To assess the fitting process and clinical feasibility of a novel large diameter rigid gas-permeable (RGP) corneal contact lens (CL) presenting regular or reverse geometry for managing keratoconus (KC). The lens was designed to rest on the peripheral cornea and vault over the cone, aiming to enhance visual quality, comfort, and corneal physiology.
Methods32 KC patients across all severity stages, including central, paracentral and peripheral cones enrolled. Lens parameters were customized from corneal measurements obtained via Oculus Pentacam HR tomography, mainly based on sagittal height and accounting for adequate tear film clearance. Visual acuity (VA), contrast sensitivity (CS), ocular complications, and user satisfaction were evaluated at baseline, and at one week and three months of lens wear.
ResultsTwenty-four patients (41 eyes) were fitted and completed the 3-month follow-up (median age of 28.5 years, 9 females). A median of 2 trial lenses per eye (interquartile range of 4.5) were needed during the fitting process. Significant improvements in median VA (baseline: 0.32 logMAR; three months: 0.01 logMAR; p = 0.002) and CS (p < 0.001 at all frequencies) were observed compared to baseline values. Ocular complications remained below clinical significance, with superior limbic keratoconjunctivitis showing significant reduction over time (p < 0.001). Patient satisfaction was high, with a median daily lens wear of 9.5 h. Most users (79.2%) reported consistent comfort with their CLs.
ConclusionThe novel large diameter RGP corneal CL demonstrated comparable efficacy to existing RGP CL designs, offering high levels of comfort and improved vision with minimal ocular complications.
Keratoconus (KC) is a chronic, bilateral, and asymmetric eye disease characterized by progressive thinning and steepening of the cornea, which can lead to irregular astigmatism and vision loss.1 The global prevalence of KC is estimated at 1.38 cases per 1000 people,2 though significant geographical variations exist, with rates reaching up to 47.9 cases per 1000 people in the Middle East.3
When vision with glasses becomes unsatisfactory, contact lenses (CLs) are the preferred treatment for managing KC, achieving success in approximately 75% of patients.4 While rigid gas-permeable (RGP) corneal CLs are the most commonly prescribed option,5 other designs may be considered if these are found to be ineffective,6 including thicker soft lenses,7 hybrid lenses, corneoscleral and scleral lenses. Over the last decade, the use of scleral CLs has increased significantly,8 due to the advantages offered by their large diameter, such as reduced lens awareness and enhanced stability.9 However, this design has some drawbacks that limit its widespread use, including limited tear exchange, complex handling by the patient, and the possible appearance of midday fogging, requiring repeated lens removal and reinsertion.10
While RGP corneal CLs have demonstrated effectiveness in managing KC,11 their primary limitation is discomfort, which can affect patient tolerance. Various fitting strategies have been developed, with the three-point technique being the most widely recommended.12 This approach aims to distribute pressure evenly across the paracentral cornea, and, to a lesser extent, on the apex of the cone. However, this technique does not always prevent excessive pressure on the central cornea, especially in advanced ectasia, where progressive changes can result in unintended increased pressure on the apex of the cone.12
Rigid gas-permeable corneal CLs for KC typically have a total diameter ranging from 8 to 10 mm.13 Most manufacturers and fitting guides recommend selecting the base curve radius of the initial lens based on central corneal curvature parameters, such as keratometry or simulated keratometry. However, this approach has notable limitations,9 particularly in cases of irregular corneas, where the corneal surface becomes increasingly asymmetrical and lacks two orthogonal principal meridians.14 For other lens designs tailored to irregular corneas, such as scleral CLs, parameters like sagittal height are more commonly used for lens calculation.
