Spectral filters are used to treat light sensitivity in individuals with traumatic brain injury (TBI); however, the effect of these filters on normal visual function has not been elucidated. Thus, the current study aimed to determine the effect of spectral filters on objectively-measured visual-evoked potential (VEP) and alpha-wave responses in the visually-normal population.
MethodsThe full-field (15°H×17°V), pattern-reversal VEP (20′ check size, mean luminance 52cd/m2) was administered to 20 visually-normal individuals. They were tested with four Intuitive-Colorimeter-derived, broad-band, spectral filters (i.e., gray/neutral density, blue, yellow, and red), which produced similar luminance values for the test stimulus. The VEP N75 and P100 latencies, and VEP amplitude, were recorded. Power spectrum analysis was used to derive the respective powers at each frequency, and peak frequency, for the selected 9–11Hz components of the alpha band.
ResultsBoth N75 and P100 latencies increased with the addition of each filter when compared to baseline. Additionally, each filter numerically reduced intra-session amplitude variability relative to baseline. There were no significant effects on either the mean VEP amplitude or alpha wave parameters.
ConclusionsThe Intuitive Colorimeter filters significantly increased both N75 and P100 latencies, an effect which is primarily attributable (∼75%) to luminance, and in some cases, specific spectral effects (e.g., blue and red). VEP amplitude and alpha power were not significantly affected. These findings provide an important reference to which either amplitude or power changes in light-sensitive, younger clinical groups can be compared.
Los filtros espectrales se utilizan para tratar la sensibilidad a la luz en individuos con lesión cerebral traumática (TBI); sin embargo, no se ha esclarecido el efecto de estos filtros sobre la función visual normal. Por ello, el estudio actual trató de determinar el efecto de los filtros espectrales sobre el potencial evocado visual (PVE) y las respuestas de las ondas alfa, medido objetivamente en la población con visión normal.
MétodosSe aplicó un PVE de campo completo (15°H×17°V), y patrón invertido (tamaño de estímulo de 20’, y luminancia media de 52cd/m2) a veinte individuos con visión normal. Se realizó la prueba utilizando cuatro filtros espectrales de banda ancha, derivados del Colorímetro Intuitivo (densidad gris/neutra, azul, amarillo y rojo), que produjeron unos valores de luminancia similares para los estímulos de la prueba. Se registraron las latencias N75 y P100, y la amplitud del PVE. Se utilizó el análisis del espectro de potencia para calcular las respectivas potencias en cada frecuencia, así como la frecuencia máxima, para los componentes seleccionados de 9–11Hz de la banda alfa.
ResultadosAmbas latencias N75 y P100 se incrementaron con la incorporación de cada filtro, en comparación a la línea basal. Además, cada filtro redujo numéricamente la variabilidad de la amplitud intra-sesión, en relación a la línea basal. No se produjeron efectos significativos sobre la amplitud del PVE medio o los parámetros de la onda alfa.
ConclusionesLos filtros del Colorímetro Intuitivo redujeron considerablemente las latencias N75 y P100, un efecto que es principalmente atribuible a la luminancia (∼75%), y en algunos casos a los efectos espectrales específicos (es decir, el azul y el rojo). La amplitud del PVE y la potencia alfa no se vieron significativamente afectados. Estos hallazgos aportan una importante referencia para poder comparar tanto los cambios de amplitud como de potencia en los grupos clínicos de personas más jóvenes, con sensibilidad a la luz.
Individuals who have suffered a traumatic brain injury (TBI) often report several post-injury visual sequelae. For example, up to 50% of patients experience persistent light sensitivity for up to one to two years, or more, post injury,1–3 the severity of which ranges from mild to marked. Some light sensitive individuals also experience difficulties in scotopic environments; thus, TBI-induced light adaptation defects have been termed “dual-system deficits” (i.e., photopic and scotopic1,4): a shift in the mean light-adaptation level (i.e., gain control) has been suggested to explain the simultaneous presence of reduced scotopic sensitivity and enhanced photopic sensitivity.2 The origin of this alteration is thought to be cortical, as the deficits persist despite normal ocular health and electroretinogram recordings.5 Thus, the visual-evoked potential serves as a logical starting point for examination of light sensitive individuals following TBI.
If cortical processing abnormalities are present, can they be remediated? Spectral filters may provide insight regarding this question. Spectral filters used in these patients are broad-band, which selectively transmit short, medium, or long-wavelength light: they are often prescribed to reduce or relieve post-injury light sensitivity.6 Further investigation is warranted in the TBI population for two reasons. First, there is a paucity of research regarding spectral filters in a TBI cohort: objective reports of filter efficacy have been derived from other clinical populations. For example, Riddell et al. found increased steady state VEP amplitudes in a group of children with a diagnosis of migraines when a specific spectral filter was worn.7 Similarly, Huang et al. documented reduced cortical hyperexcitability in extrastriate visual cortex when migraineurs wore their precision tint.8 The increase in VEP amplitude and reduction in hyperexcitability suggest that neural activity may be altered by wavelength-dependent processes; however, further investigations are needed to clarify the mechanism underlying these changes. Moreover, the effect of such filters must first be documented in visually-normal individuals, so that any changes in a given clinical population may be considered unique. Second, there is no standardized wavelength “prescription” for a given problem: confusion and skepticism have arisen from colorimetry systems which aim to reduce one symptom with a spectral bandwidth unique for each patient.9,10 Utilization of filters which selectively transmit short, medium, and long wavelength light is necessary for delineation of wavelength specific effects. Thus, prior to studies which aim to validate the use of spectral filters in clinical groups (e.g., TBI, migraine), steps must be taken to address the aforementioned concerns. Therefore, the goal of the current study was twofold: first, to collect data from a normative population, and second, to use filters with defined bandwidths, which together envelop the visible spectrum.
