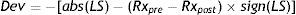To analyze the effect of seasonal changes in residual refraction 1-year after corneal refractive surgery using the SCHWIND AMARIS laser system.
Methods5740 consecutive treatments have been retrospectively reviewed. For all eyes, aspheric treatments were planned with the Custom Ablation Manager software and the ablations were performed with the SCHWIND AMARIS system (SCHWIND eye-tech-solutions). Seasonal outcomes were evaluated in terms of residual refraction stratified per treatment month, as well as stratified per year season. Student's T test comparing stratified values with global ones was used for the statistical analysis.
ResultsTreatments performed in April, June, August, September, and October showed relative undercorrections of the spherical equivalent (SE) (−0.09D), whereas treatments performed in January, February, and March showed relative overcorrections of the SE (+0.13D). Similarly, treatments performed in spring and summer showed relative undercorrections of the SE (−0.04D), whereas treatments performed in winter showed relative overcorrections of the SE (+0.10D).
ConclusionsSeasonal differences in refractive outcomes were observed among a large scale population. The effect of these environmental variables on refractive outcomes warrants further evaluation.
Analizar el efecto de los cambios estacionales en la refracción residual, transcurrido un año tras cirugía refractiva corneal empleando el sistema láser SCHWIND AMARIS.
MétodosSe revisaron retrospectivamente 5.740 tratamientos consecutivos. En todos los casos se planificaron los tratamientos asféricos con el software Custom Ablation Manager, realizándose las ablaciones con el láser excímer SCHWIND AMARIS (SCHWIND eye-tech-solutions). Se evaluaron los resultados estacionales en términos de refracción residual, estratificada por mes de tratamiento, y por estación del año. Se utilizó la prueba de t de Student para comparar los valores estratificados con los globales en el análisis estadístico.
ResultadosLos tratamientos realizados en abril, junio, agosto, septiembre y octubre reflejaron infracorrecciones relativas del equivalente esférico (EE) (-0,09D), mientras que los tratamientos realizados en enero, febrero y marzo mostraron unas sobrecorrecciones relativas del EE (+0,13D). De igual modo, los tratamientos realizados en primavera y verano reflejaron infracorrecciones relativas del EE (-0,04D), mientras que los tratamientos realizados en invierno mostraron sobrecorrecciones relativas del EE (+0,10D).
ConclusionesSe observaron diferencias estacionales en los resultados refractivos en una población a gran escala. El efecto de estas variables ambientales sobre los resultados refractivos justifica una evaluación adicional.
De Souza et al.1 evaluated the influence of temperature and humidity on laser in situ keratomileusis (LASIK) outcomes and concluded that operating room environment may influence LASIK outcomes; humidity may be more significant than temperature.
Walter and Stevenson2 determined whether environmental factors affect laser in situ keratomileusis (LASIK) enhancement rates and found out that the 2-week-preoperative mean outdoor relative humidity, procedure room relative humidity, outdoor temperature, and procedure room temperature may have to be considered during LASIK planning.
Schena et al.3 presented and discussed a theoretical model of the water vapor absorption at 193nm wavelength in order to quantitatively assess the influence of environmental parameters on the laser energy that actually reaches the corneal surface. Model simulations show that laser energy absorption (up to 7% of the available energy) occurs along the path of laser beam, into the existent space between the laser beam source and the patient's eye, and is caused by environmental temperature and relative humidity (35°C and 95%, respectively). Their findings suggest that this energy loss reduces the ablation rate, producing a significant under-correction of the treated corneas.
Dantas et al.4 evaluated excimer laser fluence after experimentally induced changes in room temperature and relative air humidity and concluded that in a setting with controlled temperature and relative air humidity, subtle changes in environmental factors do not appear to influence laser fluence and efficacy, but acknowledge that the variations seen in PMMA test ablations may not translate completely into clinical changes. Regarding tissue characteristics and specificity, stromal tissue may be more sensitive to environmental changes than PMMA because of the differences in ablation thresholds and the effects of dehydration.
