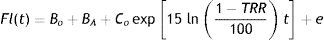Tear exchange beneath a contact lens facilitates ongoing fluid replenishment between the ocular surface and the lens. This exchange is considerably lower during the wear of soft lenses compared with rigid lenses. As a result, the accumulation of tear film debris and metabolic by-products between the cornea and a soft contact lens increases, potentially leading to complications. Lens design innovations have been proposed, but no substantial improvement in soft lens tear exchange has been reported. Researchers have determined post-lens tear exchange using several methods, notably fluorophotometry. However, due to technological limitations, little remains known about tear hydrodynamics around the lens and, to-date, true tear exchange with contact lenses has not been shown. Further knowledge regarding tear exchange could be vital in aiding better contact lens design, with the prospect of alleviating certain adverse ocular responses.
This article reviews the literature to-date on the significance, implications and measurement of tear exchange with contact lenses.
El intercambio de lágrima bajo las lentes de contacto facilita la reposición continua de fluido entre la superficie ocular y las lentes. Dicho intercambio es considerablemente menor con el uso de lentes blandas en lugar de rígidas. Como resultado, se incrementa la acumulación de desechos de la película de lágrimas y los sub-productos metabólicos entre la córnea y las lentes de contacto blandas, lo que conlleva complicaciones potenciales. Se han propuesto innovaciones en el diseño de las lentes, pero no se ha reportado ninguna mejora sustancial en lo referente al intercambio de lágrima con las lentes de contacto blandas. Los investigadores han determinado el intercambio de lágrimas tras el uso de lentes utilizando diversos métodos, principalmente la fluorofotometría. Sin embargo, debido a las limitaciones técnicas, se conoce poco acerca de la hidrodinámica relacionada con las lentes y, hasta la fecha, no se ha mostrado un intercambio de lágrima substancial con lentes de contacto. El conocimiento adicional sobre el intercambio de lágrima podría resultar esencial para ayudar a diseñar lentes de contacto mejoradas, con la perspectiva de aliviar ciertas respuestas oculares adversas. Este artículo revisa la literatura hasta la fecha acerca de la importancia, implicaciones y medición del intercambio de lágrima con lentes de contacto.
It has been estimated that there are approximately 140 million wearers of contact lenses (CL) worldwide.1,2 Despite the introduction of many new lens materials and care systems, dryness and discomfort continue to be reported by some 30–50% of lens wearers, particularly at the end of the day.3–7 As a result of this discomfort, 30–50% of wearers will at some point lapse from lens wear 8–10 and 25% of wearers will permanently cease contact lens wear.10 Of greater concern is that more severe complications such as contact lens-induced microbial keratitis and inflammatory conditions have not decreased in the past decade.11–16 Numerous factors are associated with contact lens discomfort17–22 and inflammation.2 One aspect of the lens–tear–cornea interaction that has received relatively little attention is tear exchange. Soft contact lens design and fitting have remained essentially unchanged since their introduction in the early 1970s and it has been suggested that improving tear exchange and “flushing” beneath lenses may reduce inflammatory events and could improve long-term wearing success.
In this review, we examine and discuss the current literature on tear exchange with contact lenses, including its significance, mechanism and the strategies proposed to improve both tear exchange and its measurement.
