The purpose of this research is to propose a new method for the easy, inexpensive and objective quantification of nystagmus using eye-tracking records collected during a simple reading task that could be implantable in clinical practice to assess patients with nystagmus.
MethodsThis is a prospective, observational pilot study. Eye movements of 4 nystagmus patients and 9 healthy children during a reading task (a paragraph with 82 words) on a 15′’ monitor were collected and compared. Data are time series indicating the gaze position on the screen. Two quantifiers were proposed: IndS (based on the speed of movements) and IndF (based on the variation of the gaze trajectory).
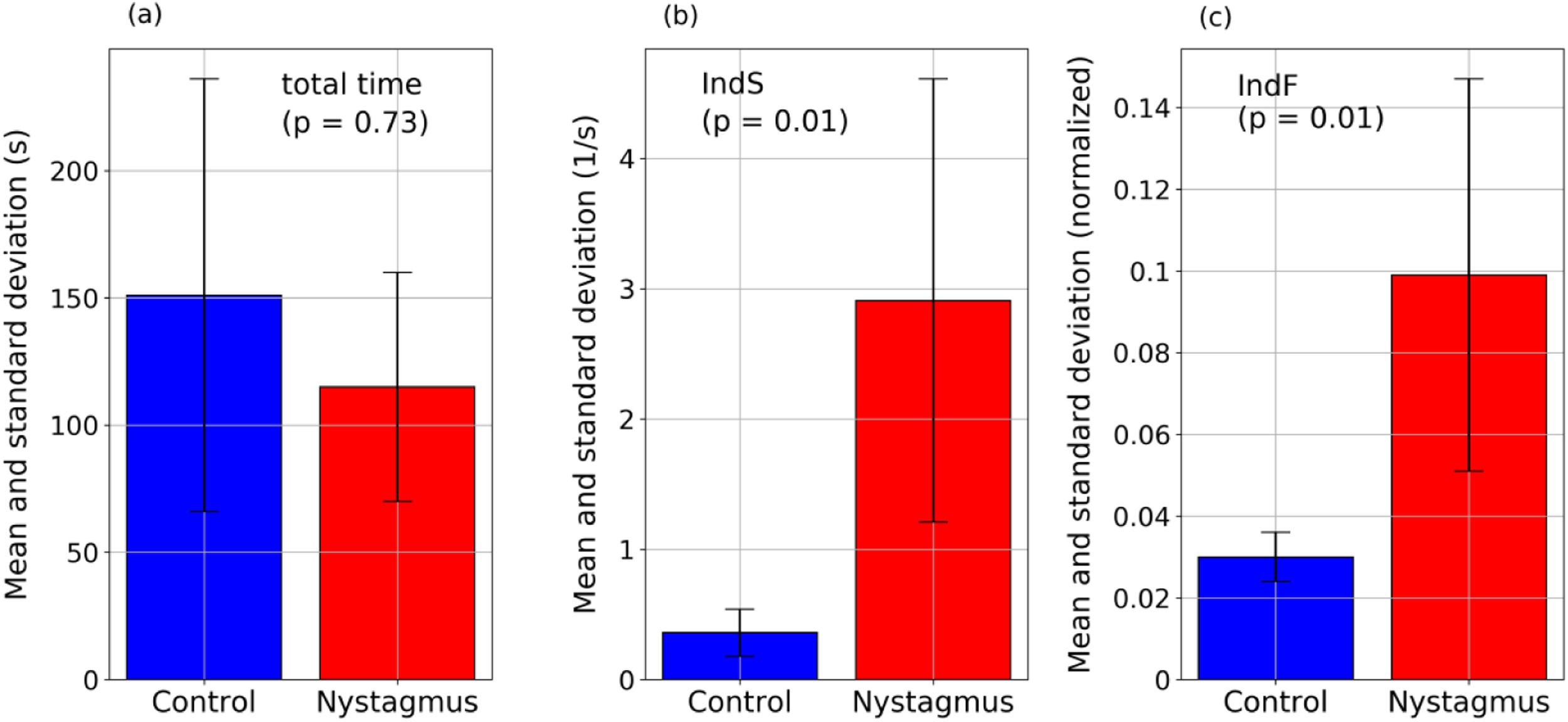
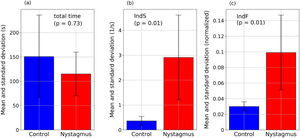
ResultsThe indices proposed reflect differences in the behavior of eye movements between the two groups. Nystagmus patients present higher values of IndS - indicating smaller number of slow movements (16% of movements with speeds <0.33 1/s for nystagmus and 85% for the control group, with p = 0.01) - and higher values of IndF - indicating higher gaze fluctuation (p = 0.01). Differences were not related with reading speed as show the mean and standard deviation: the nystagmus group required 115±45 s to complete the task and the control group 151±85 s; p = 0.73.
ConclusionsThe proposed indices provide a new method that allows an objective assessment of nystagmus, with potential use in clinical and research practice to improve the follow-up of patients by monitoring the nystagmus over time or treatment.
