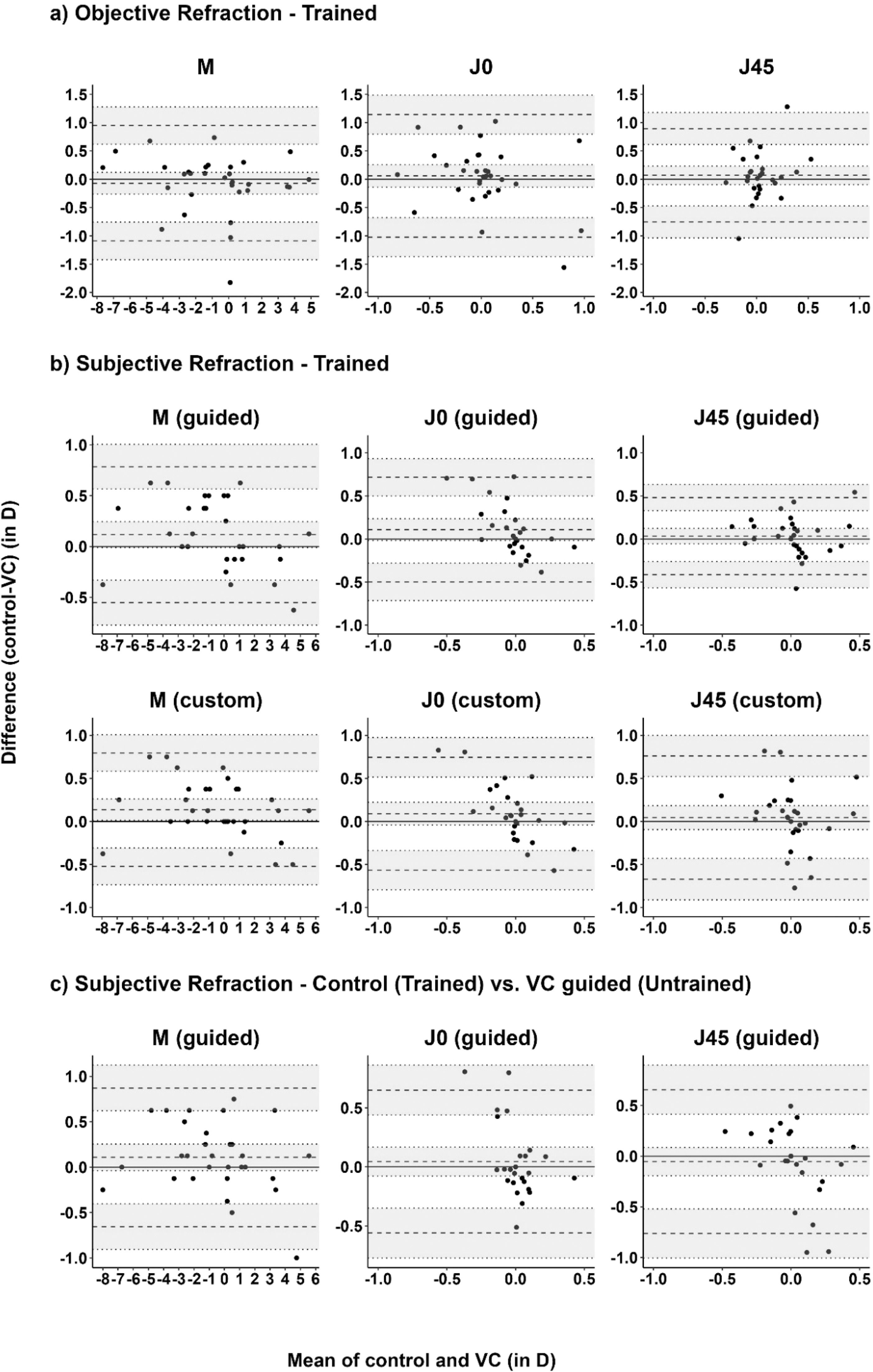
The objective refraction with both devices was compared for the first expert examiner to assess agreement. VC aberrometry yields a slightly more negative (more myopic or less hyperopic) spherical equivalent (M) than the control device with a mean difference with 95 % limits of agreement (−0.07 ± 0.84 D), see Fig. 3a. J0 and J45 of VC objective refraction were more positive than control (+0.06 ± 1.08 D and +0.07 ± 0.82 D, respectively).