Randleman et al.5 analyzed and compared retreatment rates after wavefront-optimized photorefractive keratectomy (PRK) and LASIK and determine risk factors for retreatment and found out that the retreatment rate of 6.3% in their cohort was not influenced by age, sex, corneal characteristics, or environmental factors.
Seider et al.6 determined whether procedure room temperature or humidity during LASIK affects the refractive outcomes in a large patient sample and concluded that neither procedure room temperature nor humidity during LASIK were found to have a clinically significant relationship with postoperative manifest refraction in their population. However, they stated that when evaluating all eyes in the population together, procedure room temperature and humidity did not show a clinically significant relationship with postoperative manifest refraction, although as expected, the relationships were statistically significant (P=.0094 for temperature and P<.0001 for humidity). Specifically, in the subgroup with a preoperative refractive error of +2.00 to +4.00D and aged 18–30 years, an increase in 1°C during LASIK was associated with a decrease in 1-month postoperative refractive error (more correction) of 0.048D.
We explore another approach to study the effect of temperature and humidity on refractive surgery outcomes. Studying the effects of variations in temperature and humidity during different seasons of a year, can shine more light on the statistical significance of the effect of these parameters on refractive surgery outcomes.
This retrospective chart review attempts to compare the postoperative refractive outcomes among a large population of patients that have undergone refractive surgery treatments using the SCHWIND AMARIS laser system performed at different times during the year in order to analyze seasonal changes in residual refraction 1-year after corneal laser refractive surgery.
MethodsPatient Population and Examinations5740 consecutive treatments performed between 16/08/2010 and 16/08/2012 were reviewed. The average age at the time of the surgery was 38±10 years (18–79 years). Patients included in the retrospective review had manifest refraction spherical equivalent (SE) ranging from −10.38D to +5.50D with up to 6.00D of astigmatism.7 Patients had best corrected distance visual acuity (CDVA) of 20/32 or better using the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart, stable refraction for 1 year prior to the treatment and discontinued contact lenses for at least 2–4 weeks (depending on contact lens type) prior to the preoperative evaluation.
Baseline examinations included measurement of uncorrected distance visual acuity (UDVA), CDVA, manifest refraction, corneal topography (Nidek OPD Scan, Nidek Co., Ltd., Gamagori, Japan), ultrasound corneal pachymetry, pupillometry, slit lamp examination of the anterior segment and a dilated fundus examination.
At day seven postoperatively, UDVA were measured and the patient underwent a slit lamp examination of the anterior segment. The same measurements as the baseline examination were performed at 3 months, and 1 year postoperatively.
Treatment PlanAll aspheric treatments8,9 were prepared using the SCHWIND ORK-CAM treatment planning module in Aberration-Free10,11 mode (SCHWIND eye-tech-solutions GmbH and Co. KG, Kleinostheim, Germany). The SCHWIND ORK-CAM module integrates aspheric ablation profiles that compensate as well for the peripheral loss of energy due to an increased angle of incidence on the cornea.12,13 The treatment of ocular or corneal wavefront aberrations was not intended in this study.
The sphere and cylinder values entered into the laser were based on the manifest refraction without any nomogram adjustment. Further, the flat and steep keratometry readings at 3mm diameter as measured by the topographer were used for the compensation of the loss of ablation efficiency when the laser hits the cornea in non-normal incidence.12
Optical Zone ranged from 6.0 to 7.0mm with transition zone extending to 7.3–8.5mm. Retreatments were not considered for this retrospective study.
Surgical TechniqueLASIKDrops of topical anesthetic were instilled in the upper and lower fornices. Flaps were created using an Intralase 60kHz femtosecond laser (Advanced Medical Optics, Inc.) and a 100μm nominal flap thickness.
Additional drops of topical anesthetic were instilled, and the lid margins and periocular region were disinfected with diluted povidone. A sterile drape covering the eyelashes and face was used to isolate the surgical field. A lid speculum was inserted to allow maximum exposure of the globe.
Proper alignment of the eye with the laser was achieved with a 1050Hz infrared eye tracker with simultaneous limbus, pupil, and torsion18 tracking integrated into the laser system and centered on the corneal vertex.19 The eye tracker had a typical response time of 1.7ms with a system total latency time of 2.9ms. The flap was lifted and the excimer laser ablation delivered to the stroma. Patients were asked to look at a pulsing green fixation light throughout the ablation. The flap was repositioned and the interface irrigated with a balanced salt solution to remove debris.