The human visual system involves many parts, starting with the eyes and their internal structure, followed by the optic nerve and all its connections to different parts of the brain. Therefore, there is a wide variety of pathologies that manifest in different ways depending on which part of the system is affected. Nystagmus, one of those pathologies, is defined by rhythmic and involuntary oscillations of the eyes. Usually this excessive motion reduces visual acuity because of the instability of the retinal image.1 The classification of nystagmus can be referred to the differences in nystagmus forms, such as congenital (infantile nystagmus), which appears in the first 6 months of life, or acquired, which can occur in any moment later. Infantile nystagmus may be associated to albinism, visual deprivation in early life (congenital cataracts, latent nystagmus), retinal disease, low vision, optic nerve hypoplasia, retinal dystrophies or idiopathic.1-5 Acquired nystagmus can be related to diseases of central myelin, such as multiple sclerosis, cerebellar disorders, vestibular disease, syndrome of ocular palatal tremor, among other rare conditions and tumors or drug toxicity.6-9
Although in some cases nystagmus can be asymptomatic,10 patients with nystagmus commonly present visual impairment from blurred vision caused by disabling oscillopsia, which prevents the image of an object from being held steady in the fovea, inducing significant psychological, social and quality of life impact, especially in acquired nystagmus patients.10,11
Nystagmus is clinically confirmed by direct observation of the eyes or by recording the eye movements with techniques such as electrooculography, the scleral search coil, video eye-tracking devices or other devices.1 Many features can be identified to characterize nystagmus12,13 such as: plane of movement (horizontal, vertical, torsional or a combination)14 ; amplitude of movement; frequency (cycles per grade); waveform (jerk or pendular nystagmus)8,10; conjugacy (differences in movements between both eyes); foveation periods (low velocity periods allowing the fovea to receive information) and others.7,14,15 Different models, metrics and algorithms (NAF, NAFX13 and NOFF16) have been proposed to describe and evaluate the mechanisms that produce nystagmus, its dynamics or waveform morphology and the effects of treatment, usually based on standard deviation, gain, frequencies and precision of saccades.12,13,16,17 To our knowledge, these proposals have not been implemented in clinical practice to facilitate the classification or assessment of nystagmus, probably because they require careful and precise calibration13,16 of the eye-tracking device, which is particularly difficult in infantile nystagmus patients.16,17 In addition, the characterization of nystagmus during everyday activities may also be of interest to assess appropriate forms of treatment. Therefore, new approaches to describe nystagmus could be useful and necessary to be able to characterize it during simple tasks as e.g. reading. The reading process has been described based on eye tracking tools in healthy subjects, both young and adults, and it is recognized as a highly consistent sequence of fixations and saccades.18 People with nystagmus are able to dominate the reading process even with the superposition of voluntary and involuntary eye movements.19,20 Therefore, it is of great interest to compare the behavior of subjects with and without nystagmus during reading due to the sophistication of the process carried out by patients with nystagmus to achieve the acquisition and processing of information during reading. The proposal developed in this work points in that direction.
To date, studies of eye movements during reading have been carried out in patients with infantile nystagmus in order to analyze the differences observed with different orientations of the text and with languages other than Spanish.20-22 In this work, an analysis of the eye movements of children with infantile nystagmus during reading is carried out for the first time. The purpose of this pilot study is to propose a new method to quantify nystagmus using eye-tracking records collected during a simple reading task in an easy, inexpensive and objective way that could be implantable in clinical practice to assess infantile and acquired nystagmus patients with potential to be used in clinical trials to quantify differences in treatments. The method presented here is based on a text written in Spanish but it could be easily extended to other languages or even to an arrangement of numbers or even small images simulating a text.
Material and methodsParticipants and experimental setupThree children (6–7 years old) and one adult (48 years old) with infantile nystagmus and 9 children (ages 6–7) with no ocular pathology (control group) voluntarily participated in the experiment. The study group consisted of nystagmus patients with different etiology: bilateral congenital cataract with bilateral amblyopia (visual acuity between 20/80–20/40); esotropia with high hyperopia, vertical tropia with a low refractive error and dissociated vertical deviation with high hyperopia. The adult patient presented monocular nystagmus related to hyperopic anisometropic amblyopia and exotropia. None of the nystagmus types corresponded to a pure movement pattern such as pendular, jerk or other nystagmus movement pattern.
A simple reading task was designed to record eye movements out of the null position when participants read out loud a simple paragraph (82 words) with a sufficiently large font size, which did not cause reading difficulties in their native language (Spanish). Participants were seated 60 cm away from the same 15′’ monitor (1920 × 1080 pixels) of a Windows 10 Pro Lenovo laptop (Intel Corei5 7200CPU:2.5 GHz 2.75 GHz. RAM 8GB and 64bits) in which the text was displayed. Binocular records of eye movements during the reading task were recorded using an eye tracker Tobii Pro (Tobii AB, Sweden) at a sample rate of 90 Hz in all cases. This device includes head tracking, an attribute that allows for a robust estimation of the gaze.