Bland-Altman plots showing mean difference and 95 % limits of agreement for the power vectors M, J0, J45 of a) objective refraction of control vs. VC, and b) subjective refraction comparing control, VC guided, and VC custom mode, measured by the first trained examiner, and c) subjective refraction comparing control by the first trained examiner and VC guided mode by the first untrained examiner (n = 33 participants).
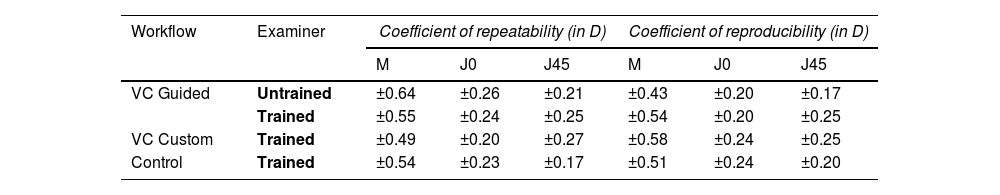
Table 2 gives an overview of the repeatability and reproducibility of the different subjective refraction protocols. Within the VC guided mode, the coefficient of repeatability for the spherical equivalent (M) was slightly lower for the trained than the untrained examiner (±0.55 D vs. ±0.64 D). The repeatability for the astigmatic components was similar throughout all workflows and examiners, ranging from ±0.17 D to ±0.27 D
Coefficient of repeatability and reproducibility (in D) for subjective refraction components among different examiners (n = 33 participants).
The reproducibility for M was similar across all workflows and examiner groups ranging between ±0.43 D and ±0.58 D The same tendency was observed for the measurement of astigmatism with reproducibility from ±0.17 D to ±0.25 D for all workflows and examiner groups.
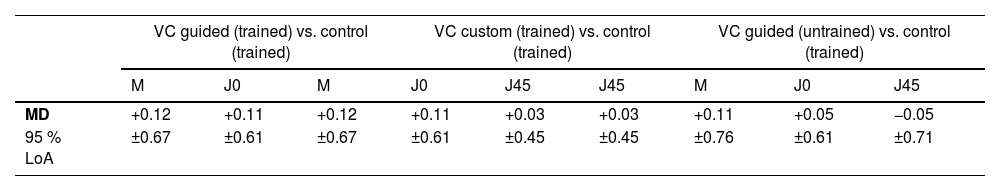
Agreement of subjective refraction modesThe mean difference between the control and both VC workflows ranges closely around zero for all refraction components (VC guided vs. control +0.03 D to +0.12 D; VC custom vs. control +0.05 D to +0.14 D), see Table 3 and Fig. 3b. The 95 % limits of agreement for the spherical equivalent, both astigmatic components and addition ranged from ±0.45 D to ±0.67 D and ±0.66 D to ±0.72 D for VC guided and VC custom refractions compared to control.