Role of tear exchange in contact lens wearWhen a contact lens is placed on the eye, the lens divides the tear film into two layers, the outermost layer that overlies the lens, which is termed the pre-lens tear film (PrLTF), and the layer between the back surface of the lens and the cornea, which is referred to as the post-lens tear film (PoLTF).23–25 The fluid circulation between the pre- and the post-lens tear film is commonly referred to as “tear exchange”, with authors also using the terms “tear turnover”, “tear pumping”, “tear flow”, “tear flushing” or “tear mixing” synonymously.26–34 The importance of tear exchange behind a contact lens remains an ongoing debate. Historically, tear exchange has been ascribed the leading role in delivering oxygenated tears to the cornea behind non-permeable, polymethyl-methacrylate (PMMA) contact lens materials.35,36 However, modern, highly oxygen transmissible silicone hydrogel (SiHy) lenses have virtually eliminated hypoxic complications, and the significance of tear exchange has been redefined. It is now considered essential that tear exchange occurs in an attempt to reduce post-lens debris, as metabolic byproducts that stagnate between the lens and cornea, particularly in extended (EW) and continuous wear (CW), can perhaps contribute to the onset of adverse events, by altering the epithelial barrier function.37–39 Despite excellent oxygen permeability properties, reviews indicate that SiHy wearers still develop a number of adverse events when lenses are worn overnight.2,40–45 These include inflammatory conditions, including contact lens peripheral ulcers, (CLPU)46–50 contact lens-induced acute red eye (CLARE)47,48,51 and infiltrative keratitis (IK).43–45,47,48,51,52 Incidence for these events varies between 1% and 5% in CW SiHy wearers, with recurrence rates being as high as 10% for CLPU, 14% with IK and 29% for CLARE.47 These rates are to be considered with caution, as up to 50% of CLPU and some IK cases have been found to be asymptomatic and thus these rates may underestimate the true rates. Corneal infiltrate incidence in CW users of a SiHy lens was 5.7%, 8.5% and 10.3% after one, two and three years of wear, respectively, as opposed to 1.6–4% in non-contact lens wearers.53 The most sight threatening complication for contact lens wearers, microbial keratitis (MK), still affects 3–5 in 10,000 daily CL wearers, a number that has remained constant over the past 20 years.54 A number of reports suggest that most of these conditions are not due to hypoxic stress, but rather due to the presence of bacterial exotoxins, which could lead to inflammatory complications if their removal is delayed due to tear stagnation beneath the lens.40,48,55
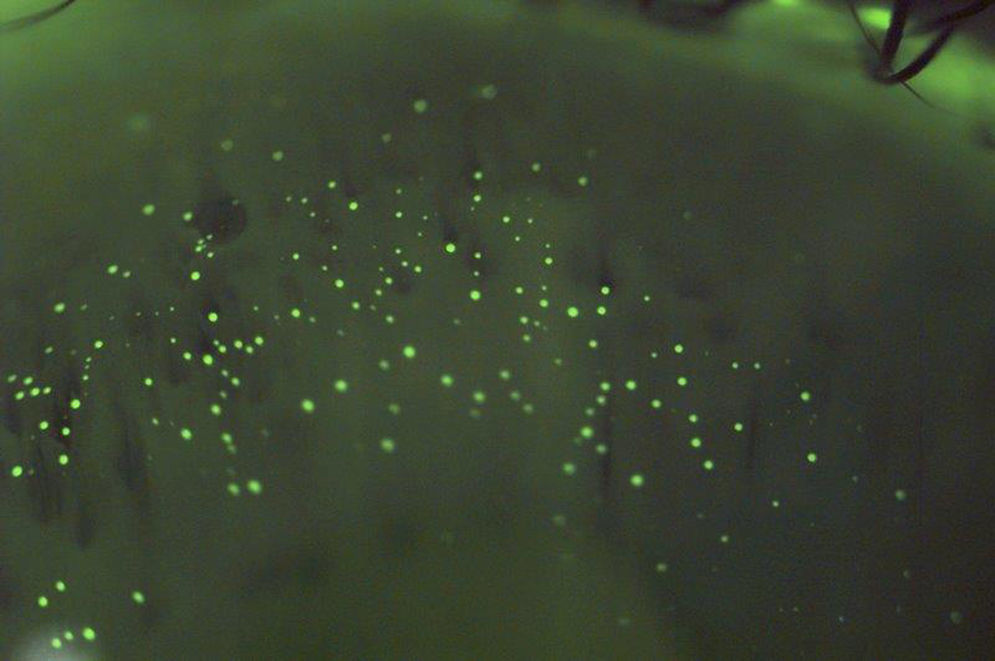
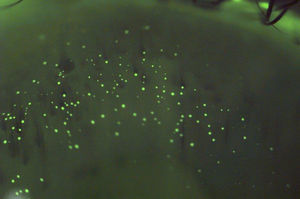
While not inflammatory in nature, “mucin balls” are a further complication observed with EW/CW of SiHy lenses.56–61 These spherical, translucent, insoluble bodies, ranging in diameter from 10 to 100¿m, are composed of naturally occurring tear film mucins and form between the back surface of a contact lens and the cornea, being rigid enough to indent and leave an imprint on the epithelial surface (Fig. 1).61 While their exact formation remains unclear,57 several factors may contribute. The composition of tears changes during sleep, with a much reduced