Informed consent was obtained from each subject or their legal guardian after approval of the study was granted by the Human Sciences Ethics Committee of the University of Valladolid. All subjects were treated in accordance with the Declaration of Helsinki.
Experimental data analysisThe recorded data were time series (signals) corresponding to the gaze position on the screen (an average between the position of the right and left eye) and having the form (ti,xi,yi) where xi,yi are the normalized coordinates of the horizontal and vertical position, respectively (ranged between 0 and 1 -dividing each pixel position between total screen pixels-), and ti is the time in which the coordinates are recorded.
To illustrate the different eye movement dynamics found in the data collected, a detection of fixations (for a child without nystagmus) and foveations (for a child with nystagmus) was carried out. Both algorithms are based on the detection of slow speed movements. Foveation periods were calculated following the ideas developed for the expanded nystagmus acuity function (NAFX),13,17 particularly the proposal of Felius et al. study.17 The detection of fixations was performed following the proposal developed in Nyström & Holmqvist study.23 The dataset was first subjected to a pre-processing that allowed determining the quality of the information collected. For each signal, the percentage of consecutive sampled points with a time variation greater than 30 ms (this is, ti+1−ti>30ms) was calculated with respect to the total number of points in the signal. Points registered with a time variation higher than 30 ms indicate that the eye tracker lost the gaze, and a high percentage of such points may compromise the analysis and conclusions to quantify or classify nystagmus patterns. Signals with less than 5% of points with time variation higher than 30 ms are considered acceptable to be analyzed.