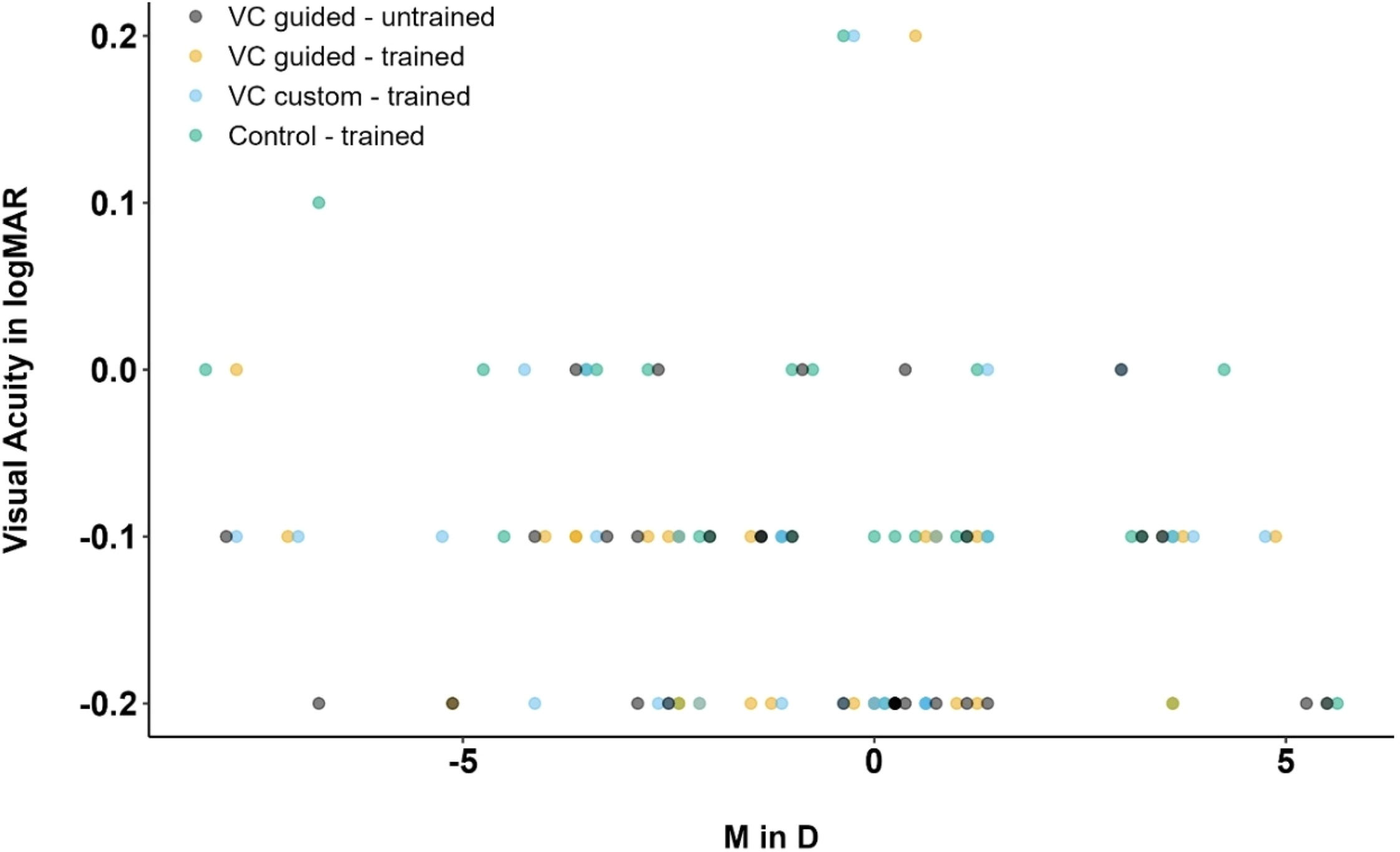
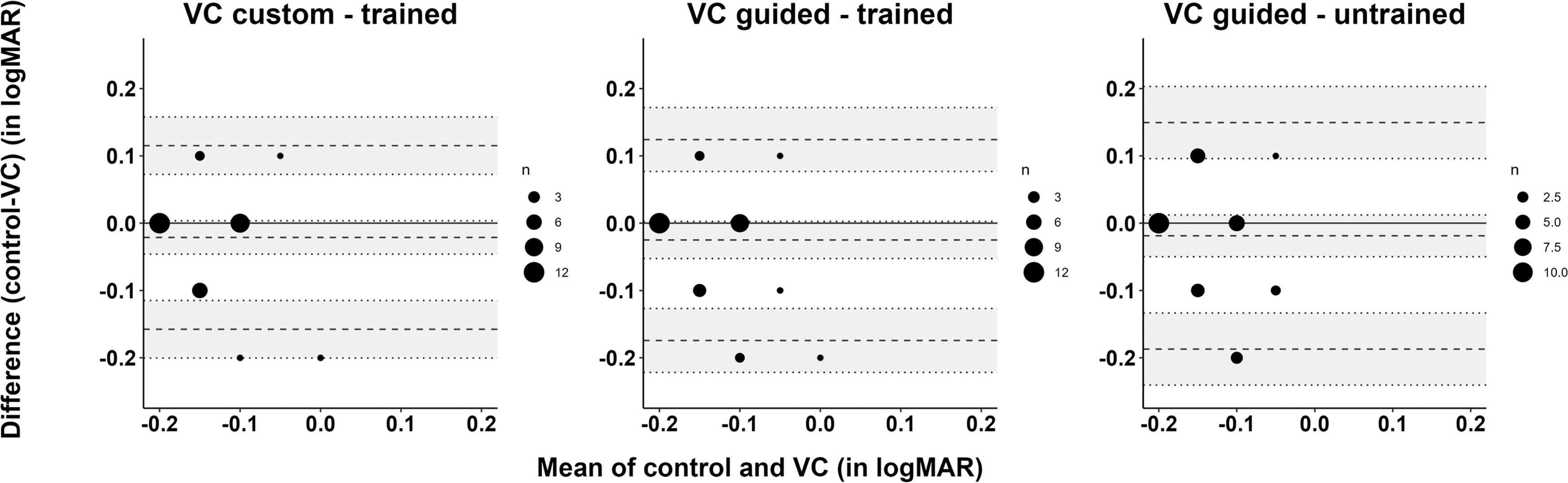
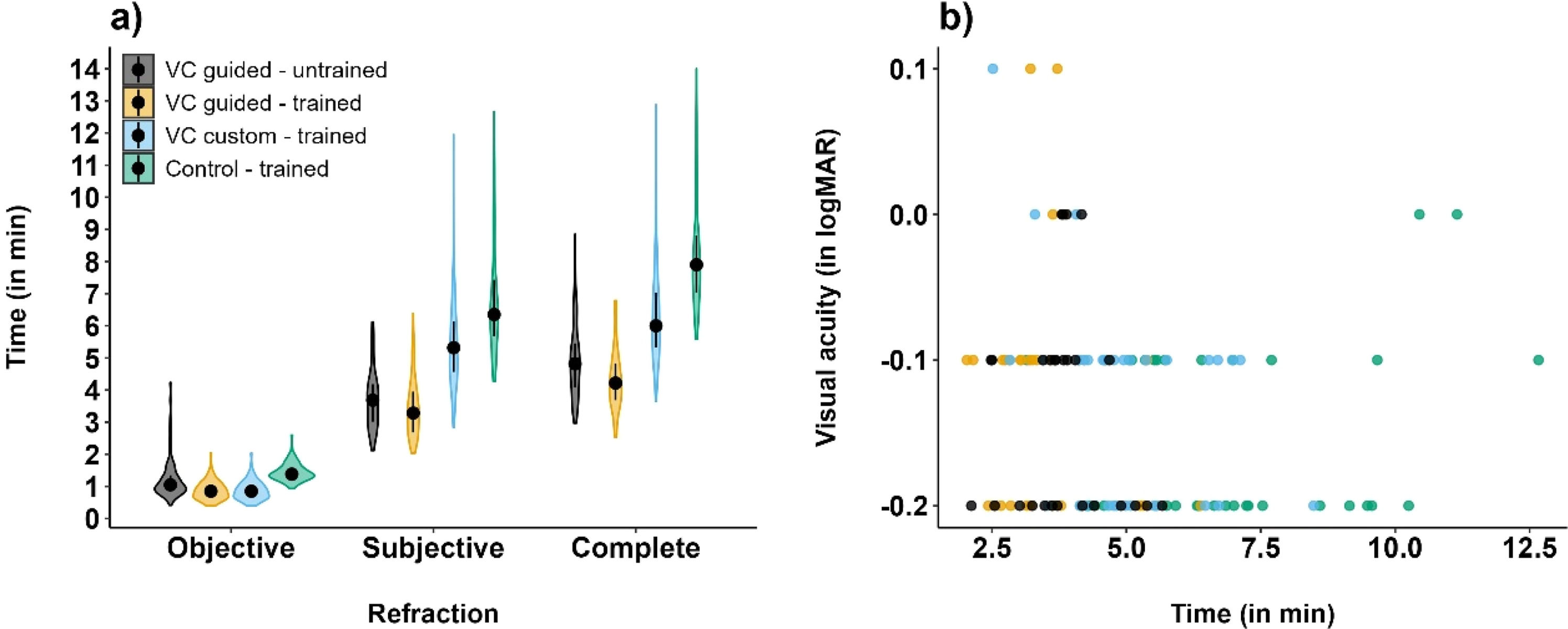
Best-corrected visual acuityAll subjective workflows led to an average visual acuity of −0.1 logMAR ranging from 0.2 logMAR to -0.2 logMAR across examiners and refraction workflows, see Fig. 4. Multivariate analysis showed that visual acuity did not significantly differ between the workflow methods (p = 0.31). Fig. 4 shows the correlation of the best-corrected visual acuity obtained by an untrained examiner following VC guided refraction mode (mean ± standard deviation: −0.18±0.07 logMAR), and by a trained examiner as a result of VC guided, VC custom, and control procedures (mean ± standard deviation, respectively: −0.13±0.08 logMAR, −0.12±0.09 logMAR and −0.12±0.09 logMAR). The untrained examiner achieved slightly better visual acuity results than the trained examiner, without a significant difference. Fig. 5 shows the agreement between the visual acuity of control, VC custom – trained, VC guided – trained and VC guided – untrained, with mean differences and confidence intervals of −0.02 [−0.16;0.12]; −0.03 [−0.17;0.12] and −0.02 [−0.19;0.15] (logMAR), respectively.