MethodsTwenty visually-normal individuals (14 females, 6 males) with a mean age of 23.3 years (SD±1.4, range 21–26) were recruited from the students and faculty at the SUNY State College of Optometry. Subjects with a history of concussion, mild-traumatic brain injury (mTBI), or seizures, as well as the presence of strabismus, amblyopia, ocular or systemic disease, and/or neurological disease, were excluded from this group. All had corrected visual acuity of at least 20/20, both binocularly and monocularly, at distance and near, as well as normal contrast sensitivity.11 Mean spherical refractive error was −2.75D, with a range from +1.25D to −6.25D. Mean astigmatic error was −1.18D, ranging from plano to −2.75D.
Written informed consent was obtained from each subject prior to commencement of the experiment. The study was approved by the IRB at the SUNY State College of Optometry. Furthermore, all procedures performed were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
VEP systemThe Diopsys™ NOVA-TR VEP system (Diopsys Inc., Pine Brook, NJ, USA) was used to present a full-field (17°H×15°V), black-and-white checkerboard pattern (64×64, equivalent to 20min arc or Snellen acuity of 20/264, at 1m). The temporal frequency was 1Hz (2reversals/s), and the mean Michelson contrast was 85% for all test conditions. The mean baseline stimulus luminance was 52cd/m2, and the ambient room lighting was 38lx. The VEP signals were amplified by a factor of 10,000, sampled at 1024Hz, and noise filtered (0.5–100Hz) by the software during data acquisition. The visual stimulus was viewed binocularly with (1) full refractive correction, and (2) either no filter in place (i.e., baseline), or a broad-band spectral filter.
Signals were obtained from three Grass gold-cup scalp electrodes (Grass Technologies, Astro-med Inc., West Warwick, RI, USA). Each electrode was 1cm in diameter and consisted of a ground, reference, and active electrode. The ground, reference, and active electrodes were placed at the Fp2 position on the right side of the forehead, at the Fpz position approximately 10% of the distance from the nasion to the inion, and at the Oz position 2.5cm above the inion, respectively. This electrode placement is slightly modified from international standards,12 per the manufacturer's instructions to reduce preparation time in clinic populations. The skin was fully cleaned prior to electrode placement. Each electrode's impedance was measured using an EIM-105 PREP-CHECK impedance meter (General Devices, Ridgefield, NJ, USA) provided by Diopsys™. Impedance of each electrode was maintained below 5kΩ, per the standards of the International Society for Clinical Electrophysiology of Vision (ISCEV).12
Each subject was instructed to sit comfortably and place his or her head in an adjustable floor-mounted, headrest/chinrest assembly centered along the midline at eye level. They were instructed to fixate and focus on a small (0.5° diameter), red, rotating circle that was in the center of the test screen, as part of the Diopsys™ software test design. Subjects were instructed to blink approximately every 5s to reduce the number of artifacts registered by the system that may occur with uncontrollable blinking. Any trial with greater than 5 artifacts was discarded.13 Each subject was informed when the trial was half completed, and when there were 5s left, to maintain attention and alertness.
Four trials were conducted for each test condition. The first condition for each subject was their baseline, in which no spectral filter was in place, but with their distance refractive correction worn. These baseline trials were assessed for normalcy of response prior to any further testing; all subjects produced responses which were within normal limits for our laboratory.13,14 Next, a counterbalanced design was used to determine the order in which each subject would be assessed with each of the filters (i.e., gray, blue, yellow, and red). Each trial was 20s.