aqueous phase, resulting in more viscous tears.62–66 The high lens modulus (stiffness) and low deposition rate of SiHy's, coupled with the sheer forces of the cornea-lens interaction may cause the mucin within the post-lens tear film to roll up into spheres.61 Their occurrence is high in patients who sleep in lenses, with 60–100% of eyes exhibiting mucin balls after 1–3 weeks of CW.46,67 The percentage of subjects who never used lubricating drops was up to three times higher in subjects with mucin balls than in those without mucin balls (p=0.0014).56 This suggests that artificially enhancing tear volume and tear replenishment helps eliminate this form of post-lens debris.61
Mucin balls are regarded by some as a contact lens complication,61 but the association with adverse ocular responses remains controversial, and they certainly do not result in any deleterious symptoms. Some theorize that they may act as markers signaling a more mucous-rich tear film, which potentially fulfills a protective role against corneal inflammatory events (CIE) and show a lower CIE incidence.60 In contrast, others have shown that the presence of mucin balls results in a 3.6× increased risk of CIE.68 A more recent report showed an association between mucin balls and micro-structural changes of the cornea, including basal epithelial irregularity and dendritic cells.67 Of note, is that in all reports, complete recovery from the mucin ball indentation occurs 1–3 days after ceasing overnight wear.56,60,69
Thus far, data from even the most oxygen permeable of lens materials indicates that inflammatory complications still occur and could actually be twice as high with SiHy lenses, regardless of whether they are worn on a daily wear or overnight basis.12–16,70 Could there be a link between inflammatory responses and reduced tear exchange? If so, could inflammatory responses be reduced if tear exchange were to be increased? In order to address this important question, it is vital that a reliable method (or methods) exist that can determine post-lens tear exchange.
Measurement of tear exchangeClinical assessment of the interactions of the tear film with the lens is typically restricted to the anterior lens surface (or PrLTF). This may include a general examination of tear film quality with a slit-lamp,71 non-invasively measuring the tear break up time (NIBUT) by using a projected grid over the front surface of the lens,72–74 determination of tear film stability using interferometry,23,24,75 assessment of the optical quality of the specular reflection from the front surface of the lens at high magnification76 and the measurement of front surface aberrations as an indicator of tear film thinning using aberrometry.77
One factor that has received much attention is precorneal tear film thickness in the absence of lens wear, with numerous reports on its measurement, employing diverse techniques such as optical pachometry,78 interference79–81 and optical coherence tomography (OCT).82–85 Until recently, there was little agreement on the true thickness of the precorneal tear film, with values ranging from 1 to 45¿m.81,84,86 The large variation in reported values appears to stem not only from the diversity of measurement methods, but also from the fact that tear film thickness is a dynamic value, with a rapid decrease after the blink over the time