Then, two quantifiers were calculated for each signal. The first one, named IndS, is defined in terms of the speed of the eye movements (ratio between displacement and time). It is the value of the percentile 85 of the averaged eye velocities given by v¯i=15∑j=−2+2vi+j, where vi=(xi+1−xi)2+(yi+1−yi)2ti+1−ti. Patients with nystagmus do not have the same proportion of low-speed movements (foveations) as subjects without the pathology; hence, IndS is expected to be higher in the presence of nystagmus.
The second index, named IndF, is defined as the sum of the standard deviations of the position with respect to a centered moving average of 21 consecutive positions. This is:
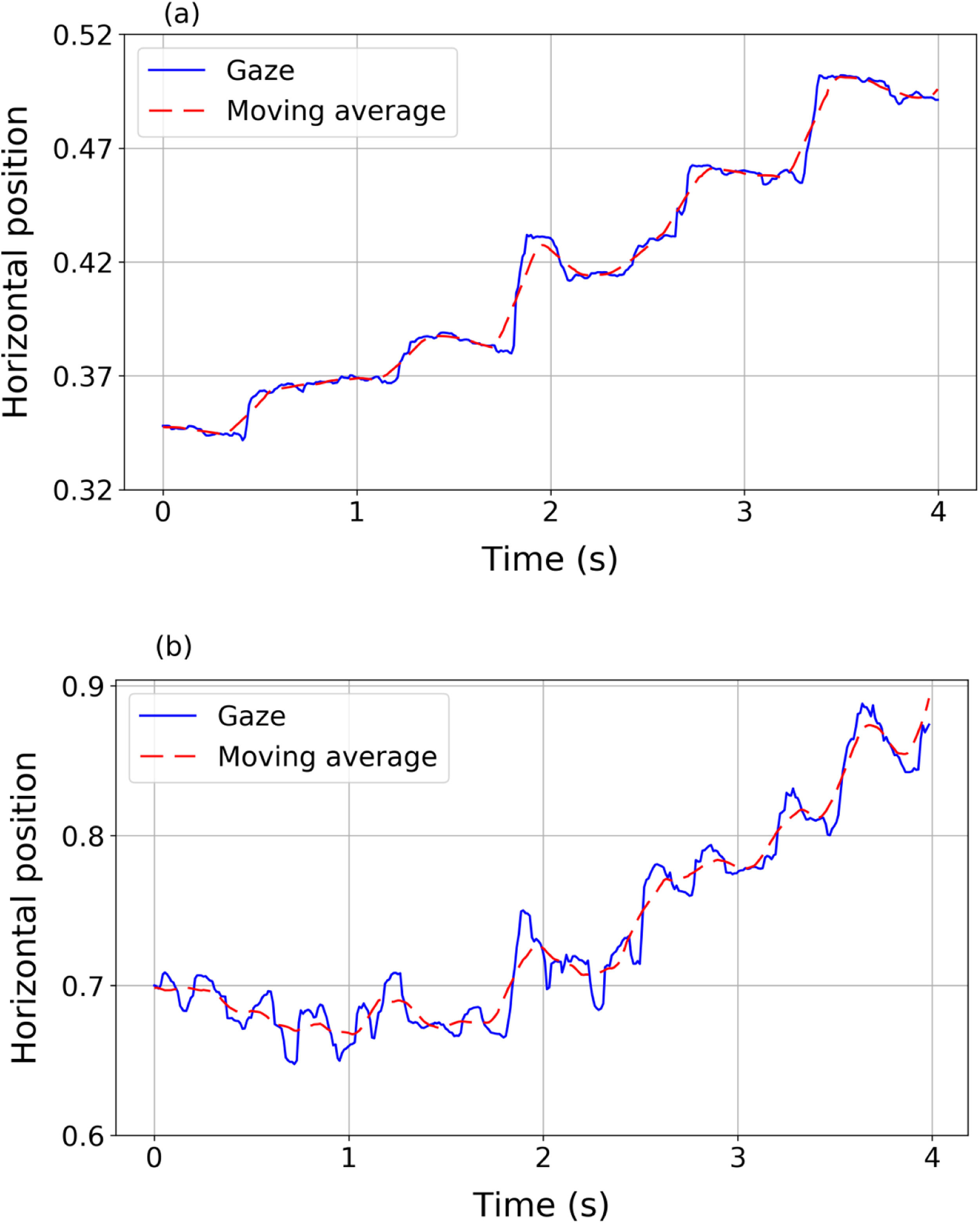
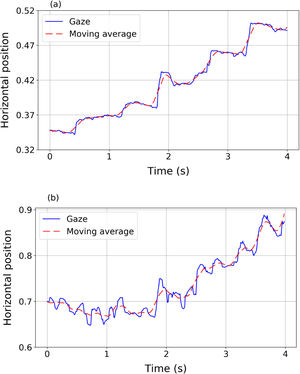
where x¯i=121∑j=−10+10xi+jand y¯i=121∑j=−10+10yi+j define the moving average. The moving average of a signal is a new signal that smoothly describes the path of the original one.24Fig. 1 shows two examples of horizontal gaze trajectories (blue solid line) and their resulting moving averages (red dashed line) in a segment of 4 s. Panel (a) corresponds to a volunteer without nystagmus and panel (b) corresponds to a volunteer with nystagmus. The proposed index IndF measures the difference between the data and the averaged trajectory. IndF should be higher when the eye movements present very abrupt and erratic changes in the advancing direction of the text, as occurs in nystagmus patients.ResultsPreprocessing dataFrom the preprocessing it was found that one nystagmus signal had 4.2% of the recorded points with a time variation higher than 30 ms. The remaining three nystagmus signals showed less than 1.5% of points not respecting the correct sample rate. For the control group, it was found that one signal had 2.1% of points with a time variation higher than 30 ms while the remaining eight signals had less than 0.3% of these points. Therefore, all data met the quality criteria to be considered acceptable.
The adult participant required 28 s to complete the reading task, while the mean time for children with nystagmus was 115±45 ranged from 68 to 158 s and 151±85 ranged from 57 to 317 s for the control group. This difference, related to the fact that the adult is an expert reader, is of interest because it will be shown that even in the case of expert readers the indices proposed capture the presence of anomalous oscillations in eye movements during reading.