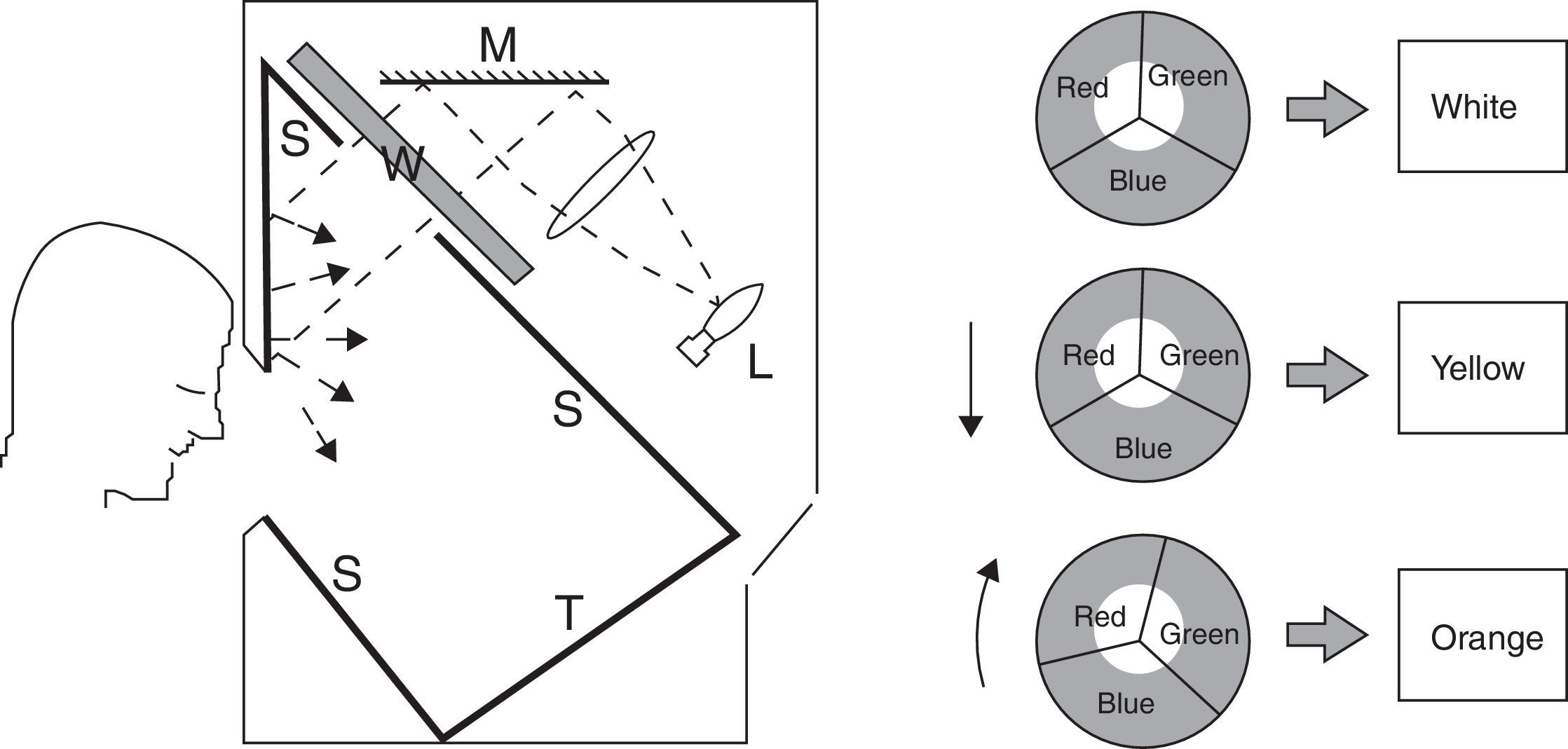
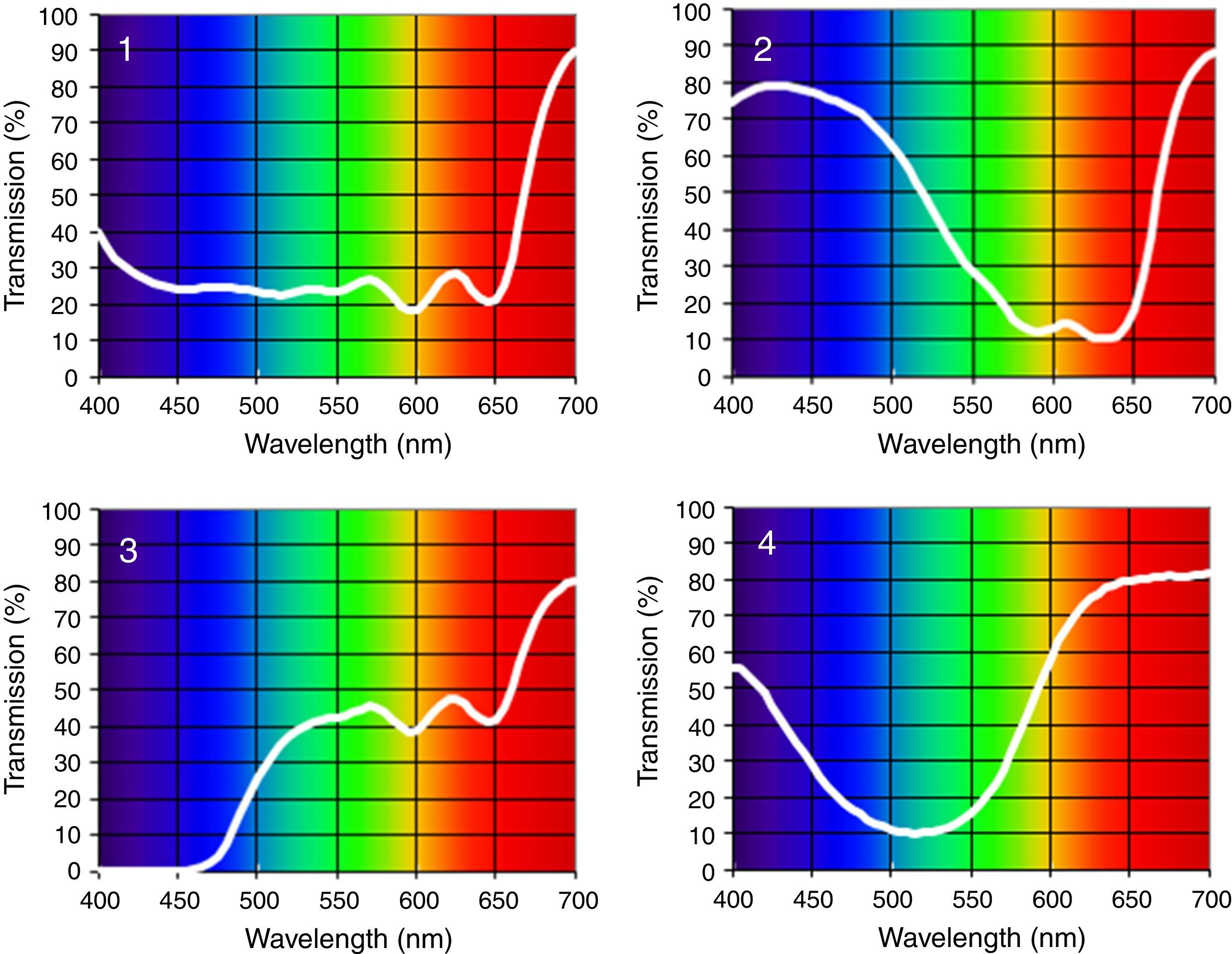
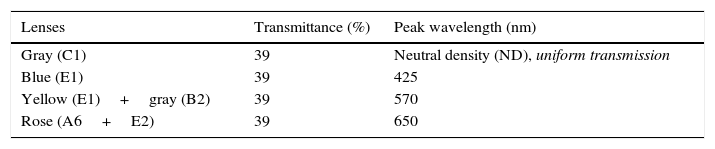
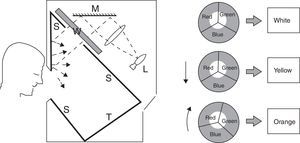
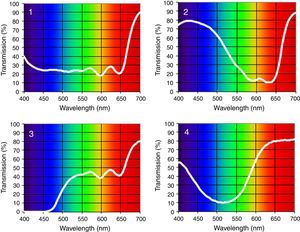
Four spectral filters were used: gray, blue, yellow, and red. The spectral filters were chosen to probe three distinct bandwidths (i.e., short, medium, and long-wavelengths) which together covered the entire visible spectrum, whereas the gray lens was included as a spectrally-invariant control (see Table 1). Each spectacle lens was 70mm in diameter and was part of the Intuitive Colorimeter System9 (Fig. 1). This system determines the optimal hue, saturation, and luminance combination that reduces a patient's light sensitivity, visuoperceptual distortions, and/or improves reading efficiency in a range of diagnostic groups (e.g., visual stress,7 stroke,15 mTBI6). Each lens had a mean transmission of 39% in the visible spectrum (400–700nm), as measured with the OptiSource Multimeter Spectrophotometer (OptiSource International, Bellport, NY). The luminance of the screen was 19, 18, 23, and 18cd/m2 for the gray, blue, yellow, and red filters, respectively, and it was measured with the PR-524 Litemate (Photo Research, Incorporated, Chatsworth, CA). To construct the filters to be worn during VEP testing, each filter (or filter combination, as required to achieve the desired spectral transmission curve9) was placed into a black spectacle holder provided with the Intuitive Colorimeter System. This spectacle holder was then held steadily by each subject, while viewing the VEP stimulus. See Fig. 2 for the transmission spectra of each filter.
Cross-sectional view of the Intuitive Colorimeter. A beam of white light from a tungsten-halogen lamp (L) is reflected from a mirror (M) and passes through a wheel (W) and into a box with matte white inner surfaces (S). The wheel is divided into three sectors, each covered with a different filter so as to transmit light of a different color (red, green, or blue). The colored light is mixed as it is reflected and scattered from the inner surfaces of the box. Calibrated text (T) is mounted on one surface of this box and viewed through a window in the front. Viewing distance is approximately 40cm (2.5D). Saturation increases with eccentricity of the white beam, whereas hue changes with rotation9.