Recent studies based on corneal sagittal height measurements,15 as well as the analysis of peripheral corneal angles manually measured from Scheimpflug images obtained with the Oculus Pentacam tomographer (Oculus Optikgeräte GmbH, Wetzlar, Germany),16 suggest that the peripheral cornea of eyes with KC exhibits a symmetry of revolution comparable to that of healthy eyes. Consequently, a large diameter RGP corneal CL design that rests on the peripheral cornea while vaulting over the cone could be successfully fitted in these eyes. This design would provide stable support outside the area most affected by the ectasia and might offer comfort benefits comparable to scleral lens designs, with the added advantages of better tear exchange and easier lens handling. Several clinical cases have recently been reported in which this novel lens design was successfully fitted in eyes with KC, demonstrating favorable outcomes in terms of vision, comfort, and the absence of ocular complications.17
The aim of this study was to assess the clinical feasibility of this newly designed large diameter RGP corneal CL, which rests on the periphery of the cornea and displays an apical clearance, over a follow-up period of three months in a final sample of 24 patients with KC. The study included eyes with central, paracentral and peripheral ectasias, as well as those across all severity stages, including advanced KCs. Outcomes were evaluated in terms of visual quality, ocular physiology, and comfort during use.
METHODSStudy design and participantsA prospective clinical study was conducted to determine the viability of a novel large diameter RGP corneal CL design for eyes with KC. Patients were recruited from those attending the optometry clinic at the Universitat Politècnica de Catalunya between March 2022 and March 2024, all with a confirmed ophthalmological diagnosis of KC, irrespective of type and stage of the condition. Due to the asymmetric nature of KC,1 which was verified with an intraclass correlation analysis, as reported below, all eyes fitted with CLs were included in the subsequent data analysis. Exclusion criteria were eyes with a history of keratoplasty, refractive surgery, corneal pathologies other than KC, or signs of CL induced corneal warpage.
The study adhered to the tenets of the Declaration of Helsinki (1975, revised in Tokyo in 2013). Written informed consent was obtained from all participants or their legal tutors after providing a detailed explanation of the purpose, potential risks, and consequences of the study. Participants were informed that they could withdraw from the study at any time without repercussions. Ethical approval was granted by the Fundació Assistencial Mútua de Terrassa Ethics Committee for Clinical Research with Drugs (code n. E/22–023).
Contact lens design and parameter selectionRigid gas-permeable corneal KAKC-I CLs (Hecht Contactlinsen GmbH, Freiburg, Germany and Conóptica SL, Barcelona, Spain), manufactured with Boston EO material (Dk = 58 units), and with diameters ranging from 10.30 mm to 11.50 mm were used in eyes with KC. These lenses feature four concentric spherical zones with diameters D0, D1, D2, and D3, and corresponding radii of curvature R0, R1, R2, and R3. The central zone D0 serves as the optic zone, functioning as a dome over the area most affected by KC. The adjacent zone (D0 – D1) transitions to the alignment zone, which is located around the circumference defined by diameter D1. The subsequent zones (D1 – D2, and D2 – D3) create a gradual progression toward the edge lift, which begins at the circumference of diameter D3 (Fig. 1).
The determination of the lens parameters was tailored for each patient. For the initial trial lens, the diameters were based on the horizontal visible iris diameter (HVID), measured using the MYAH topographer (Topcon Healthcare, Barcelona, Spain). The total diameter (DT) was set at 90% of the HVID, whereupon the diameters of the concentric zones (D3, D2 and D1) were calculated by subtracting 0.6 mm, 1.2 mm, and 2.8 mm from DT, respectively. The diameter of the central zone (D0) was at least 1 mm smaller than D1, and could adopt one of the following four values: 6.0 mm, 6.5 mm, 7.0 mm, and 7.5 mm. The widest possible D0 was chosen to ensure adequate pupillary coverage in low-light conditions. In order to determine the radii of curvature, the average sagittal heights of the eyes for circles concentric to the pupil corresponding to the previously determined diameters D0, D1, D2 and D3 of the CL were first obtained with the Oculus Pentacam model HR (High Resolution, software version 1.21r65). Secondly, adequate tear thickness values were added to calculate the sagittal heights of the CL for each diameter and at the geometric centre. Finally, the radii of curvature for each zone were obtained through geometrical calculations based on sagittal height and diameter.
Tear thickness values were selected based on clinical experience. Thus, fixed values were considered at the outer transition zone points: 60 µm at the beginning of the edge lift (D3), and 15 µm and 5 µm at the end (D2) and beginning (D1) of the alignment zone, respectively. The topographic location and elevation of the cone apex was used to select adequate tear thickness at the end of the optical zone (D0) and at the geometric centre of the lens, with values ranging from 20 to 60 µm.