Patients received topical antibiotic drops four times a day for one week and corticosteroid drops four times a week, after which they were tapered over one week tapering off in one week. They also used ocular lubricants as needed.
PRK/TransPRKTopical anesthetic drops were instilled in the upper and lower fornices. The lid margins, cul-de-sac, and periocular region were disinfected using diluted povidone. A sterile drape was used to isolate the surgical field. A lid speculum was inserted to allow maximum exposure of the globe.
In the alcohol-assisted PRK, a 9.0mm laser-assisted subepithelial keratectomy corneal cone was placed centrally on the cornea and filled with 19% ethanol (1mL of 96% ethanol diluted with 4mL of distilled water). Downward pressure was applied to create an epithelial indentation. After 30s of exposure, the alcohol was absorbed with a small sponge, the alcohol cone was removed, the epithelium was debrided, and the cornea was thoroughly rinsed with a balanced salt solution. The area of the epithelial incision was then dried with a small sponge.
In the transepithelial PRK procedure, treatment was preceded by standardized wet-sponge application. A polyvinyl alcohol sponge (Merocel, Medtronic, Inc.) was dipped in a balanced salt solution and left to expand maximally and then applied with 3 slow, painting-like movements on the corneal surface. This step prevents uneven wetting, avoiding the risk for an uneven ablation.
Proper alignment of the eye with the laser was achieved with a 1050Hz infrared eye tracker with simultaneous limbus, pupil, and torsion18 tracking integrated into the laser system and centered on the corneal vertex.19 The eye tracker has a typical response time of 1.7ms with a system total latency time of 2.9ms. Patients were asked to look at a pulsing green fixation light throughout the ablation. After the ablation, mitomycin-C 0.02% was applied to the stromal bed for 30s and the bed was irrigated with 30mL of a balanced salt solution to remove debris.
A soft bandage contact lens (Cibavision Night&Day, base curve 8.4–8.6mm) was applied for 3–4 days. Patients received topical ofloxacin drops 6 times a day for one week. Fluorometholone drops were started three times a day after epithelial closure and contact lens removal; the drops were tapered over nine weeks. Ocular lubricants were prescribed as needed.
Excimer LaserThe laser ablation algorithm used a flying spot laser delivery system that operates at 750Hz with a super-Gaussian beam profile of 0.54mm Full Width Half Maximum.14 Depending on the planned refractive correction, approximately 80% of the corneal ablation is performed with a high fluence level (∼500mJ/cm2), thus decreasing treatment times.14 Fine correction is performed for the remaining ∼20% of the treatment using a low fluence level (∼250mJ/cm2) which reduces the ablation volume per pulse delivered in order to smooth out the ablated area.14 Spot placement is randomized in order to prevent heat buildup between laser pulses.15–17 Additionally, an aspiration system with laminar flow dynamics is incorporated to reduce debris and heat buildup.
Data AnalysisRefractive outcomes were analyzed using Microsoft Excel software (Microsoft, Redmond, Washington, USA). Seasonal outcomes were evaluated in terms of residual refraction stratified per treatment month, as well as stratified per year season. For that, treatments were cumulated either per month in the year or per season. The difference between attempted (laser settings) and achieved (preoperative minus postoperative refraction) was considered the refractive deviation. Since myopic and hyperopic undercorrections have a different sign, we determined refractive deviation as follows:
where Dev is deviation, LS is laser settings, Rx is refraction (pre- and post-operative, respectively), abs is the function for absolute value, and sign is the sign function.With this definition negative deviations represent always undercorrections (irrespective of the sign of the original refraction) and positive deviation represent always overcorrections.
Example: For the case preoperative manifest SE of −2.00D, laser settings SE of −2.25D, and postoperative manifest SE of −0.25D, a deviation of −0.50D is obtained. And for the case preoperative manifest SE of +2.00D, laser settings SE of +2.25D, and postoperative manifest SE of +0.25D, a deviation of −0.50D is obtained.