Several electrophysiological parameters were recorded. First, the N75-P100 VEP amplitude and N75/P100 latencies were recorded for each of the 5 test conditions for each subject. Second, embedded alpha wave (i.e., 8–13Hz bandwidth) responses and their respective Fourier-transformed power values (amplitude squared, uV2) were also included in the analysis14: these values are automatically derived by the customized Diopsys™ software. A previous experiment had demonstrated the central 9–11Hz bandwidth component to provide the largest and most repeatable alpha responses, and thus only these three discrete frequency bins were analyzed. Third, an additional metric, the alpha peak frequency (APF), was also calculated in the following manner for each filter condition, over all frequencies: ∑[(Power)×(Frequency)]/∑[(Power)].16 APF is a gravity-weighted mean, and thus it represents the frequency at which overall power is maximal. For each condition, the trial value that either exceeded 1 SD from the mean of the four trials, or was the most deviant one, was deleted for that subject to control for outlier effects.17 The three remaining trials for each condition were used to calculate the mean and standard deviation (SD). The mean amplitude, latency, and selected alpha power values were calculated for each subject, and then the group average was determined. The group mean values±1 SEM were analyzed and graphically displayed for each parameter as a function of filter type. GraphPad Prism Version 5 Software was used for all of the subsequent analyses. A p-value of p≤0.05 was considered to be statistically significant in all cases.
The data were analyzed in several ways. First, a one-way, repeated-measures ANOVA for the factor of filter condition was used to investigate differences in the group mean VEP amplitude with each filter. Similarly, two one-way, repeated-measures ANOVAs for the factor of filter condition were used to investigate filter-induced differences in the group mean N75 and P100 latencies. A two-way, repeated-measures ANOVA for the factors of filter condition and frequency was used to investigate group mean power differences in the alpha wave bandwidth with the gray, blue, yellow, and red filters. Last, a one-way, repeated measures ANOVA was used to investigate the effect of each filter on the alpha peak frequency.
Variability was assessed both within and between sessions using the coefficient of variation (CV=SD/mean).18 The CV can range from 0.00 to 1.00, with 0 representing the lowest level of variability/best repeatability. First, several one-way, repeated-measures ANOVAs were used to investigate within session variability across filter conditions for the parameters of VEP amplitude, N75 and P100 latency, alpha power, and alpha peak frequency. Second, CV's were compared between two of the subjects for two separate test sessions separated by fourteen days to assess repeatability.