Contact lenses with a geometry based on increasing radial values, or with reverse geometry, were employed according to corneal profile and topographic location of the cone apex. Increasing radial geometry is particularly suited for topographically centred ectasias, as these CLs feature a steeper central dome to accommodate the corneal shape. In contrast, reverse geometry lenses are designed with a flatter profile and a higher dome in the paracentral area, making them the preferred choice for eyes with topographically decentred ectasias.
To complete the characterization of the CLs, additional parameters were defined: the sagittal height from the plane described by the circle D1 (SD1), i.e., the height of the CL dome from the alignment zone; and the sagittal height from the plane described by the circle D3 (SD3), which corresponds to the total sagittal height of the CL, excluding the edge lift.
Fitting and follow-up procedureAll patients attended an initial baseline visit, followed by one or more evaluation visits, if parameter adjustments were required to optimize fit. Follow-up visits were scheduled at one week (1 W), 15 days and 1 month after lens delivery, and three months later (3 M), although only data from the 1 W and 3 M visits was collected for the purposes of this study. All procedures, including lens fitting and handling training sessions, were performed by the same practitioner and employed the same instrumentation. Briefly, these CL are inserted following the same procedure as an orthokeratology CL. First, a couple of preservative-free artificial tear drops are added into the bowl of the CL to improve comfort and avoid the formation of trapped air bubbles; then, patients are instructed to tilt their heads parallel to the surface of a counter top and to insert the CL by looking directly into the bowl of the CL during the process. Lens removal follows the same procedures as a typical RGP CL. Patients already wearing CLs were required to refrain from using them for one week before participating in the study to ensure reliable topographic measurements.
At the baseline visit, all participants underwent a comprehensive optometric examination that included topographic analysis using the Oculus Pentacam HR, measurement of HVID and pupillary diameter under photopic and scotopic lighting conditions with the Topcon MYAH and slit-lamp anterior ocular surface evaluation (the Efron Grading Scales were used to grade any observed ocular complication18). Monocular visual acuity (VA) and contrast sensitivity (CS) at 3, 6, 12 and 18 cycles per degree (cpd) were determined with a logMAR chart and the CSV-1000 test (VectorVision®, Greenville, OH, US) under photopic conditions (58 cd/m2), respectively, while patients used their habitual correction. In addition, to assist in the assessment of the visual performance with the new CL design, best-corrected visual acuity (BCVA) was obtained while patients wore a KC RGP corneal CL from a trial lens set with the best over-refraction in a trial frame.
At the evaluation visit, CL fit was assessed at least 15 min after lens insertion. The following criteria were defined to evaluate the suitability of the fitting: (1) Visual acuity with the CLs was equal to or greater than the BCVA obtained with the trial lens, and could not be further improved with over-refraction; (2) DT was correct to avoid invading the limbal area, ensuring the lens edge remained within the boundaries of the visible cornea at all times; (3) Adequate vertical movement with blinking was observed, and rapid re-centring of the CL with reference to the geometrical centre of the cornea; (4) Fluorescein pattern showed no bearing at the central cornea or cone apex and uniform alignment across the periphery; (5) Edge lift ensured tear exchange and comfort and showed a symmetrical pattern along the entire perimeter of the lens. In addition, patients were required to demonstrate adequate CL handling and care practices. A 0.05% concentration povidone-iodine based maintenance system was provided to the patients (Cleadew, OPHTECS Europe B.V., Elst, The Netherlands) with the recommendation of wearing the CLs for two hours the first day and to gradually increasing wear time each day over the course of the first week.
If the adaptation was deemed unsatisfactory, new CLs were ordered with the necessary design adjustments, including: (1) An increase or decrease in sagittal height if the dome was found to have insufficient or excessive clearance, respectively; (2) A change in Dᴛ of up to 0.5 mm, or adjustments to radii R₃ and/or R₂, if tear exchange or lens movement was not clinically satisfactory; (3) Adjustments in D₁ by up to 0.5 mm to prioritize a more peripheral bearing or to obtain a wider alignment zone. The number of CLs required to achieve optimum fit for each patient was recorded.