The time for the different objective and subjective workflows including visual acuity testing was measured and added up to the complete examination time (Fig. 4). The complete examination time is statistically significantly shortest with VC guided mode (4.2 min and 4.8 min for trained and untrained examiners), followed by VC custom mode (6.0 min) and control procedure (7.9 min), all p < 0.05. While the objective refraction procedure times were about 1 min (1.1 min, 0.9 min, 0.9 min and 1.4 min for VC guided – untrained, VC guided – trained, VC custom – trained and Control – trained; all p < 0.05, except VC guided – trained vs. VC custom – trained (n.s.)), the subjective refraction times showed a greater disparity, averaging 3.7 min, 3.2 min, 5.3 and 6.5 min, respectively (all p < 0.05, except VC guided – untrained vs. VC guided – trained (n.s.)).
Moreover, 87 % of all refraction measurements in the VC guided mode were completed on the first run-through. 8 % of cases needed one restart but were then completed successfully, whereas 5 % of cases required a second restart or a trained examiner, and thus, were defined as workflow cancellations per study protocol Fig 6.
a) Time duration (in minutes, median, and IQR) for the individual refraction workflow parts and the complete examination time. Differences were all statistically significant (p < 0.05) unless marked as not significant (n.s.); b) Correlation of time duration (in minutes) and visual acuity (in logMAR) for the different testing conditions (n = 33 participants).
This study assessed the repeatability, reproducibility, visual acuity, and efficiency of the novel VC wavefront aberrometry and its two integrated subjective refraction workflows for examiners with and without optometry background. The VC’s objective and subjective refraction values were compared against the control procedures based on conventional closed-field wavefront aberrometry in combination with a phoropter and projection display.
In summary, binocular open-field autorefraction with VC results in good repeatability for the spherical equivalent (≤ ±0.34 D) and astigmatic components (≤ ±0.19 D). These values are within the same range as the reported findings by Carracedo et al. investigating the repeatability of a similar open-field aberrometer setup (M ±0.41 D, J0 ±0.18 D, and J45 ±0.18 D) in 99 healthy eyes of participants aged 8 to 69 years.14 In the current study, the sample size (n = 33 participants) was determined based on power estimates for assessing repeatability and reproducibility using repeated measures designs. A minimum of 30 participants was estimated to detect clinically relevant differences (±0.25 D) with a standard deviation of 0.30 D, 80 % power, and α = 0.05, accounting for multiple measurements across three trained and three untrained examiners. Several other studies, testing the repeatability of various wavefront aberrometers using Hartman-Shack also observed similar values between ±0.25 D to ±0.72 D for M, and between ±0.12 D to ±0.28 D for J0 and J45 (for pupil diameters of 3 to 4 mm) in non-cycloplegic, healthy adult participants.15–17 The repeatability of objective refraction can be affected not only by the measurement accuracy of the device itself but also by variations in patient accommodation fluctuations, tear film quality, and fixation stability.18 Especially in hyperopes with larger accommodative fluctuations, a better control of accommodation is expected with a binocular open-field setup (VC) as opposed to a closed-field monocular setup such as the i.Profiler+,19 which was used as a control procedure. However, the mean spherical equivalent refractive errors measured with the VC were on average similar to the control device by (−0.07 ± 0.84 D). A more positive (in D) tendency with a mean difference for M of +0.20 ± 0.77 D was reported in a previous study also comparing a binocular open-field setup against a monocular wavefront aberrometer.14 For J0 and J45 vectors, their study reported mean differences and 95 % limits of agreement of +0.02 ± 0.22 D) and -0.02 ± 0.11 D, respectively, which is similar to the results of the current study.