ResultsVEP amplitudeThe group mean amplitudes for each condition were 14.52μV, 15.62μV, 15.21μV, 14.37μV, and 14.58μV for the baseline, gray, blue, yellow, and red filters, respectively. There was no significant effect of the spectral filters on the VEP amplitude (F[4,19]=1.56; p=0.194). The effect of spectral filters was significant for the group mean amplitude variability (F[4,19]=2.45; p=0.05): Dunnett's multiple comparison test revealed a significantly lower intra-session amplitude variability for the blue filtered condition, as compared to baseline (p<0.05).
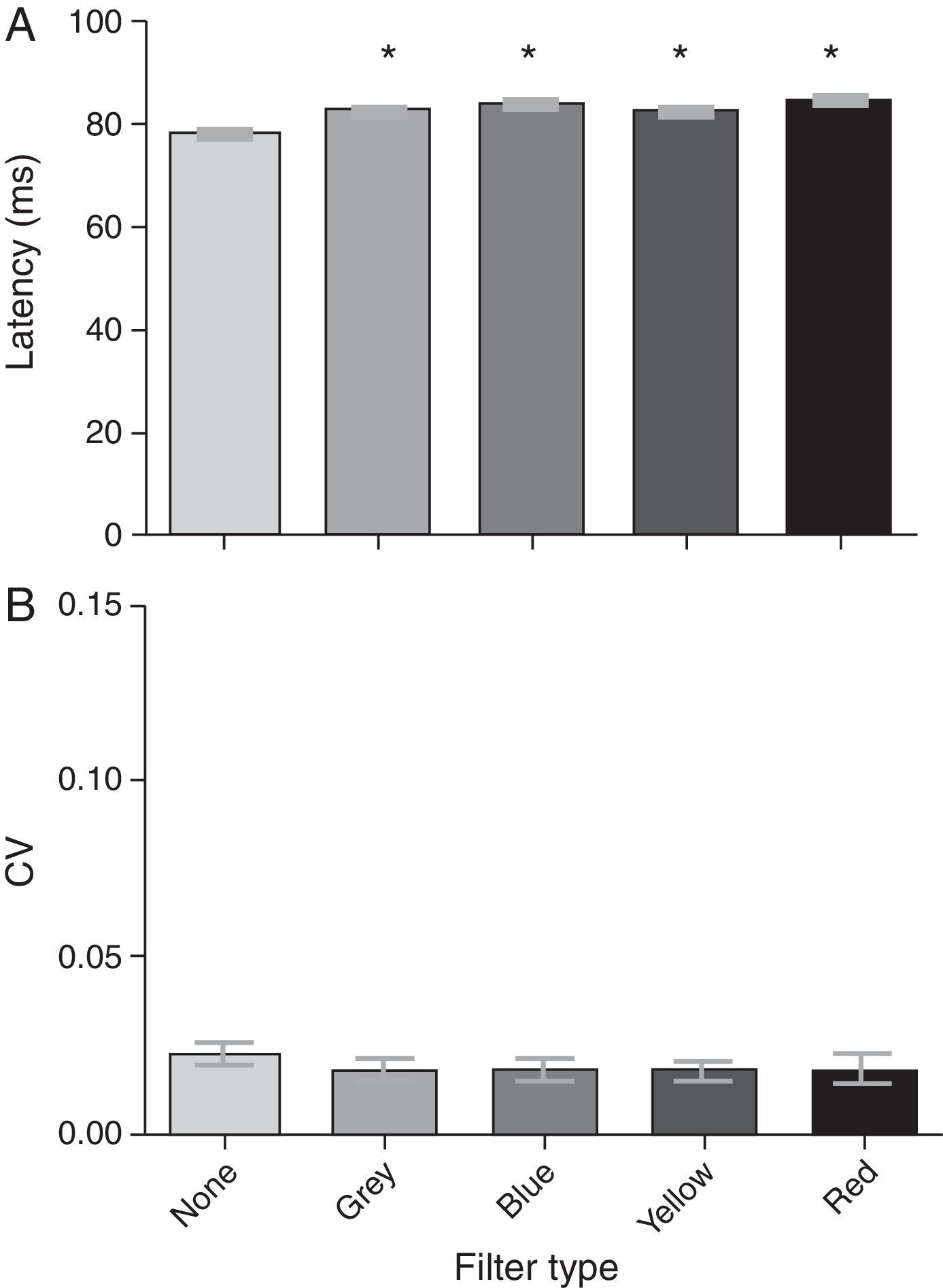
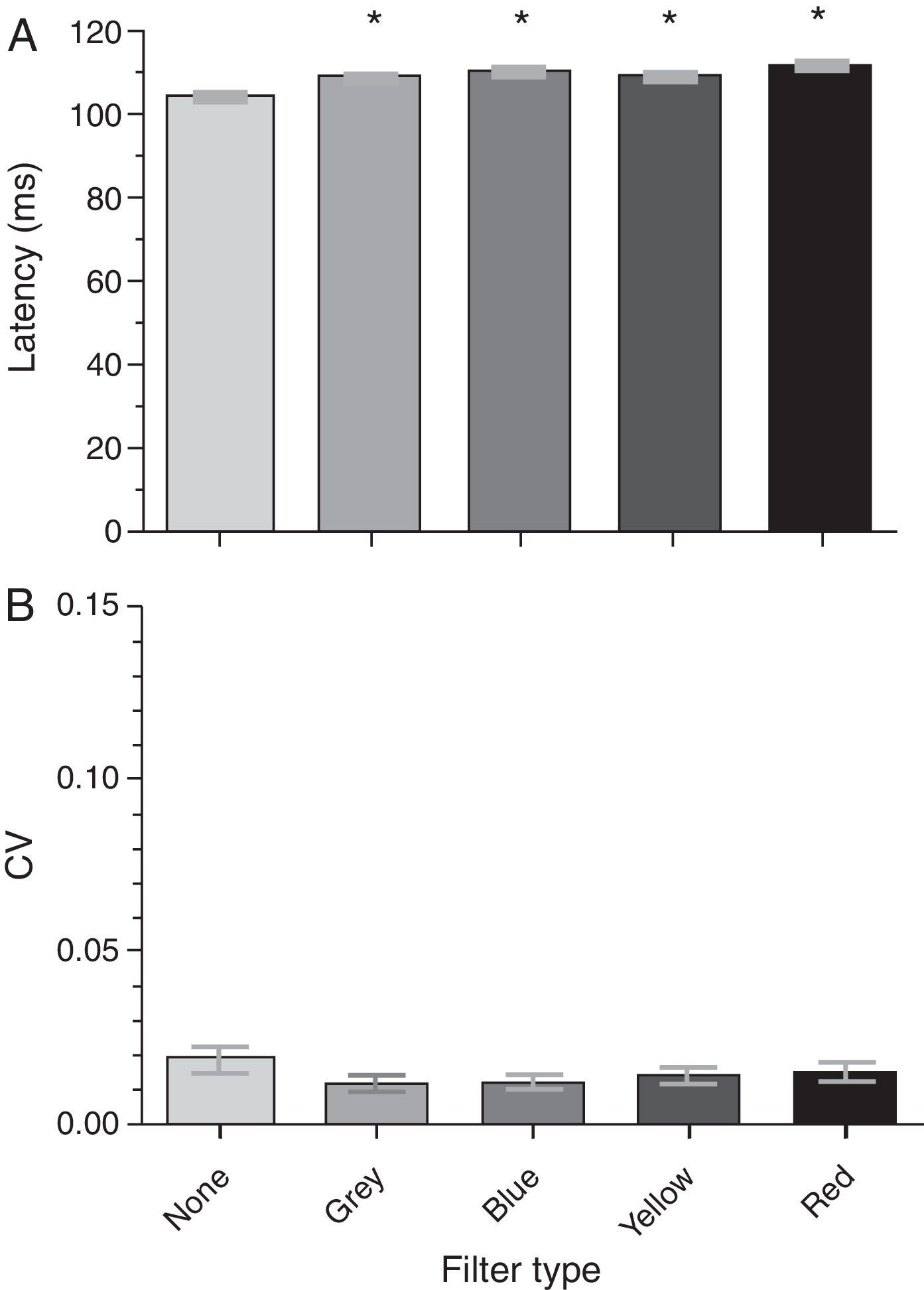
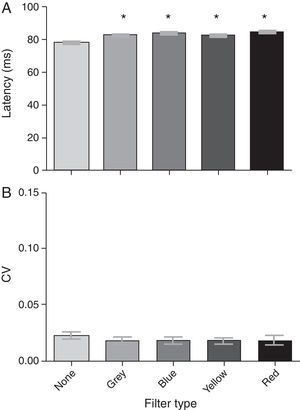
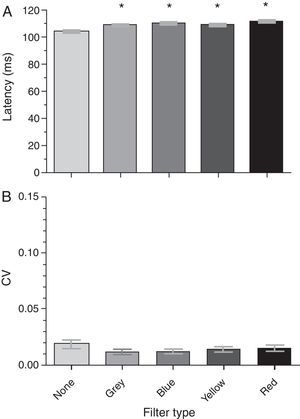
VEP N75 latencyThe group mean N75 latencies for each condition were 77.86ms, 82.89ms, 83.68ms, 82.34ms, and 84.56ms for the baseline, gray, blue, yellow, and red filters, respectively (Fig. 3a). There was a significant effect of the filters on the N75 latency (F[4,19]=76.64; p<0.0001): it increased with the addition of each filter when compared to baseline (i.e., no filter). A post hoc Tukey test revealed significant comparisons (p<0.05) between several conditions (see Table 2a); all latency values with the filters were similar and increased with respect to the no-filter condition. There was no significant effect of any spectral filter on the N75 latency variability (Fig. 3b) (F[4,19]=1.39; p=0.244).
The group mean P100 latencies for each condition were 104.26ms, 109.41ms, 110.18ms, 108.93ms, and 111.43ms for the baseline, gray, blue, yellow, and red filters, respectively (Fig. 4a). There was a significant effect of the spectral filters on the P100 latency (F[4,19]=10.11; p<0.0001): it increased with the addition of each filter when compared to baseline (i.e., no filter). A post hoc Tukey test revealed significant comparisons (p<0.05) between several conditions (see Table 2b). There was no significant effect of any spectral filter on the P100 latency variability (Fig. 4b) (F[4,19]=1.78; p=0.142).
There was no interaction between frequency and filter type on mean alpha power (F[8,57]=0.89; p=0.529). There was no effect of either frequency (F[2,57]=1.50; p=0.231) or filter type (F[4,57]=1.24; p=0.295) on mean alpha power alone. The mean alpha peak frequencies for each condition were 10.03, 9.98, 10.00, 10.01, and 9.96Hz, for the baseline, gray, blue, yellow, and red filter conditions, respectively. The values did not vary significantly with filter type (F[4,19]=1.34; p=0.244). Regarding within-session variability, there was neither an interaction between frequency and filter type (F[8,57]=1.87; p=0.065), nor effects of frequency (F[2,57]=0.33; p=0.717) or filter type (F[4,57]=1.865; p=0.117), on alpha power variability.