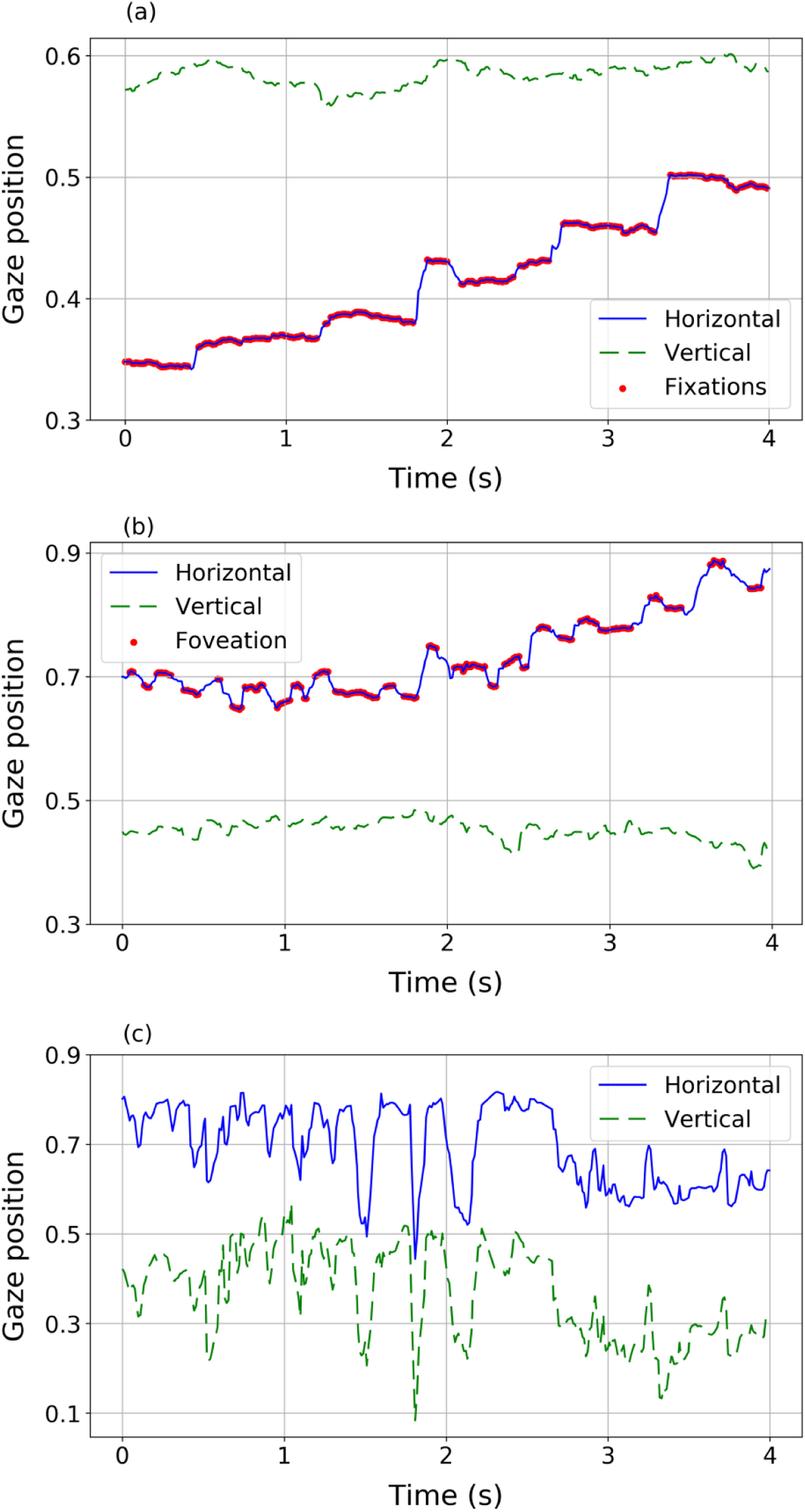
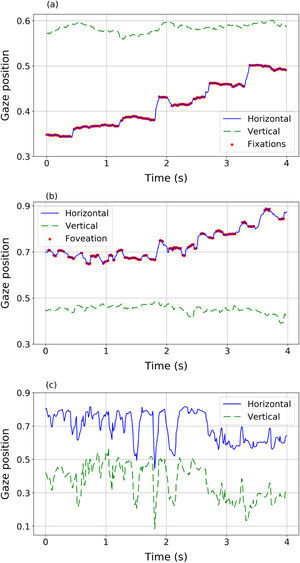
Eye movementsEye movements of nystagmus patients during reading present different behaviours and different features compared to healthy subjects (Fig. 2). The eye movements of a volunteer without nystagmus (Fig. 2a) show very little fluctuation in vertical position (indicating that the reader is reading one line in the text) while horizontally the eyes move from left to right (increasing values of the horizontal variable) with a sequence of saccades (blue solid line) and fixations (red dots). However, nystagmus patients show really different patterns of movement (Fig. 2b and c). For example, signals in panel (b) and (c) describe two different situations, corresponding to two different nystagmus patients. In both plots, higher fluctuations (compared to the trajectory plotted in panel 2a of a control volunteer) in horizontal and vertical movements can be observed. The signal in panel (2b) shows slow movements (red dots) where foveations may be occurring. In panel (2c) fluctuations without clearly identifiable foveations are observed, showing big differences among nystagmus patients.
Different eye movement dynamics during reading a line of text: Top (panel a): a sequence of fixations and saccades performed by a volunteer without nystagmus (control). Middle (panel b) a sequence of fast and slow phase movements (nystagmus patient #1). Bottom (panel c) disordered movements performed by nystagmus patient #2 unable to detect foveations.
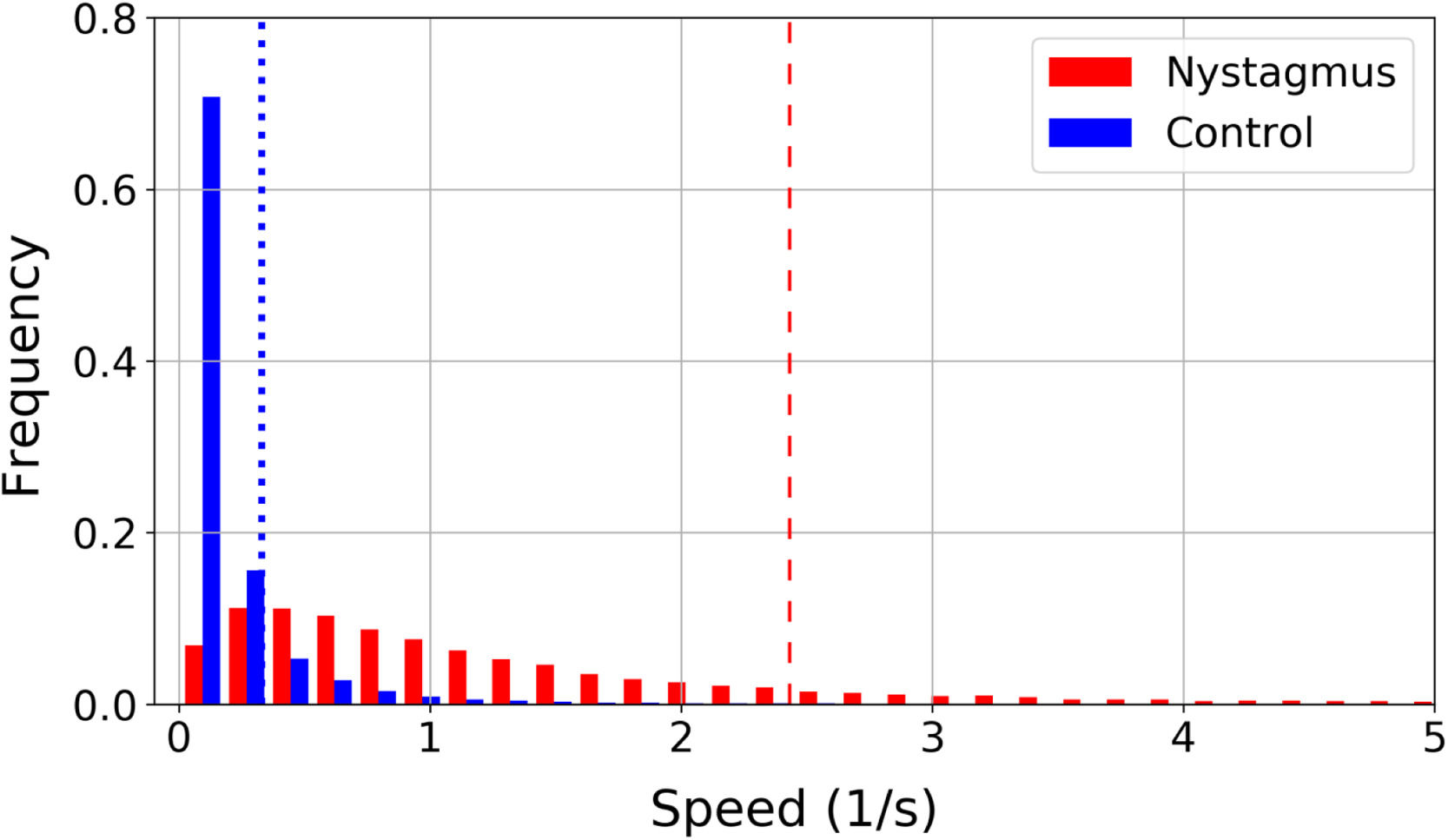
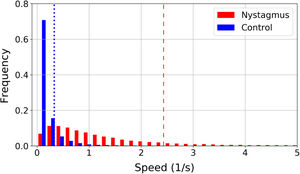
In patients with nystagmus slow movements are less frequent than in the control group. This is illustrated in a normalized histogram in Fig. 3, where the vertical lines represent the 85th percentile of all speeds in each group: 0.33 1/s for the control group (blue dotted line) and 2.43 1/s for nystagmus (red dashed line). The 85% of the speed values found in the control group are located in a range from 0 1/s to 0.33 1/s, while to cover the 85% of the values found in children with nystagmus a larger interval must be considered with a range from 0 to 2.43 1/s. In children with nystagmus, the range of speeds from 0 1/s to 0.33 1/s encompasses 16% of the data, thus slow movements are less frequent.