Reproducibility results for both aberrometers fell in the same range as the repeatability values (≤ ±0.30 D). Moreover, they were similar between untrained (±0.28 D for VC guided) and trained examiners (±0.21 D for VC guided and ±0.30 D for control). These findings were expected, as there is only minimal examiner input needed during the objective refraction procedure. Previous studies reported similar findings for the spherical equivalent with reproducibility of ±0.38 D for objective conventional autorefraction in adults.20
The repeatability of subjective refraction does not vary much between workflows and examiners: for the spherical equivalent up to ±0.55 D and ±0.64 D for the trained and untrained examiners, respectively. The numbers are within ranges of previous studies that range between ±0.29 D and ±0.75 D of spherical equivalent.21–26 The repeatability for astigmatism measurements ranges from ±0.17 D to ±0.26 D for both examiners and across all refraction procedures, which is in good agreement with other studies reporting a repeatability from ±0.11 D to ±0.37 D22–27
No clinically relevant differences across refraction workflows and examiner groups were found for reproducibility. Values ranged from ±0.43 D to ±0.58 D (M), from ±0.17 D to ±0.25 D (J0, J45). This is in accordance with previous literature that found inter-examiner reproducibility for spherical equivalents and astigmatism between ±0.48 D and ±0.78 D and between ±0.14 D to ±0.40 D, respectively.28–34 In general, there was very good agreement between the two subjective VC refraction processes and the control procedure.
For the subjective VC guided mode compared to the control, the mean difference in spherical equivalents was +0.12 D with 95 % limits of agreement of ±0.67 D, which is commonly found when comparing algorithm-based procedures to the conventional manual subjective refraction in healthy populations.24,26,35–40 The mean difference for the spherical equivalent was +0.11 ± 0.61 D for the VC custom mode compared to the control, which is similar to the VC guided mode by trained examiners and untrained examiners and no relevant bias from a clinical perspective, where only differences of more than ±0.25 D or ±0.50 D are considered significant.21 These findings show the system’s robustness under real-world and low-expertise conditions in eye-healthy participants. Special attention needs to be paid to the directionality of the offset of spherical equivalent, as algorithm-based workflows tend to lead to over-minus, likely caused by not enough accommodation control in the questioning techniques.24,35,37 Consequently, the algorithm in the VC guided mode seems to have good control over accommodation without overcorrection of myopia or undercorrection of hyperopia. The differences in J0 and J45 results are in the same range as the spherical equivalent with a maximum 95 % limits of agreement of ±0.72 D, as reported by other studies as well.24,26,35,39 These studies varied in methodology, as different refraction procedures were used, such as custom-built24 or semi-automated systems,26,39 while others integrated ocular aberrometry.35 However, these studies included healthy participants with a broader age range, compared to the current study.
Consequently, both trained and untrained examiners achieved comparable repeatability and reproducibility in objective and subjective measurements, suggesting that the automated guidance and structured workflow of the VC system enable reliable refraction results in healthy adult participants, even when performed by non-optometrists.