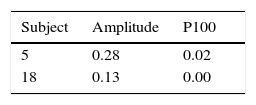
Between session repeatabilityThe coefficient of variation calculations for two key clinical parameters (i.e., VEP amplitude and P100) are presented for the two repeat-tested subjects in Table 3. The values were low, thus suggesting excellent inter-session repeatability for each subject, for both parameters.
DiscussionThe goal of the current study was to determine how spectral filters (i.e., bandwidths of visible light) alter neural activity in visually-normal individuals, thus providing a basis for future clinical investigations. This problem can be approached mechanistically through consideration of the manner in which spectral filters alter incoming light. Specifically, both the amount (i.e., luminance) and type (i.e., spectral composition) of light are changed: their relative contributions must be determined. The results of the present study can be classified with these two mechanisms in mind, and further, into those findings related to cumulative neuronal activity (i.e., amplitude, power) and those related to temporal processing (i.e., latency). First, regarding cumulative neuronal activity, neither the mean VEP amplitude, nor the mean alpha power in the selected 9–11Hz bandwidth, was significantly influenced by the filters. Second, the within-session VEP amplitude variability decreased with the addition of spectral filters. Third, significant differences were found in measures related to neuronal processing time (i.e., VEP latency measures). Each finding is expanded below.
First, the VEP amplitude and alpha power are both reflective of the activity of neuronal populations; thus, the synchronous neural activity related to the VEP stimulus, and within the selected alpha bands, does not seem to be either luminance or spectrally-dependent, at least in visually-normal individuals. Specifically, increases and decreases in both VEP amplitude and alpha power are expected if neural activity becomes relatively more synchronized or desynchronized, respectively. The chromatic filters used in the present study did not change the VEP amplitude in the normative population. Thus, whereas spectral modification seems to neither synchronize nor desynchronize neuronal populations in visually-normal individuals, modification of incident light in neurological populations (e.g., TBI1 or migraine7,8) may increase synchronization of visuocortical neuronal populations. Whether this change is attributable to luminance, spectral transmission, or a combination of both, remains to be determined.