At all follow-up visits, the suitability of the adaptation was reassessed based on the previously described criteria. Visual acuity and CS were measured while patients wore the CLs, using the same tests and lighting conditions as at the baseline visit. After removing the CLs, ocular health was evaluated. Additionally, at the 3 M follow-up visit the number of hours of daily lens wear was recorded, and user satisfaction was assessed with both a 10 cm vertical Visual Analogue Scale (VAS) and the multiple choice question “Over the past three months, how often have you felt satisfied with your contact lenses?" (Possible answers were: a) No response or not certain; b) Never; c) Infrequently; d) Sometimes; e) Most of the time; f) Always).
Data collection and analysisKeratoconus were classified according to the position of the cone apex as central (apex within the central 3 mm zone), paracentral (apex within the 3–5 mm zone), or peripheral (apex outside the central 5 mm zone).19 For severity, the Topographic Keratoconus Classification (TKC) Index, provided by the Oculus Pentacam software, was used, which evaluates parameters of the anterior corneal surface and assigns one of four increasing severity levels: TKC1, TKC2, TKC3, and TKC4.
The IBM SPSS Statistics v.28 (IBM Corp. NY, US) was used for statistical analysis. Only participants who completed the 3 M follow-up period were included in the analysis. Data normality was assessed with the Kolmogorov-Smirnov test. As the data did not follow a normal distribution, results are presented as median, maximum and minimum values. The Friedman non-parametric test for repeated measures was employed to determine the statistical significance of changes in parameters over time and, when appropriate, pairwise comparisons were conducted with the Conover post-hoc test. The interocular symmetry between the keratoconic eyes of the same patient was assessed with Intraclass Correlation Analysis,20 focusing on the variables rPUPIL (the distance from the corneal apex to the pupillary centre) and dPC (the distance from the pupillary centre to the cone apex). A p-value <0.05 denoted statistical significance.
RESULTSSample demographics and ocular parametersFifty-seven eyes from 32 participants were initially recruited for this prospective study. Eight participants (25%) failed to complete the 3 M follow-up, of whom two discontinued due to an unsuccessful adaptation, and the remaining six for personal reasons unrelated to the adaptation process. None of the patients discontinued due to poor visual quality, discomfort or severe adverse events associated with CL wear. The final sample included 41 eyes from 24 participants (9 females) diagnosed with KC, with a median age of 28.5 years (range from 15 to 65 years). Seven patients (13 eyes) were previous CL wearers, including 2 scleral CLs, 7 RGP CLs and 4 soft CLs. None of these patients presented clinically significant corneal or conjunctival alterations at the baseline visit related to their previous CL use. Table 1 presents a summary of relevant ocular parameters of the final study sample.
Ocular parameters and keratoconus position and severity of the eyes included in the study (n = 24). Median (minimum; maximum) or absolute and relative frequencies are reported.
TKC: topographic keratoconus classification.
Distance from the corneal apex to the pupillary centre (rPUPIL) ranged from 0.07 to 1.06 mm, with a median value of 0.32 mm. The distance from the pupillary centre to the cone apex (dPC) varied between 0.25 and 3.83 mm, with a median of 1.20 mm. An analysis of interocular asymmetry revealed intraclass correlation values of 0.20 for rPUPIL and 0.28 for dPC. According to these results, it was deemed safe to include both eyes of the same patient in subsequent analyses.
Contact lens parameters and fitting processThirty-two (78.0%) of the CLs had increasing radial values geometry and the remaining 9 (22.0%) featured a reverse geometry design. Increasing radial values geometry was used in 28 eyes with central KC, and 2 eyes with paracentral and 2 eyes with peripheral KC (to preserve the predefined tear film thicknesses values); Reverse geometry CLs were employed in 6 eyes with paracentral and 3 eyes with peripheral ectasias. Table 2 displays a summary of the parameters of these CLs, including DT, D1, SD3 and SD1 (D3 and D2 are not reported, as these values are derived by subtracting a fixed amount from DT, as described in the Methods section).