that the eye remains open. A recent publication shows that the precorneal tear film thickness measures 6¿m immediately after the blink and halves within 16s.82 The timing of the measurement is therefore crucial for any thickness assessment. Over the past decade, there has been general agreement on a range of 2–5¿m for the precorneal tear film.82–85 Once a contact lens is inserted, this value initially doubles,83 resulting in a 2–5¿m thickness for both PrLTF and PoLTF, with the latter quickly decreasing, sometimes to values <1¿m. Some authors note that the PoLTF determined was below the ∼3¿m depth resolution of the measurement system being used and had to be mathematically computed.80,83
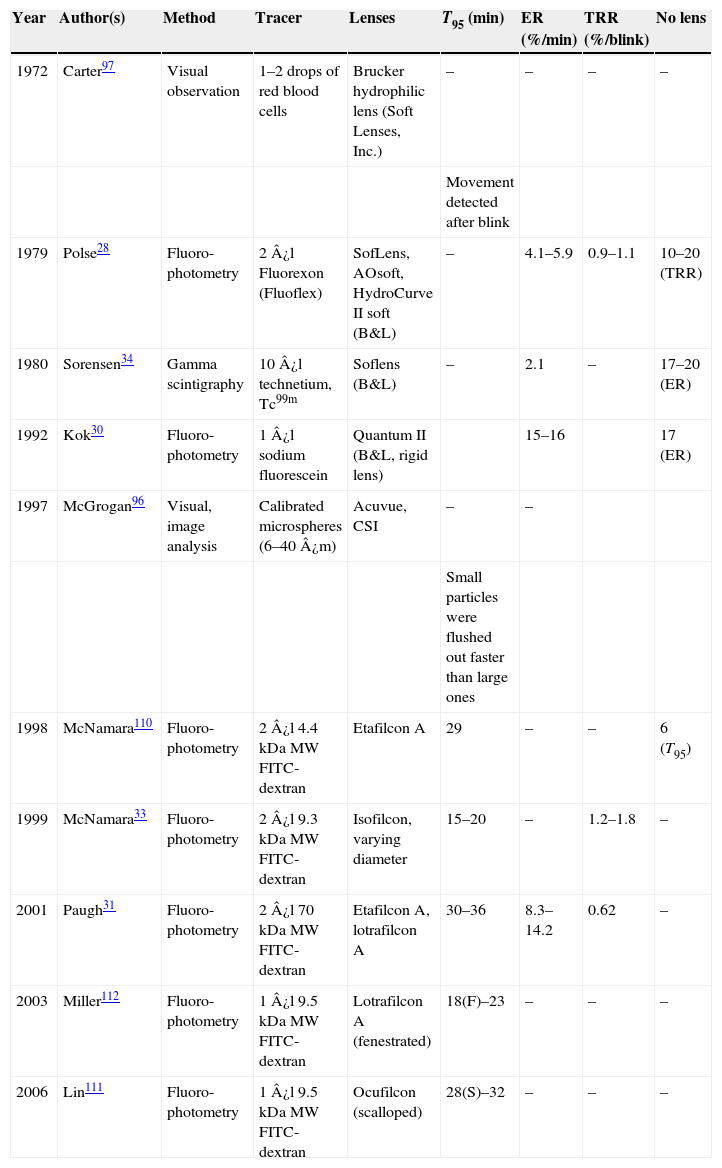
As described above, much work has been published on PrLTF and PoLTF. However, of possibly greater relevance is data on how that thickness changes over time and what percentage of the tears are exchanged during wear. To date, technological limitations have stood in the way of an accurate and non-invasive, direct measurement method for determining tear exchange. Many publications have reported on the experimental measurement of tear exchange,26–34,87–97 but the majority of these studies have been undertaken in non-lens wearers, measuring physiological tear turnover rate. In the case of subjects wearing lenses, the approaches used can be broadly grouped into three fundamental categories: mathematical models, particle-based methods and inferential methods. Commonly reported measurement units for tear exchange are “tear turnover time” (T95 – which represents the time in minutes for 95% of the tear volume to be eliminated from under the lens), “elimination rate” (ER – which represents the percentage of tear-volume eliminated per minute) and “tear replenishment rate” (TRR – which represents the percentage of tear-volume eliminated per blink).31,33
Mathematical modelsTheoretical approaches seek to calculate PoLTF mixing using mathematical models. Most of these were focused on determining tear flow, with the primary aim being the improvement of oxygen concentration and distribution under the lens.98–101 Various blinking models are proposed, ranging from simplified descriptions of flat surfaces with single layer tear volumes, to complex estimates of lid pressure and blink velocity, viscosity gradients in the typical three-layer tear model and highly sophisticated mathematical models.98–104 Although the true relationship between modeling and clinical results remains to be determined, some mathematical models resulted in possible predictions for consideration. Chauhan and Radke105 published a model that predicted the volume of the post-lens fluid squeezed out during the blink to be greater than the amount of fluid drawn back under the lens. The contact lens would therefore eventually be in contact with the cornea. This coincides with experimental findings, where the PoLTF is reduced below the imaging resolution.80,83 Maki and Ross106 calculated the amount of tear fluid exchange in response to variations in the back surface shape of soft lenses. They found that the amount of tear fluid exchange was sensitive to the thickness profile of the contact lens, with a stiffer, thicker lens being more efficient at exchanging the post-lens tear film, as the mechanical suction pressure gradients are larger.