Cumulated treatment refractive outcomes (either per month or per season) were compared to the global treatment refractive outcomes. Student's T test comparing stratified values with global ones was used for the statistical analysis. A P value less than 0.05 was considered statistically significant. Postoperative data for one-year follow-up are reported here.
ResultsStratificationOf the year 2010, 1182 treatments were included (21%) (second half of the year), 3053 treatments from 2011 (53%) (full calender year), and 1505 treatments from 2012 (26%) (first half of the year). The month and season distributions can be found in Table 1.
Monthly and Seasonal Distribution of the Treatments.
| Group | Number of Treatments | % |
| January | 493 | 9 |
| February | 483 | 8 |
| March | 477 | 8 |
| April | 452 | 8 |
| May | 428 | 7 |
| June | 417 | 7 |
| July | 440 | 8 |
| August | 549 | 10 |
| September | 541 | 9 |
| October | 481 | 8 |
| November | 498 | 9 |
| December | 479 | 8 |
| Winter | 1453 | 25 |
| Spring | 1296 | 23 |
| Summer | 1533 | 27 |
| Autumn | 1458 | 25 |
UDVA ranged from +0.1 LogMAR to +2.0LogMAR (20/25–20/2000), whereas CDVA ranged from −0.2LogMAR to +0.2LogMAR (20/12–20/32).
1-Year PostoperativeUDVA ranged from −0.2LogMAR to +1.5LogMAR (20/12–20/632), whereas CDVA ranged from −0.2LogMAR to +0.2LogMAR (20/12–20/32). At one-year postoperatively, 84% of the eyes could see uncorrected 0.0LogMAR or better (20/20), whereas 1% of the eyes lost 2 lines of CDVA.
Refractive OutcomesCorrelation of the achieved versus attempted refractive corrections shows only −2% undercorrection at SE. The global refractive deviation from target refraction was −0.07±0.43D for SE with 0.31±0.33D for Ast. 85% and 80% of eyes were within 0.50D of target SE and Ast, respectively.
Seasonal DifferencesThe mean laser settings SE, Ast, and cardinal and oblique astigmatism components were −3.09±2.35D, 0.99±0.87D, +0.20±0.55D, and +0.01±0.29D, respectively, corrected to deviations of −0.07±0.43D, 0.31±0.33D, −0.05±0.18D, and −0.01±0.13D, postoperatively. See Table 2 for further details.
Monthly and Seasonal Distribution of the Refractive Outcomes.
| Group | SE | Ast | C+ | Cx |
| Global | ||||
| Preop | −3.09±2.35 | 0.99±0.87 | +0.20±0.55 | +0.01±0.29 |
| Dev | −0.07±0.43 | 0.31±0.33 | −0.05±0.18 | −0.01±0.13 |
| January | ||||
| Preop | −3.37±2.33 | 0.93±0.83 | +0.15±0.52 | +0.02±0.30 |
| Dev | −0.01±0.41 | 0.28±0.30 | −0.04±0.15 | −0.02±0.13 |
| February | ||||
| Preop | −2.93±2.49 | 1.01±0.88 | +0.18±0.58 | −0.01±0.28 |
| Dev | −0.06±0.57 | 0.36±0.34 | −0.03±0.20 | 0.00±0.14 |
| March | ||||
| Preop | −3.00±2.22 | 1.01±0.93 | +0.14±0.61 | 0.00±0.29 |
| Dev | +0.03±0.49 | 0.32±0.37 | −0.02±0.20 | −0.01±0.14 |
| April | ||||
| Preop | −3.13±2.27 | 0.98±0.83 | +0.19±0.55 | +0.01±0.26 |
| Dev | −0.12±0.39 | 0.31±0.26 | −0.05±0.16 | −0.01±0.12 |