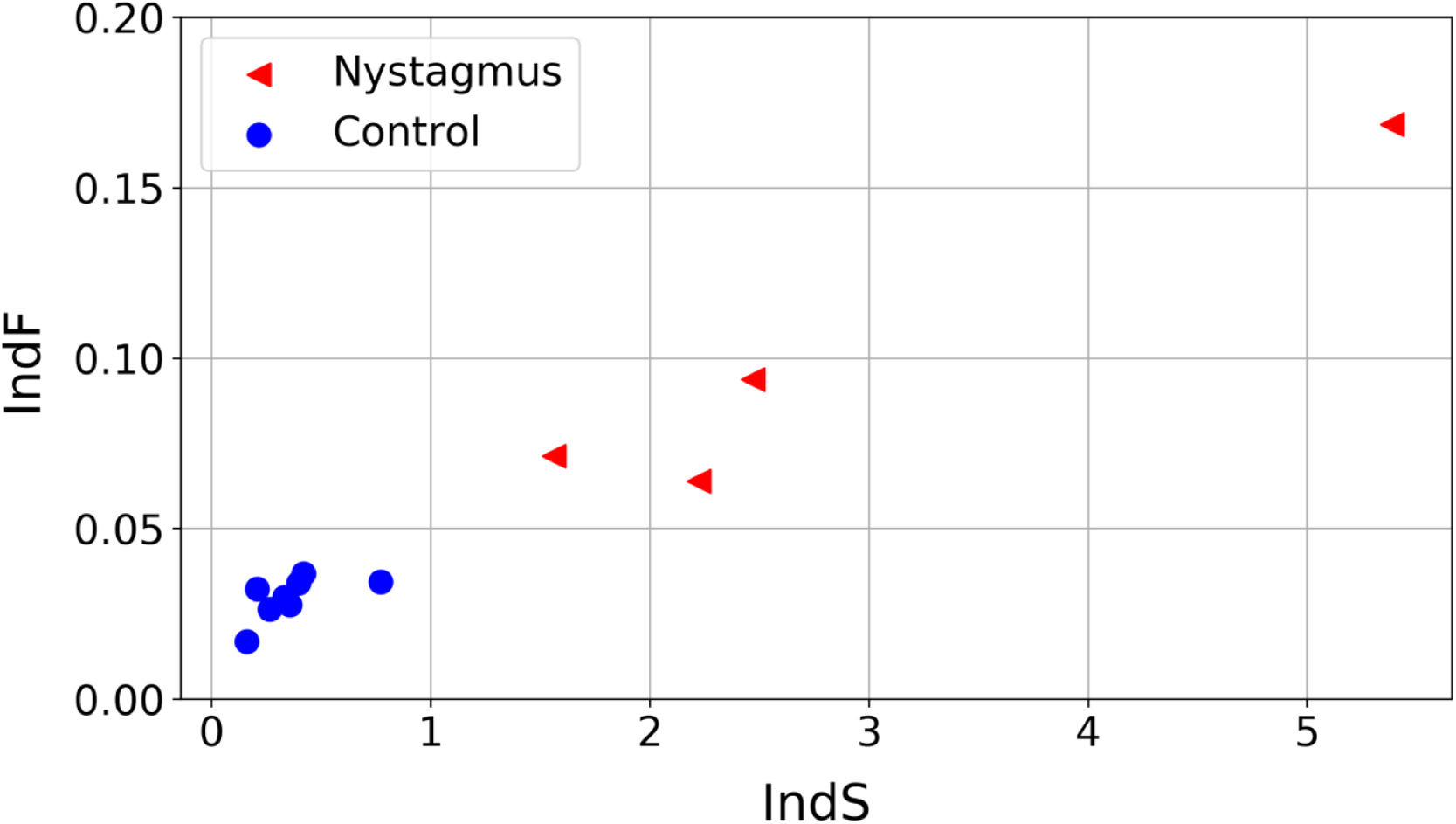
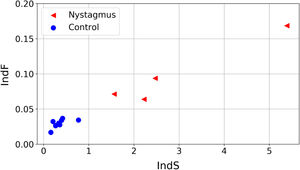
The values obtained for the proposed quantifiers IndS and IndF are plotted in Fig. 4 and summarized in Fig. 5. Each element plotted in Fig. 4 corresponds to one of the registered signals. It can be observed that the values of IndS in patients with nystagmus are higher than those obtained for the control group. This indicates that patients with nystagmus have a lower number of slow movements, which are associated with fixations (absent in these individuals) or foveation periods. On the other hand, for the children in the control group the values of IndS are clearly lower, reflecting a higher number of slow movements coming from fixations. The fluctuation of nystagmus signals is higher than that of the control group reflecting the fact that abrupt changes in the gaze trajectory are more frequent in patients with nystagmus. The results presented in Fig. 4 could be of clinical interest since they allow classifying the eye movements of nystagmus patients compared to normal or expected movements, with a simple reading task. Besides, this analysis could help with monitoring the development of nystagmus during growth in children as well as differences between treatments. The plot shows that if the oscillations of nystagmus decrease, the indices values will get closer to the coordinate origin.
Fig. 5 summarizes the principal results of this work: the comparison of time required to solve the task and values of the proposed indices between the two groups. It represents the mean values of the total time, IndS and IndF. For the total time the adult was not included since his reading speed is not comparable to that of children. The figure also includes the p-value obtained from the Mann Whitney U test when testing if the groups are significantly different. It was found that the differences in reading time are not significant but the differences in the proposed indices are (p < 0.05).