Particle-based methodsObservational methods that track particle movement in tears are potentially a more pertinent approach, as minuscule particles should, in theory, follow the natural motion of tears. In 1972, Carter instilled patients’ own heparinized blood (to avoid adverse reactions) onto the ocular surface and visualized red blood cell movement behind a hydrophilic contact lens.97 The movement of the erythrocytes in the PoLTF, similar to the tear film particle movement observed in the PrLTF,107 was pronounced after the blink and reduced in the inter-blink phase. Human red blood cells range in size from 5 to 8¿m,108 which, according to more recent PoLTF estimates mentioned above, is close to the upper range of current precorneal tear film thickness estimates (1–5¿m). A radioactive Technetium tracer was used by Sorensen and coworkers in 1980, to monitor tear flow with and without a contact lens.34 However, the Gamma tracer was absorbed by the contact lens material, rendering the results unreliable. More recently, McGrogan and colleagues instilled high precision calibrated polystyrene microspheres on the back surface of lenses to measure the rate of particular expulsion as influenced by contact lens fit.96,109 While no detailed quantitative measures on their work are available, they report that only small particles (1–6¿m in diameter) flushed out over time, with larger ones (40¿m) being static. Again, the majority of these beads appear to have exceeded the PoLTF thickness. It was shown that lens fit has a major influence on particle movement: steep lenses showed little particle movement in the PoLTF, even when the lens was mechanically displaced on the eye. Flat fitting lenses demonstrated major particle movement in the lens periphery following a blink. Of note is that in areas of contact between the lens and cornea, particles were embedded in the pre-corneal mucus layer, being visible several days after the experiment. The mucus layer is thick enough to protect beads of 10¿m from the mechanical abrasion of the lid.
Inferential methodsFluorophotometry is the most frequently used method to determine tear expulsion under contact lenses.28,29,31,33,37,110–112 Developed and described by Maurice in 1963,113 it was modified and improved by others26,92,110,114 and used for various purposes, including study of tear physiology, turnover, thickness, dynamics,32,91,115 corneal pH110,116 and epithelial permeability.117 The basic fluorophotometer involves the modification of a conventional ophthalmic slit lamp bio-microscope attached to a computer-aided video capturing device, to obtain and quantify the decay of an instilled fluorescent dye over time. In 1985, a dedicated scanning fluorophotometer (The Fluorotron Master, Ocumetrics Inc., Mountain View, California) was introduced and later modified by others.33,118–120 Either device produces a blue excitation beam which coincides with the absorption spectrum of the dye. At the intersection of the excitation beam and the emission beam, an area between 0.05 and 0.1mm wide and up to 2mm high is formed on the ocular surface and its fluorescent intensity measured.