| May | ||||
| Preop | −2.99±2.33 | 0.89±0.81 | +0.24±0.49 | +0.02±0.27 |
| Dev | −0.05±0.38 | 0.30±0.32 | −0.05±0.17 | −0.02±0.13 |
| June | ||||
| Preop | −3.18±2.20 | 1.11±0.93 | +0.22±0.63 | 0.00±0.29 |
| Dev | −0.16±0.43 | 0.36±0.32 | −0.07±0.18 | −0.02±0.14 |
| July | ||||
| Preop | −2.95±2.42 | 0.92±0.84 | +0.23±0.45 | −0.01±0.26 |
| Dev | −0.05±0.41 | 0.27±0.32 | −0.04±0.15 | −0.01±0.14 |
| August | ||||
| Preop | −3.38±2.40 | 0.99±0.82 | +0.17±0.52 | +0.02±0.27 |
| Dev | −0.14±0.40 | 0.30±0.30 | −0.05±0.16 | −0.01±0.13 |
| September | ||||
| Preop | −2.87±2.47 | 0.95±0.88 | +0.20±0.54 | 0.00±0.28 |
| Dev | −0.12±0.41 | 0.31±0.34 | −0.06±0.19 | −0.02±0.12 |
| October | ||||
| Preop | −3.32±2.37 | 1.00±0.90 | +0.28±0.54 | +0.01±0.29 |
| Dev | −0.10±0.37 | 0.29±0.31 | −0.05±0.17 | −0.02±0.11 |
| November | ||||
| Preop | −2.79±2.33 | 1.08±0.94 | +0.20±0.62 | 0.00±0.30 |
| Dev | −0.09±0.43 | 0.34±0.38 | −0.07±0.20 | −0.02±0.13 |
| December | ||||
| Preop | −3.13±2.22 | 0.95±0.86 | +0.23±0.51 | +0.01±0.31 |
| Dev | −0.07±0.40 | 0.29±0.33 | −0.05±0.17 | −0.02±0.13 |
| Winter | ||||
| Preop | −3.11±2.36 | 0.98±0.88 | +0.16±0.57 | 0.00±0.29 |
| Dev | +0.03±0.49 | 0.32±0.34 | −0.03±0.19 | −0.01±0.14 |
| Spring | ||||
| Preop | −3.10±2.27 | 0.99±0.86 | +0.22±0.56 | +0.01±0.27 |
| Dev | −0.11±0.40 | 0.32±0.30 | −0.05±0.17 | −0.02±0.13 |
| Summer | ||||
| Preop | −3.08±2.44 | 0.96±0.85 | +0.20±0.51 | +0.01±0.27 |
| Dev | −0.11±0.41 | 0.30±0.32 | −0.05±0.17 | −0.01±0.13 |
| Autumn | ||||
| Preop | −3.07±2.32 | 1.02±0.90 | +0.23±0.56 | +0.01±0.30 |
| Dev | −0.09±0.40 | 0.31±0.34 | −0.06±0.18 | −0.02±0.13 |
SE, spherical equivalent; Ast, astigmatism; C+, cardinal astigmatism component; Cx, oblique astigmatism component.
The SE planned in January, August, and October was slightly higher than the SE planned in September and November (P<.05). The Astigmatism planned in June and November was slightly higher than the Astigmatism planned in May (P<.05). The cardinal astigmatism component planned in October was slightly higher than the cardinal astigmatism component planned in January and March (P<.05). Similarly, the cardinal astigmatism component planned in Autumn was slightly higher than the cardinal astigmatism component planned in Winter (P<.05).
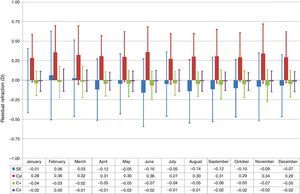
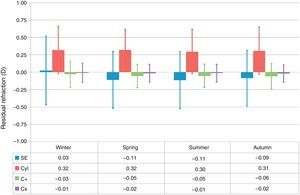
Treatments performed in April, June, August, September, and October showed relative undercorrections of the SE (P<.005), whereas treatments performed in January, February, and March showed relative overcorrections of the SE (P<.05) (Fig. 1). Similarly, treatments performed in spring and summer showed relative undercorrections of the SE (P<.0005), whereas treatments performed in winter showed relative overcorrections of the SE (P<.0001) (Fig. 2).