Best-corrected distance visual acuities for VC were shown to be generally very high (0.2 logMAR to -0.2 logMAR) and independent of refraction workflows and examiner group. Especially, independent of the refractive error, untrained examiners reached good visual acuity in participants using the VC guided mode. Additionally, visual acuities were slightly better across all VC workflows and examiners compared to the control procedure. This supports the usage of the guided refraction procedure and the use of final results as prescription values, where access to eye care professionals is limited. Two other studies got similar results when comparing visual acuity derived from algorithm-based vs. conventional subjective refraction.35,39 Nonetheless, as only healthy adults were included in the current study, further studies are needed to confirm these results in broader populations, including those with complex refractive errors, binocular problems, accommodative issues, or older and younger age. Time efficiency is another important factor in performing refraction procedures. Even though it is not clinically relevant, it affects daily work. The VC guided workflow is the shortest workflow with on average less than 5 minute duration for the complete chair time throughout all examiner groups. The slowest workflow was observed for the control protocol, with an average chair time of about 8 minutes, which is in good accordance with previous findings.4 This difference in time is mainly due to three factors: a) there is no need to change devices and re-center the eye with the VC system between objective and subjective refraction; b) the objective procedure works binocularly instead of monocularly sequentially, and c) the time efficiency of the implemented algorithm for the subjective guided mode. The same tendency was apparent in a previous study with another algorithm-based refraction procedure.39 However, it is noteworthy that the time and operator efficiency of the guided mode come with the compromise of potential workflow restarts or cancellations in more difficult refraction cases. While 95 % of cases could be completed successfully in the guided mode, 5 % of measurements were unable to be finished per study definition as a second restart was forced due to inaccurate measurements, fixation instability, or accommodation during the VC objective refraction procedure. This minority requires the support of an experienced refraction examiner, either to troubleshoot the issue or to initiate a custom refraction process. Furthermore, although the test durations were significant across the procedures, except for objective VC guided trained vs. VC custom trained, and subjective VC guided – untrained vs. VC guided trained, the average differences are small and therefore negligible.
Nonetheless, as the current study measured only healthy eyes, the findings cannot be generalized to individuals with ocular pathologies or more complex refractive characteristics. Therefore, the performance of the VC device has yet to be tested for participants with color vision deficiency, binocular problems, media opacities, or corneal irregularities, such as after refractive surgery or in keratoconus. Here, previous work in for example keratoconus participants revealed an expected drop of repeatability by factors 2 to 3.23 These pathologic conditions may affect accuracy, device usability, or the examiner’s ability to follow guided instructions. Furthermore, populations with neuro-ophthalmic disorders, reduced cognitive ability, or physical limitations, such as poor head control, may face challenges with the device interface or test duration. The current study also excluded children and older adults, whose refractive responses and cooperation levels may differ, thus limiting the broader clinical applicability of these results. Furthermore, eyes were not cyclopleged for accommodation control, which could have led to more stable results in hyperopes and fewer cancellations in the VC guided mode. This workflow is aborted if the discrepancy between objective and subjective spherical results exceeds 1.00 D. However, cycloplegia is usually not applied to adults, thus, the non-cyclopleged results can be seen as more realistic from a clinical point of view. Another limit is the test used for binocular balance with red-green dissociation, a test where the right and left eye see different non-fused stimuli though red and green filters, which the participant is able to fuse. This red-green presentation of stimuli might lead to an imbalance of the accommodation amplitude.41 However, the same test was applied to all subjective procedures. The VC guided procedure included one more limitation, as cylindric values from objective refraction smaller than 0.7 D were disregarded in the VC guided subjective procedure. This could have led to small reductions in visual acuity; however, all participants reached a good visual acuity within the range of 0.0 to -0.2 logMAR following the VC guided mode.
ConclusionThe novel VISUCORE 500 wavefront aberrometry system provides reliable refraction measurements with good repeatability, reproducibility, and efficiency, with small examiner-dependent variability. Comparable results between trained and untrained examiners underscore the system's robustness and clinical applicability. These results support its use in healthy eyes with uncomplicated refraction cases and limited availability in eye care professionals. Therefore, further investigations are needed to assess its reliability in eyes with ocular pathologies, younger and older populations, and higher refractive errors to fully assess the system’s clinical utility. These findings suggest that the VC is a practicable and reliable solution for the increasing demand for refractive measurements in health care.
Data availabilityData is available upon reasonable request.
FundingThis work was done in an industry-on-campus-cooperation between the University of Tübingen and Carl Zeiss Vision International GmbH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Further funding was received from Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen.
DisclosuresAR: None, KB: Carl Zeiss Vision International GmbH (E); ND: Carl Zeiss Vision International GmbH (E); JM: None; SW: Carl Zeiss Vision International GmbH (E)
The authors thank Linus Emmerich and Dr. Martin Dechant for their support during measurements.