Next, the within-session variability of the VEP amplitude decreased with the addition of spectral filters; that is, the mean CV values were all numerically lower than baseline for the spectral filter conditions. This difference may be reflective of an increased signal-to-noise ratio. With no filter in place, the amount of synchronous activity, and thus amplitude, is likely to fluctuate slightly between successive trials. If activity is limited to those neuronal cells responsible for processing a narrower bandwidth of light (e.g., 600–650nm as in the red filter), a partial saturation effect may occur, thus minimizing amplitude fluctuations and producing measurements which fall within a closer range between trials. In fact, populations of cells optimally responsive to luminance, color, or luminance and color have been shown to exist, and exhibit anatomical segregation, in the macaque primary visual cortex (V1).19 Furthermore, the luminance-color sensitive cells possess spatial tuning curves whose peaks closely correspond to the spatial frequency of the current VEP test stimulus (i.e., 2.26cpd, and peaks of 2.56 and 2.73cpd to achromatic and equiluminant chromatic stimuli, respectively).19 Thus, spectral modification of incoming light has the potential to target specific neuronal populations in the human primary visual cortex.
Last, significant differences were found in measures related to neural processing time (i.e., VEP latency measures). Each filter increased both the N75 and P100 latencies as compared to baseline: this effect is primarily due (∼75%) to the reduction in luminance imposed by each colored filter. For example, neural processing time has been shown to increase with decrements in luminance (e.g., the Pulfrich phenomenon20); similarly, VEP latency measurements increase when luminous intensity is decreased.21–23 With no filter in place, the mean luminance of the VEP screen was 52cd/m2: this was reduced to an average of 19.5cd/m2 across the Intuitive Colorimeter filters (i.e., gray, blue, yellow, and red). This nearly three-fold reduction (∼0.30 log units) in luminance translated to ∼5ms increases in N75 and P100 latency (i.e., 77.86ms to mean 83.37ms, 104.26ms to mean 109.98ms): similar values were found in a prior investigation (i.e., 16.6ms increase in P100 latency per 1.0 log unit reduction in retinal illuminance21), and in a recent investigation in our laboratory.24
Significant differences were also found when comparing latency values obtained with each of the filters. For example, both the N75 and P100 latency values were prolonged more so by red than any of the other filters (i.e., red>blue>gray>yellow, in ms for both N75 and P100 latencies). In fact, the red-filtered N75 and P100 values were significantly different than a majority of the respective values obtained with other filters in place [see Table 2a (N75) and Table 2b (P100)]. The significant latency differences between filters may be attributed to changes in luminance and/or spectral transmission. Regarding luminance, although each filter transmitted a constant percentage of visible light (i.e., 39%), the mean luminance of the VEP screen differed very slightly when measured through each of the filters: red and blue were the dimmest (i.e., 18cd/m2), followed by the gray (19cd/m2), and yellow (23cd/m2). Following interpolation of the present data with a previous database concerning the luminance-P100 latency relationship,24 the luminance reducing characteristics of each spectral filter accounted for 85 (gray), 70 (blue), 80 (yellow), and 60% (red) of the latency increase from baseline. Thus, the remaining increase (∼25%) can be attributed to the spectral aspect of each specific-filter. Whether the prolonged N75 and P100 latencies may be attributed to changes in spectral transmission depends on the threshold luminance decrement needed to increase latency. Exact luminance matching is ideal; however, the significant difference in mean P100 latency between the blue and red filters, which were equiluminant, suggests spectral properties per se also influence latency measurements.
The present VEP amplitude, latency, and selected-alpha wave measurements will serve as a comparative, normative database in young adults for future colorimetric experiments: specifically, our laboratory plans on investigating the effect of spectral modification on electrophysiological responses in the acquired brain-injury population (e.g., mTBI and stroke). Wavelength-specific benefits have been suggested to occur in both populations (e.g., in contrast-sensitivity, reading, and light sensitivity1,7,15); however, VEP data regarding color and ABI remains limited, and to our knowledge, the present study is the first to report normative data.
In conclusion, several basic physiological principles have been confirmed and suggested by the current study to provide an outline/reference by which subsequent studies may be based upon or compared. Specifically, application of spectral filters in visually-normal individuals alters temporal information processing in a predominantly luminance-dependent manner. Conversely, the current study suggests cumulative neuronal activity (measured by the pattern-VEP) is both luminance and wavelength independent. Thus, investigations which document departures from the normative luminance-latency relationship (e.g., steepening or shallowing of the slope) and/or changes in VEP amplitude or alpha power may highlight potential objective biomarkers for neurobiological disease.21 Similarly, colored-filter induced changes in neurophysiological parameters (e.g., amplitude and latency) may also be reflective of beneficial therapeutic effects. It is our hope that through the establishment of electrophysiological phenomena in a younger normative population, and later an older cohort, a clearer picture as to the mechanism by which colored filters improve quality of life in select clinical populations is afforded.
DisclosuresAll authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank Cerium Optical Products (Kent, United Kingdom) for loan of the Intuitive Colorimeter and Diopsys for loan of the VEP system.