Box-and-whisker plot of the residual refraction for treatments performed at each month of the year. The box-edges indicate the first and third quartiles (covering 50% of the total outcomes), and the dash inside the box is the median. The whiskers indicate the minimum and maximum values. Treatments performed in April, June, August, September, and October showed relative undercorrections of the SE (P<.005), whereas treatments performed in January, February, and March showed relative overcorrections of the SE (P<.05). Treatments performed in February and June showed relative undercorrections of the Ast (P<.01), whereas treatments performed in January and July showed relative overcorrections of the Ast (P<.05). Treatments performed in June and November showed relative undercorrections of the cardinal astigmatism component (P<.05), whereas treatments performed in February and March showed relative overcorrections of the cardinal astigmatism component (P<.05).
Box-and-whisker plot of the residual refraction for treatments performed at each season of the year. The box-edges indicate the first and third quartiles (covering 50% of the total outcomes), and the dash inside the box is the median. The whiskers indicate the minimum and maximum values. Treatments performed in Spring and Summer showed relative undercorrections of the SE (P<.0005), whereas treatments performed in Winter showed relative overcorrections of the SE (P<.0001). Similarly, treatments performed in Autumn showed relative undercorrections of the cardinal astigmatism component (P<.05), whereas treatments performed in Winter showed relative overcorrections of the cardinal astigmatism component (P<.0005).
Treatments performed in February and June showed relative undercorrections of the astigmatism (P<.01), whereas treatments performed in January and July showed relative overcorrections of the astigmatism (P<.05) (Fig. 1).
Treatments performed in June and November showed relative undercorrections of the cardinal astigmatism component (P<.05), whereas treatments performed in February and March showed relative overcorrections of the cardinal astigmatism component (P<.05) (Fig. 1). Similarly, treatments performed in autumn showed relative undercorrections of the cardinal astigmatism component (P<.05), whereas treatments performed in winter showed relative overcorrections of the cardinal astigmatism component (P<.0005) (Fig. 2).
DiscussionThis study compares the postoperative clinical outcomes of treatments performed at difference times during the year in a retrospective chart review of 5740 consecutive treatments performed with the SCHWIND AMARIS system using aspheric non-wavefront-guided profiles. Seasonal outcomes were evaluated in terms of residual refraction stratified per treatment month, as well as stratified per year season.
The diurnal fluctuation of biomechanical and morphological corneal properties is well known.18–20 However, in this study, there is no information about the moment of the day in which the surgeries were performed. However, pooling together 5740 consecutive treatments during a 2-year period it is very unlikely that in different months or seasons the moment of the day in which the surgeries were performed is significantly different.
De Souza et al.1 evaluated the influence of temperature and humidity on laser in situ keratomileusis (LASIK) outcomes and found that the linear regression coefficient showed that lower temperature levels were associated with lower spherical equivalent refractions at 60 days after LASIK (r2=.14; P=.03) but not at 15 days after LASIK (P=.98). The evaluation of humidity indicated an influence at 15 days after LASIK (r2=.44; P=.04), as well as at 60 days (r2=.45; P=.0002).
Walter and Stevenson2 determined whether environmental factors affect laser in situ keratomileusis (LASIK) enhancement rates and found out that LASIK enhancement rates strongly correlated with the following variables: procedure room humidity (P=.003; odds ratio [OR]=1.093; 95% confidence intervals [CI], 1.030–1.160), 2-week preoperative mean outdoor humidity (P=.011; OR=1.054; 95% CI, 1.012–1.096), outdoor temperature (P=.0059; OR=1.039; 95% CI, 1.011–1.068), and age (P=.0497; OR=1.034; 95% CI, 1.001–1.070). The percentage of correction strongly correlated with the following variables: procedure room humidity (P=.021), 2-week preoperative mean outdoor humidity (P=.001), outdoor temperature (P=.0052), and room temperature (P=.017).
This has been further discussed by Probst21 and by Schipper.22
Well we acknowledge the importance of environmental temperature and relative humidity during surgery, but was not recorded in a single case record in this retrospective review. We just wanted to know whether different seasons through the year lead to different refractive outcomes, if so one of the hypothesis or indicators explaining the findings could be environmental temperature and relative humidity during surgery.
