To analyze by spectral-domain optical coherence tomography the anatomical relationship of a specific type of posterior chamber phakic intraocular lens (pIOL) implanted in cases of high to moderate myopia with the adjacent intraocular structures and to correlate it with clinical outcomes.
MethodsProspective observational case series including 18 eyes with high to moderate myopia (spherical equivalent between −5.88 and −15.75D) of 9 patients (age range, 29–49 years) undergoing bilateral Phakic Refractive Lens (PRL, Zeiss) implantation. Postoperative visual acuity, refraction, contrast sensitivity (CS), and ocular higher order aberrations (HOA) were evaluated. Furthermore, the in vivo position of the PRL was analyzed by means of spectral domain optical coherence tomography (3D OCT-1000, Topcon). Central (CENV), temporal (TEMV) and nasal vault (NASV) were measured. Correlation of these anatomical parameters with clinical data was also investigated. Mean follow-up was 47±25 months.
ResultsA statistically significant visual and refractive improvement (p<0.01) was found postoperatively. Mean postoperative CENV, NASV, and TEMV were 157.88±69.93μm, 168.29±65.02μm, and 188.59±55.99μm, respectively. Significant difference was found between NASV and TEMV (p=0.01). No significant correlations were found between ocular aberrometric and anatomical parameters (p≥0.12). CENV, NASV and TEMV correlated significantly with 6-cycles per degree (cpd) photopic CS as well as with 18-cpd low mesopic CS (r≥0.57, p≤0.04). Furthermore, NASV also correlated significantly with preoperative anterior chamber depth (r=−0.65, p=0.01).
ConclusionsThis posterior chamber pIOL shows a trend to nasal position, with potential impact on refractive and visual outcomes, particularly in eyes with deep anterior chambers.
Analizar, mediante tomografía de coherencia óptica de dominio espectral, la relación anatómica entre un tipo específico de lente intraocular fáquica de cámara posterior (pIOL) implantada en casos de miopía alta a moderada, y las estructuras intraoculares adyacentes, así como su correlación con los resultados clínicos.
MétodosEstudio prospectivo observacional que incluye a 18 ojos con miopía de alta a moderada (equivalente esférico comprendido entre -5,88 y -15,75 D), pertenecientes a 9 pacientes (rango de edad comprendido entre 29 y 49 años), sometidos a implantación bilateral de lentes refractivas fáquicas (PRL, Zeiss). Se evaluó, a nivel postoperatorio, la agudeza visual, la sensibilidad de contraste (SC) y las aberraciones oculares de alto orden (AAO). Además, se analizó en vivo la posición de las lentes refractivas fáquicas por medio de tomografía de coherencia óptica de dominio espectral (3D OCT-1000, Topcon). Se midieron las distancias lente-cristalino a nivel central (CENV), temporal (TEMV) y nasal (NASV). También se investigó la correlación de estos parámetros anatómicos con los datos clínicos. El seguimiento medio fue de 47 ± 25 meses.
ResultadosDurante el periodo postoperatorio se observó mejora visual y refractiva estadísticamente significativa (p<0,01). La media postoperatoria de las distancias lente-cristalino a nivel central, nasal y temporal fue de 157,88 ± 69,93μm, 168,29 ± 65,02μm, y 188,59 ± 55,99μm, respectivamente. Se halló una diferencia estadísticamente significativa entre las distancias lente-cristalino a nivel nasal y temporal (p=0,01). No se hallaron correlaciones significativas entre los parámetros oculares aberrométricos y anatómicos (p≥0,12). Las distancias lente-cristalino a nivel central, nasal y temporal presentaron una correlación significativa con la sensibilidad de contraste (SC) fotópica para 6 ciclos por grado (cpd), así como con la SC mesópica baja para 18 cpd (r≥0,57,p≤0,04). Además, la distancia lente-cristalino a nivel nasal presentó una correlación significativa con la profundidad preoperatoria de la cámara anterior (r=-0,65, p=0,01).
ConclusionesEsta lente intraocular fáquica de cámara posterior muestra una tendencia a presentar una posición nasal, con un impacto potencial sobre los resultados refractivos y visuales, especialmente en ojos con cámaras anteriores profundas.