Tear mixing under lenses is estimated during lens wear by measuring the rate at which a high molecular weight fluorescent dye is depleted from the PoLTF. In order to estimate mixing, baseline autofluorescence readings for the cornea and lens are obtained first. The fluorescent dye is then pipetted onto the back surface of a contact lens, which is immediately applied to the cornea. After the lens is in place, the fluorescent intensity is measured at a set interval of time for approximately 30min, while the subject's blink rate is controlled by a metronome. Mathematically, the rate of fluorescein decay is estimated by fitting an exponential curve to the measured fluorescent intensity values. The analysis model is:
where Fl(t) is the tear film fluorescence at the time t measured in minutes from the point at which the dye is instilled; BO=baseline autofluorescence (contact lens+cornea) before instilling the dye; BA=residual fluorescence of dye that is either absorbed by or is adherent to the contact lens surface; CO=initial fluorescence intensity beyond that which is already included in BA after instilling the dye (when t=0); e=error in the fluorescence intensity measurements. The equation is solved for TRR (tear replenishment rate), which is the percentage at which the fluorescence Fl changes with each blink. Alternatively, the exponential decay in fluorescence can be represented by T95, the time in minutes required for 95% of the fluorescein to be depleted from underneath the lens.33,110In spite of the reported “gold standard” status that fluorophotometry has earned for determination of tear exchange over time, the method suffers from a number of issues.26,87 Initial drawbacks of this method included the extended measurement time and the inhibition of the blink reflex, both of which induce reflex lacrimation. A later, “refined method” featured a shorter measurement and a metronome-controlled blink rate.26 One cause for the potential unreliability of measurements relates to the fluorescent dye employed. Early reports28,29 employed a fluorescent dye (Fluorexon, molecular weight 710Da), which was shown to be absorbed into the lens matrix.121 Paugh et al.31 also suggested the possibility of corneal and scleral penetration with Fluorexon, which can lead to errors of up to 25% in the ER measurement. Therefore, a larger, non-penetrating FITC-dextran tracer (molecular weight 7–9.5kDa) was selected in later studies, which did not penetrate the lens material.27,31,111,112 However, fluorescein-based dyes may stain corneal and conjunctival cells, and this can lead to measurement errors.122 It is also unclear how fluorescein affects the composition, osmotic concentration and thus production of tears. Fluorescein is known to have a concentration dependent quenching character. Its fluorescent efficiency is proportional to concentration only up to a concentration of 0.2%. Once this level is exceeded, the efficiency decreases with the inverse square of the concentration88,123 and thus the fluorescence will vary across the time that the assessment is made, as fluorescein is flushed from the ocular surface. Another drawback of fluorescein dyes is that they homogeneously dissolve in the tear film after a few initial blinks. This prevents the precise characterization of flow dynamics in the PoLTF, such as direction, velocity or stagnation. In addition, instillation may cause a stinging sensation with more sensitive patients, which could trigger an increase in tear production. A relatively large volume of fluorescent dye is instilled beneath the lens (1–5¿l), potentially triggering faster physiological flushing. Another assumption in fluorophotometric procedures is that the thickness of tears beneath the contact lens remains constant,33 but as discussed previously, there are recent results that disagree with this.80,82,83 In addition, only a small area (at most a column of 0.1 by 2mm) on the ocular surface is analyzed for fluorescent intensity, as opposed to the entire ocular surface. This is relevant, in light of reports that show major differences in tear turnover between the center and the periphery of flat fitting lenses.109 It is therefore prudent to consider the invasiveness of the measurement, with volume, composition and concentration potentially being different from physiological values.
Other potential errors relate to the interpretation of the data obtained. Tear exchange measured with soft lenses is often described as a double exponential curve,31–33,111,112 with up to 70% of the fluorescent intensity decaying within the first 5min of the measurement.31 Authors often disregard the readings of this initial period in order to avoid reflex tearing due to the instillation of fluorescein or insertion of the CL, and to allow for physiological tearing to settle. The consequent readings are linear over the next 25min, until less than 5% fluorescence is reached and the measurement ended. Thus, only a portion of the data are used, which may not reflect what is truly occurring beneath the lens.