DiscussionEye tracking is a powerful technology to register eye movements non-invasively while the subject being evaluated performs a visual task. It has been used in different disciplines, including eye diseases25-28 and nystagmus15,27,28 assessment. Different models have been proposed to characterize the morphology of nystagmus waveform,1,12,13,16,17 which require precise calibration13,16 and complex preprocessing of the data,16,17 a difficult task to accomplish with infantile patients of nystagmus. A new, easy, objective and inexpensive method to assess and characterize nystagmus is necessary to improve eye care in this group of patients.
In this pilot study a new approach to assess eye movements in nystagmus during a simple reading task is proposed. The eye movements of a healthy person during reading can be characterized as a consistent sequence of fixations and saccades performed in a particular manner that allows the subject to extract information needed to achieve the task.18 It is expected that the eye movements of a patient with nystagmus cannot fit with normal reading dynamics and the involuntary eye movements affect the naturally pure sequence of fixations and saccades across the reading task. Remarkably, even with the difficulty of controlling their eye movements, some previous studies have shown that patients with nystagmus develop strategies to read with similar performance than a person without nystagmus when certain orientation and text size conditions are established.17,20
In the present work, two quantifiers were proposed to characterize the profile of eye movement speeds (IndS) and the fluctuation of the gaze trajectories (IndF) allowing to distinguish normal eye movements from those with nystagmus. These indices were defined from the raw data (ti,xi,yi) of eye movements registered during a simple reading task. Future applications of these quantifiers could be to assess nystagmus of different forms or etiologies, to propose classification rules or to assess the impact of treatments or therapy.