This retrospective chart review attempts to compare the postoperative refractive outcomes among a large population of patients that have undergone refractive surgery treatments using the SCHWIND AMARIS laser system performed at different times during the year in order to analyze seasonal changes in residual refraction 1-year after corneal laser refractive surgery in a retrospective study.
All treatments were performed in the exact time range of two years (between 16/08/2010 and 16/08/2012) to avoid biased statistics on month or seasonal treatment rates. As per Table 1 the treatments were evenly distributed during this period.
The SE planned in January, August, and October was slightly higher than the SE planned in September and November (P<.05), but the range difference in mean values was 0.59D. The astigmatism planned in June and November was slightly higher than the astigmatism planned in May (P<.05), but the range difference in mean values was 0.21D. The cardinal astigmatism component planned in October was slightly higher than the cardinal astigmatism component planned in January and March (P<.05), but the range difference in mean values was 0.14D. Similarly, the cardinal astigmatism component planned in Autumn was slightly higher than the cardinal astigmatism component planned in Winter (P<.05), but the range difference in mean values was 0.08D.
Treatments performed in April, June, August, September, and October showed relative undercorrections of the SE (P<.005), whereas treatments performed in January, February, and March showed relative overcorrections of the SE (P<.05), but the range difference in mean values was 0.22D. Similarly, treatments performed in spring and summer showed relative undercorrections of the SE (P<.0005), whereas treatments performed in winter showed relative overcorrections of the SE (P<.0001), but the range difference in mean values was 0.14D.
Treatments performed in February and June showed relative undercorrections of the astigmatism (P<.01), whereas treatments performed in January and July showed relative overcorrections of the astigmatism (P<.05), but the range difference in mean values was 0.09D.
Treatments performed in June and November showed relative undercorrections of the cardinal astigmatism component (P<.05), whereas treatments performed in February and March showed relative overcorrections of the cardinal astigmatism component (P<.05), but the range difference in mean values was 0.05D. Similarly, treatments performed in autumn showed relative undercorrections of the cardinal astigmatism component (P<.05), whereas treatments performed in winter showed relative overcorrections of the cardinal astigmatism component (P<.0005), but the range difference in mean values was 0.03D.
We have observed overcorrections in winter, undercorrections in summer, with a seventh of a diopter difference from winter to summer. This is in good agreement with de Souza et al.1 and Walter and Stevenson2 since in wintertime relative humidity is lower in The Netherlands. Our results are in contradiction to Dantas et al.4 and Randleman et al.5 who found that subtle changes in environmental factors do not appear to influence laser performance, however, our review cohort is much larger. Chatterjee and Shah23 were unable to demonstrate any significant difference in patients grouped according to season at time of treatment in a similar size cohort. Finally, our findings in a large scale population are in partial agreement with Seider et al.6 who found statistically significant relationships with temperature and humidity (P=.0094 for temperature and P<.0001 for humidity), but with due to the small values of the differences, they did not consider it a clinically significant relationship.
However, Seider et al.6 found, in the subgroup with a preoperative refractive error of +2.00D to +4.00D and aged 18–30 years, an increase in 1°C during LASIK was associated with a decrease in 1-month postoperative refractive error (more correction) of 0.048D. This means that 30%, 43%, and 58% difference in relative humidity or 5°C, 8°C, and 10°C difference in temperature represent 0.25D, 0.37D, and 0.50D difference in outcomes, respectively.
Given the number of treatments and their even distribution through the year, we cannot find another reasonable explanation for out findings, but we can also not confirm it is the environmental change. We are now recording temperature and humidity systematically and we may be able to answer this question in two years from now (so beginning of 2016, 1 year to have sufficient treatments, 1 year for follow-up and analyses). However, likely temperature and humidity are not constant (the AC system is not working 24/7), rather it is switched on 1h before switching on the laser, and keeps the temperature in the range 20–24°C and relative humidity in the range 30%–50%. So it could well be, that in winter actual temperature is closer to the 20°C end and relative humidity closer to the 30% level, whereas in summer actual temperature could be closer to the 24°C end and relative humidity closer to the 50% level. This on its own and according to Seider's paper,6 would already account for ∼0.2D which is also in-line with our findings.