Finally and perhaps most importantly, fluorophotometry is not a direct but an inferential tear exchange measurement. What is actually being measured is the decay of an instilled fluorescent dye, not tear exchange per se; this is then mathematically extrapolated to describe fluid replenishment. The method does not discern between PrLTF and PoLTF fluorescence; the sum of both is being recorded. In actuality, a more appropriate term for fluorophotometry values would be “tear expulsion rate”. Exchange implies a two-way (in/out) circulation of fluid, but what fluorophotometry actually measures is only the decrease in fluorescence when fluorescein is instilled onto the posterior surface of the lens and the method provides no knowledge of fresh tears moving under the lens. An experiment conducted by Chen et al. underpins the phenomenon of tear expulsion, as opposed to tear exchange: PrLTF and PoLTF thickness are measured before and after instillation of a 35¿l drop of artificial tears. Before instillation, PrLTF and PoLTF measure 2.3±1.7¿m and 1.4±1.5¿m respectively. Upon instillation, both layers were measured every 2min: while the PrLTF decreased from 29.5 to 15.1, 9.5, 7.3, 5.3 and 4.3¿m after 10min, the values of the PoLTF remained relatively constant between 0.9 and 1.4¿m over this time. Three minutes after lens insertion, with no added drop of artificial tears, the PoLTF was not detectable in any of the 9 subjects.83
Published dataAn overview of the published methods to-date and the results obtained for tear exchange is provided in Table 1.
Publications on tear exchange with contact lenses. Values are reported ranges and compared to the physiological no lens value, where applicable.
| Year | Author(s) | Method | Tracer | Lenses | T95 (min) | ER (%/min) | TRR (%/blink) | No lens |
|---|---|---|---|---|---|---|---|---|
| 1972 | Carter97 | Visual observation | 1–2 drops of red blood cells | Brucker hydrophilic lens (Soft Lenses, Inc.) | – | – | – | – |
| Movement detected after blink | ||||||||
| 1979 | Polse28 | Fluoro-photometry | 2¿l Fluorexon (Fluoflex) | SofLens, AOsoft, HydroCurve II soft (B&L) | – | 4.1–5.9 | 0.9–1.1 | 10–20 (TRR) |
| 1980 | Sorensen34 | Gamma scintigraphy | 10¿l technetium, Tc99m | Soflens (B&L) | – | 2.1 | – | 17–20 (ER) |
| 1992 | Kok30 | Fluoro-photometry | 1¿l sodium fluorescein | Quantum II (B&L, rigid lens) | 15–16 | 17 (ER) | ||
| 1997 | McGrogan96 | Visual, image analysis | Calibrated microspheres (6–40¿m) | Acuvue, CSI | – | – | ||
| Small particles were flushed out faster than large ones | ||||||||
| 1998 | McNamara110 | Fluoro-photometry | 2¿l 4.4kDaMW FITC-dextran | Etafilcon A | 29 | – | – | 6 (T95) |
| 1999 | McNamara33 | Fluoro-photometry | 2¿l 9.3kDaMW FITC-dextran | Isofilcon, varying diameter | 15–20 | – | 1.2–1.8 | – |
| 2001 | Paugh31 | Fluoro-photometry | 2¿l 70kDaMW FITC-dextran | Etafilcon A, lotrafilcon A | 30–36 | 8.3–14.2 | 0.62 | – |
| 2003 | Miller112 | Fluoro-photometry | 1¿l 9.5kDaMW FITC-dextran | Lotrafilcon A (fenestrated) | 18(F)–23 | – | – | – |
| 2006 | Lin111 | Fluoro-photometry | 1¿l 9.5kDaMW FITC-dextran | Ocufilcon (scalloped) | 28(S)–32 | – | – | – |
Despite the many shortcomings of fluorophotometry discussed above, results are relatively consistent across publications. Tear turnover time (T95) averages at 30min for soft lenses,28,30,31,33,110,124 as opposed to approximately 5min for rigid lenses.30,32,89–91,125 These latter values are broadly equal to the tear turnover time with no lens on the eye.30 Interestingly, McGrogan109 also found that micro-beads clear from the pre-corneal tear film within 5min.