The number of CLs needed to achieve optimal fit ranged from 1 to 10, with a median of 2 and an interquartile range (IQR) of 4.5. As expected, the skill of the practitioner in determining lens parameters improved over time, given the novelty of the CL design and of the method of calculation. Thus, during the second year of the study, while minimum and median values remained unchanged, the IQR and maximum values decreased to 2 and 6, respectively.
Visual acuity and contrast sensitivityTable 3 presents a summary of the values of VA obtained at each visit. Statistically significant differences were found in monocular VA over time (p < 0.001). A post-hoc pairwise analysis revealed a significant improvement in VA with the new CL at the 1 W (p = 0.004) and the 3 M (p = 0.002) follow-ups, as compared with that obtained at the baseline visit. No difference was found between 1 W and 3 M visits.
Monocular CS values at baseline and each follow-up visit are displayed in Fig. 2. A statistically significant change in CS at all frequencies was found (all p < 0.001). Pairwise analysis revealed an improvement in CS over baseline values at the 1 W (p < 0.001 at 3, 6 and 18 cpd; p = 0.004 at 12 cpd), and the 3 M (p < 0.001 at all frequencies) visits. No differences were found between 1 W and 3 M CS values. With the new CL, CS values were equivalent to those reported in the literature for healthy eyes with the CSV-1000 test.21
Monocular contrast sensitivity values at baseline (BAS) and three-months (3_M) at spatial frequencies of 3, 6, 12 and 18 cycles per degree (cpd). The shaded area represents normal contrast sensitivity values at each spatial frequency.21 (*) denote atypical values, and correspond to the same patient.
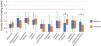
Fig. 3 presents a box plot of the various ocular complications observed at the baseline visit and after 3 M of CL use. It also includes a reference red line denoting the numerical threshold for clinical significance in the assessed ocular complications, based on the Efron Grading Scale.18 None of the observed complications reached clinical significance at any visit. Comparative analysis of the grades of ocular complications at the three visits revealed statistically significant differences for conjunctival staining (p = 0.007), which increased at 1 W (p = 0.007) and 3 M (p = 0.007), relative to baseline values, and for superior limbic keratoconjunctivitis (p < 0.001), which decreased at the 3 M follow-up, when compared with baseline (p < 0.001) and 1 W (p = 0.001). No statistically significant differences were found for any other ocular complication.
Severity of the ocular complications observed at baseline and at the 3-months follow-up, as determined with the Efron Grading Scales.18 A reference red line is shown to denote the threshold for clinical significance for ocular complications (grade 3). Statistically significant differences in severity between baseline and three months are denoted by *.
The degree of wearer satisfaction after 3 M of using the CLs, assessed with a 10 cm VAS, ranged from 4.8 to 10, with a median score of 9.1 and an IQR of 1.6. Nine users (37.5%) reported feeling “always” satisfied with their CLs, 10 (41.7%) selected “most of the time”, 3 (12.5%) answered “sometimes” and 2 (8.3%) chose “infrequently”. The number of hours per day that patients wore the CLs also emerged as a potential indicator of user satisfaction, ranging from 4 to 16 h, with a median of 9.5 h and an IQR of 6 h.
DISCUSSIONThis study evaluated the clinical outcomes of a novel large diameter RGP corneal CL for KC patients, designed to rest uniformly on the peripheral cornea and to vault over the cone. While earlier studies suggested that this design could be a successfully alternative for KC,15–17 none had systematically assessed its clinical feasibility with a relatively large sample of patients.17
The CLs used in this study had DT values ranging from 10.30 to 11.50 mm, which are significantly larger than those found in conventional designs of corneal RGP CLs.13 The diamter D₁, representing the circle around which the alignment zone is located and therefore defining the width of the dome, ranged between 7.5 and 8.7 mm. This ensured that central, paracentral, and even peripheral ectasias were enclosed within the dome. For paracentral and peripheral ectasias, reverse geometry designs were prioritized to achieve a flatter yet higher dome over the paracentral cornea. This approach minimized excessive bearing at the apex in these off-center ectasias. Using this strategy, CLs were successfully fitted to all eyes in the study sample, including those with paracentral and peripheral KC. The CLs employed in this study were based on the KAKC-I design. Although this CL has been available for a number of years, the novelty of the present research resided in employing the elevation data of the Pentacam, as well as certain predefined tear film thickness values, to independently calculate each curve of the CL to best fit any type of KC. This allowed for reverse geometry designs, when necessary, which are not commonly available for KC patients. As such, the CLs described in this study were custom made for each patient.