If we set the level of clinical relevance at 0.13D (half of the typical step of the manifest refraction, but objective refractions and aberrometer refractions may already have finer resolution), then 15% difference in relative humidity or 3°C difference suffices to represent a clinical difference. Our differences in relative correction from winter to summer account 0.14D, just above this level, but sufficient for us to qualify it as clinically relevant.
Similarly, Wernli et al.24 investigated the relevance of initial temperature of the polymethylmethacrylate (PMMA) plates used as a target for photoablation during calibration of excimer lasers performed in daily clinical routine, and found out that the ablation depth increased linearly from 73.9 to 96.3μm within a temperature increase from 10.1°C to 75.7°C (increase rate of 0.3192μm/K). The linear correlation was found to be significant (P<.05) with a coefficient of determination of R2=0.95. Based on these results and assuming a standard room temperature of 20°C, optimal plate temperature was calculated to be 15–25°C to maintain an ablation within 0.25D. Since for systems nowadays the cornea-to-PMMA factor lies at about ∼2, the temperature of PMMA plates for clinical laser calibration should be controlled ideally within a range of approximately ±5°C to avoid visually significant refractive error (±0.25D) due to calibration error.
A limitation is the retrospective nature of the study. Further, the treatments were performed by eleven different surgeons and two AMARIS 750S units adding some extra variability to the cohort. A number of confounding factors may be argued in our review. We have combined myopic (5288 eyes) with hyperopic treatments (452 eyes), LASIK (3549) with PRK and TransPRK treatments (2191 eyes), and considered both eyes of the patients (2909 OD eyes and 2831 eyes OS). We have re-run the analyses for only myopic eyes (5288 eyes; P<.0005, difference 0.18D), only hyperopic eyes (452 eyes; P<.05, difference 0.29D), only LASIK treatments (3549 eyes; P<.0005, difference 0.08D), only PRK and TransPRK treatments (2191 eyes; P<.005, difference 0.20D), only OD (2909 eyes; P<.005, difference 0.14D), only OS (2831 eyes; P<.01, difference 0.15D), only LASIK myopic eyes (3198 eyes; P<.005, difference 0.12D), and only LASIK myopic right eyes (1613 eyes; P<.01, difference 0.13D) and the qualitative results were the same: treatments performed in summer showed relative undercorrections of the SE, whereas treatments performed in winter showed relative overcorrections of the SE.
We have re-run the analyses for only high myopic eyes from −6.00D (597 eyes; P<.05, difference 0.28D), only moderate myopic eyes from −3.00D to −5.99D (2247 eyes; P<.05, difference 0.20D), only low myopic eyes from 0.00D to −2.99D (2444 eyes; P<.005, difference 0.12D), only low hyperopic eyes from 0.00D to +1.99D (303 eyes; P<.05, difference 0.19D), only moderate hyperopic eyes from +2.00D to +2.99D (95 eyes; P<.05, difference 0.35D), and only high hyperopic eyes from +3.00D (54 eyes; P<.05, difference 1.01D) and the qualitative results were the same, treatments performed in summer showed relative undercorrections of the SE, whereas treatments performed in winter showed relative overcorrections of the SE.
To make sure that the relative over/undercorrections were not related to the different SE planned at different months/seasons, we looked at the slope of the correlation, which also confirmed these differences (1.004 vs. 0.982).
In conclusion, seasonal differences in refractive outcomes were observed among a large scale population. The explaining hypothesis is related to environmental factors but undetermined whether temperature or humidity. An overplanning of 0.13–0.25D in summertime can be applied to overcome these differences. Since this effect is likely proportional and not fix, 0.13–0.25D means ∼5% overplanning in summer. The effect of these environmental variables on refractive outcomes warrants further evaluation.
Financial DisclosureThis work has been conducted without external financial support.
Conflict of InterestTobias Ewering and Samuel Arba-Mosquera are employees of SCHWIND eye-tech-solutions.