Each quantifier captures different features that characterize the eye movements in nystagmus: speed (IndS) and fluctuation (IndF). Higher values of IndS indicate a lower number of slow movements. Higher values of IndF indicate more disordered signals with abrupt changes in the movement direction determined by the task being performed. IndF is defined in terms of a standard deviation, a metric already used on foveation periods to evaluate the type or intensity of nystagmus.12,13 Alternatively, in this proposal the variation of the gaze trajectory was measured while the subject was performing a cognitive task (reading).
An interesting finding is that these quantifiers are independent of the time required to solve the task and of the reading level. This is illustrated by the fact that the adult with nystagmus finished the task in 28 s (close to half of the time required by the fastest child) and, as it is already established, adults with nystagmus usually develop strategies to achieve normal levels in reading19,20 suggesting that the indices proposed capture the presence of anomalous oscillations in eye movements during reading even in the case of expert readers. Therefore, despite the differences with respect to the rest of the participants, the values of IndS and IndF obtained located the adult with nystagmus near the rest of the nystagmus group (Fig. 4).
Measurements conducted in this study have some advantages compared to previous reports based on the recognition of waveforms and the detection of foveation periods.12,13,16 First, eye movements were recorded in a non-invasive way while subjects were performing a simple cognitive task (reading) with great freedom of movement, which is an ideal condition to evaluate children with nystagmus. Second, a precise calibration of the eye tracker is not required to register the eye movements because the metrics proposed are calculated from the raw dataset. Furthermore, there is no need for an elaborated preprocessing of the data, as required by waveform or foveation assessment, because data artifacts have a low impact on the proposed IndS and IndF indicators. In fact, signals with less than 5% of points with a time variation higher than 30 ms can be considered acceptable. Finally, measurements are not affected by the time necessary to complete the task since the adult participant required just 28 s while the mean time for children with nystagmus was 115 s.
Clinical implicationsNystagmus patients can be managed with pharmacological, surgical and optical treatments. Commonly, infantile nystagmus is treated with optical or surgical methods because pharmacological treatments are associated with significant side effects (dizziness, paresthesias, incoordination drowsiness, incoordination, lethargy, headache) and long-term treatment, even lifelong.10,29 However, pharmacological treatment is often more effective in patients with acquired nystagmus, for whom optical or surgical options are considered if pharmacological treatment fails or is insufficient, but a customize option should be recommended to each patient.10,29 The goal of treatments in infantile or acquired nystagmus is to reduce the slow phase velocity of the nystagmus. Treatments rarely achieve the abolishment of nystagmus movements, and patients often do not achieve clinically normal visual function (Snellen visual acuity 20/20). Additionally, few treatments have been evaluated in well-designed clinical trials or studies with placebo group15,29 and there is a lack of objective assessment methods. Therefore, the treatment effect is usually subjective or indirectly measured in the short-term (postural stability measured by postural sway, motor performance, visual function, patient's opinion or questionnaires).6,10,29 The method developed in this study could be appropriate to be used in clinical practice, since it would allow an objective evaluation of the effect of treatment in patients and facilitate the comparison of different treatments in randomized, blinded clinical trials.
Study limitationsThis new method to quantify nystagmus eye movements will require further research to complete its clinical validation, increasing the sample size and including a wide variety of types of nystagmus in order to explore if cut-off values could be proposed to classify different types of nystagmus.
The proposed experiment does not cover illiterate patients or children who have not yet learned to read, so a different visual task must be designed in order to implement the IndS and IndF in the evaluation of this population. Small well known images could be used instead. However, to study reading allows one to verify precisely that the subject is really following the sequence step by step.
In conclusion, the new method developed in this pilot study provides objective measures of nystagmus, calculated in a simple manner, from the recording of eye movements of patients performing a quotidian task like reading. This method is potentially implantable in clinical practice and could help to improve the follow-up of patients by monitoring the nystagmus over time or treatment.
Authors' contributionsMM. Design of the work, analysis and interpretation of data, have drafted the work or substantively revised it. JADP. Design of the work, analysis and interpretation of data, have drafted the work or substantively revised it. IS. Design of the work, acquisition, analysis and interpretation of data, have drafted the work or substantively revised it. RGL. Design of the work, interpretation of data, have drafted the work or substantively revised it. GG. Design of the work, analysis and interpretation of data, have drafted the work or substantively revised it. RM. Design of the work, acquisition and interpretation of data, have drafted the work or substantively revised it.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We are grateful to the insightful comments and suggestions provided by the professionals at the Centro Integral de Neurociencias Aplicadas. This research was developed as a part of the projects UNS PIP 24/F078, CONICET PIP KE3 11220200102879CO and by Agencia Nacional de Promoción Cientifica y TecnológicaPICT - 2020 - SERIE A - 02450.