The reasons behind this large difference between soft and rigid lenses relate to lens size and material stiffness, and thus lens movement with the blink.126 Soft lenses are approximately 5–6mm larger in diameter than rigid lenses and are much less mobile. Soft lenses typically have an on-eye displacement during the blink of 0.3–0.5mm,127,128 whereas rigid lenses displace 1–2mm.129 Material stiffness or rigidity is typically reported by referring to Young's modulus.130 For soft materials, this ranges from 0.24MPa for a mid-water content hydrogel such as etafilcon A to 1.4MPa for lotrafilcon A, the SiHy material with the highest reported rigidity.131 In comparison, RGP materials have reported modulus values between 1500 and 2000MPa.126,132–134 A reported 5% improvement in ER with a SiHy material compared to hydrogels is arguably due to the slightly higher modulus, which would result in increased lens movement, increasing the tear exchange under the lens.31 Indeed, Carnt et al.68 found that a 0.2mm decrease in lens movement led to a 4.5× higher risk of developing a CIE, potentially indicative of reduced post lens tear fluid clearance.
Supporting evidence for the role of tear flushing in inflammatory responses could be surmised from the data obtained with rigid lenses. It is likely that the high tear exchange rate with RGP lenses facilitates the much needed flushing of post-lens debris and allows tears to replenish uninterruptedly, leading to the low level of inflammatory events seen with rigid materials.11,135–137 With this in mind, a number of attempts have been made to improve tear exchange with soft lens materials. Miller et al.112 suggested that fenestrating SiHy lenses may enhance tear exchange, and they were able to increase it by 28%. However, as reported previously,138 fenestrations significantly reduce in-eye comfort and over the longer-term, tear film debris tends to clog up the micro-perforations and therefore such lenses may not be clinically efficacious. McNamara's approach was to use small diameter lenses (12.0mm), as opposed to conventional 13.5mm lenses, and achieved a 0.6% improvement in TRR; this was significant, but still modest when compared to rigid lens values.33 Lens movement remained unchanged for both lens diameters and varying the base curve of a soft lens had no influence on tear replenishment.29 Finally, “scalloping” the back surface of a lens by creating channeled grooves did not lead to an improved tear-mixing rate for hydrogel lenses.111,139 This lens design achieved a marginally faster tear-mixing rate only in Asian eyes, presumably due to the higher upper-eyelid tension.111
ConclusionOver a span of 40 years, only a handful of publications have attempted to measure tear exchange under contact lenses (Table 1), the majority employing fluorophotometry as the method of choice. While this technique has become the “gold standard”, as pointed out above there are a number of issues that exist that may limit its usefulness in helping design soft lenses that enhance tear exchange. Most notable is that this method really only determines tear expulsion from behind the lens rather than true exchange. A method that is able to evaluate tear movement from both the tear film in front of the lens and movement of tear film from behind the lens onto the ocular surface would be more valuable. Of all methods described in the literature, particle-based approaches seem to be the most reliable, as they provide direct and not inferential data. So far, technological limitations have impeded the exploration of this method. A particle-based characterization of tear exchange would require particles that (a) did not adsorb to the lens material or ocular surface, (b) were biocompatible and non-toxic, (c) were small enough to prevent any comfort issues that may induce reflex tearing, (d) allowed natural tear flow without any mechanical interference and (e) were suitably visible or “traceable” using non-invasive imaging techniques to allow in vivo examinations. Subsequent image analysis would enable statistical analysis of particle motion that would provide characterization of the true tear flow pattern over time. Quantum dots have been used to determine tear film dynamics in non-lens wearers,140 and may prove useful once a commercial source becomes available.
The literature on tear exchange with contact lenses demonstrates conclusively that tear exchange with rigid materials is considerably higher than that which occurs with soft lenses. Good lens mobility on eye is essential and stimulates efficient tear exchange. Deposits, debris and metabolic by-products need to be flushed from the ocular surface in a timely manner, in order to minimize the risk of inflammation. So far, soft lens design innovations intended to enhance tear exchange have not proven clinically feasible and tear exchange values have remained essentially unchanged.
A better understanding of tear movement and replenishment with a lens on eye could aid improved soft contact lens design and perhaps reduce inflammatory events. Future research needs to address the topics of direction, velocity and stagnation of the tear fluid behind a contact lens, in order to facilitate more efficient tear exchange, and a method that could reveal the impact of subtle design variations would prove valuable.