In terms of visual quality, the CLs used in this study provided logMAR VA values ranging from 0.15 to 0.00 at both 1 W and 3 M, with a median value of 0.01, which may be considered clinically normal for this type of patients. Besides, statistically and clinically significant improvements in VA were evidenced, when compared with baseline values. Similarly, CS values fell within or near the normal range21 at all spatial frequencies and were also significantly better than at baseline. These findings are comparable to those reported with other RGP CL designs for KC, including conventional corneal,22 corneoscleral,23 and scleral lenses.24 It must be noted that while the improvement in VA was more significant in new CL wearers, experienced users achieved visual results similar to or superior to those obtained with their previous CLs.
Regarging ocular health, as shown in Fig. 3, none of the complications observed during the three visits reached grade 3 or higher on the Efron Grading Scale, which correspond to clinically significant moderate or severe grades.18 At 3 M of lens use, no notable increases were observed in typical corneal lens-related complications.25 Other complications remained stable, except for fluorescein conjunctival staining, which increased from a median value of 0.5 (range from 0 to 1.8) at baseline to 1.2 (range from 0 to 2.1) at the 3 M follow-up. This finding may be attributed to the fact that the majority of patients were naïve to RGP CLs, known to cause initial changes in blink rate and blink completeness, often resulting in altered tear film dynamics and 3&9 o'clock staining.26 As the Efron Grading Scale employed in this study does not contemplate 3&9 o'clock staining as an independent entity,18 it was not possible to separate this complication from other causes of fluorescein conjunctival staining during data collection. Conversely, a significant reduction in superior limbic keratoconjunctivitis after 3 M of lens use was observed, which could be attributed to the improved fit provided by the new lens design, particularly in eyes previously fitted with other CL types.
Users reported high levels of satisfaction after 3 M of wearing the lenses, as reflected in both the VAS scores (median 9.1 out of 10) and the responses to the questionnaire regarding the frequency of satisfaction during lens use (79.2% of patients reporting “always” or “most of the time”). The reduced CL discontinuation and the number of daily hours of lens wear also support these positive results, with a reported median of 9.5 h per day and some users wearing the lenses for up to 16 h.
It must be noted, however, that this prospective study was not devoid of limitations. The sample size for eyes with severe grade 4 KC, as classified by the TKC scale, and/or with the cone apex located in the paracentral or peripheral corneal areas was relatively small. Therefore, it is not safe to generalize the success of the new CL design to these specific types of KC, or to provide an inferential statistical analysis comparing outcomes amongst typologies and degrees of severity. Additionally, the follow-up period, ending at 3 M, was relatively short. A longer-term study would provide more robust data on the suitability and safety of this CL design over time. Future research shall address these limitations and explore further adjustments in initial CL parameter selection by means of simulated fluorescein analysis to reduce the number of CLs required to achieve optimal fit for patients at different stages of the disease and cone location. This should allow the development of a trial CL set to expedite the fitting process and to reduce the dependence on custom lens fitting using tools such as the Oculus Pentacam.
In conclusion, the findings reported in this study suggest that the visual performance of the new CL design is at least comparable to that of other RGP CL corneal or scleral designs for KC. Furthermore, none of the observed ocular complications were clinically relevant, with users reporting high levels of comfort and tolerance after 3 M of CL wear. This type of large diameter corneal CL design has been shown to be a safe and valid alternative for patients with KC, thus increasing the arsenal of options available to practitioners for the successful and rewarding management of this particular, and often complex, condition.