The Phakic Refractive Lens (PRL) is a posterior chamber phakic intraocular lens (pIOL) that was designed to float freely in the aqueous humor contained into the posterior chamber, without pressure on the ciliary structures or contact with the anterior surface of the crystalline lens. Several authors have reported good visual and refractive outcomes,1–8 although they all agree in the need of controlling the long-term behavior of this pIOL, especially its interaction with the zonule.1–8 This model of pIOL should theoretically rest on the zonule without damaging it. However, there is some scientific evidence showing that this theoretical position is not achieved in all cases.9–17 Indeed, Pitault et al.12 found in their series that this ideal position was only present in 57% of the evaluated eyes. It has even shown that the PRL position at the level of the zonule and at the level of the ciliary sulcus can coexist with the same implant in the same eye.16,17 Furthermore, the stabilization of the PRL over the zonule has been observed to promote the rotation of the pIOL whereas its position in the ciliary sulcus leads to an increase in the anterior convexity of the pIOL, causing a reduction in the anterior chamber depth and a narrowing of the iridocorneal angle.16,17
Recent advances in ophthalmic anterior segment imaging such as Scheimpflug photography technology, very high-frequency ultrasonography and anterior segment optical coherence tomography (AS-OCT) have been used to evaluate the position and vault of pIOLs as well as their dynamics with intraocular changes.18–20 As with other types of pIOL, an inadequate vault or position of the haptics of the PRL may lead to undesired consequences as cataract, pigmentary glaucoma or even zonular dehiscence and prolapse to vitreous chamber.5,14 Koivula et al.11 evaluated the distance between the posterior surface of the PRL and the anterior surface of the crystalline lens using the Scheimpflug photography technology. They confirmed a significant reduction of this distance during the first postoperative year, with stabilization and no significant changes afterwards. Furthermore, the time-domain optical coherence tomography technology has been used to evaluate the movement of the PRL during the accommodation process.10 Specifically, a significant anterior displacement of the PRL with accommodation was observed, the distance with the anterior crystalline lens surface being preserved only with one specific model of PRL.10 Rotations of more than 10° have been even observed in the initial postoperative period with this modality of pIOL.10 However, the influence of PRL position on the clinical outcomes obtained still remains unclear. Our research group recently reported a case of spontaneous PRL decentration with a decrease in measured ocular aberrations, and without degradation of the visual quality.9
The aim of the current study was to analyze by means of spectral-domain optical coherence tomography the anatomical relationship of the PRL implanted in cases of high to moderate myopia with the adjacent intraocular structures and to correlate it with the clinical outcomes obtained.
MethodsPatientsThis prospective observational case series included 18 eyes with high to moderate myopia (spherical equivalent between −5.88 and −15.75D) of 9 patients in whom corneal photo-ablative surgery was contraindicated. In all these cases bilateral PRL implantation (Phakic Refractive Lens; IOLTech/Carl Zeiss Meditec AG, Jena, Germany) was performed by the same surgeon (JBM) without complications at Oftalmar, Medimar International Hospital (Alicante, Spain). Inclusion criteria for the study were subjects with moderate to high myopia in one or both eyes and ages between 18 and 50 years. Exclusion criteria were age less than 18 years old, corneal astigmatism higher than 3D, previous corneal or intraocular surgery, anterior chamber depth less than 3mm, glaucoma, history of uveitis, crystalline lens opacity, active retinal disease, scotopic pupil diameter higher than 7mm and any other ocular disease. LASIK (laser assisted in situ keratomileusis) was contraindicated in all the cases included in this study because the estimated post-surgical central keratometry was less than 36D and the residual stromal bed thickness below 250μm. Likewise, photorefractive keratectomy (PRK) was also contraindicated for these amounts of myopic correction given the high risk of developing an important degree of corneal haze. Informed written consent in accordance with the tenets of the Declaration of Helsinki was obtained from all patients before any procedure. Local ethics committee approval was obtained for this investigation.
PIOL descriptionThe PRL® (Carl Zeiss/Meditec AG, Germany) pIOL is a monofocal and biconcave spherical lens. It has a monobloc design and is made of a new-generation of ultra-thin hydrophobic silicone with refractive index of 1.46 and specific gravity of 0.99. A small proportion (0.2%) of porcine collagen has been added to this silicone material to make the pIOL material lighter, more hydrophilic and permeable to gas and nutrients. In addition, the pIOL has spherical, thin and flexible haptics in order to adapt itself to the anatomy and dynamic changes of the eye. The available optical power of the myopic implant ranges from −3D to −20D in 0.5-D increments. The optic diameter of this pIOL varies from 4.5 to 5mm depending on the refractive power. There are two models for myopia: PRL™ 100 with a total diameter of 10.8mm and PRL™ 101 with a total diameter of 11.3mm.
Preoperative and postoperative examination protocolPreoperative ophthalmologic examination included uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA) using LogMAR acuity charts under photopic conditions (85cd/m2), refraction (objective, manifest and cycloplegic), slit-lamp biomicroscopy, Goldman applanation tonometry, pupil size measurement under scotopic conditions (Colvard pupillometer (OASIS Medical, Glendora, USA), corneal topography (Pentacam, OCULUS Optikgeräte, GmbH Wetzlar, Germany), ultrasound pachymetry (DHG 5100, DHG Technology Inc., Exton, PA), IOL-Master optical biometry (Carl Zeiss/Meditec AG, Germany) to measure the white-to-white distance, axial length and anterior chamber depth (from epithelium), binocularity evaluation, and funduscopy.
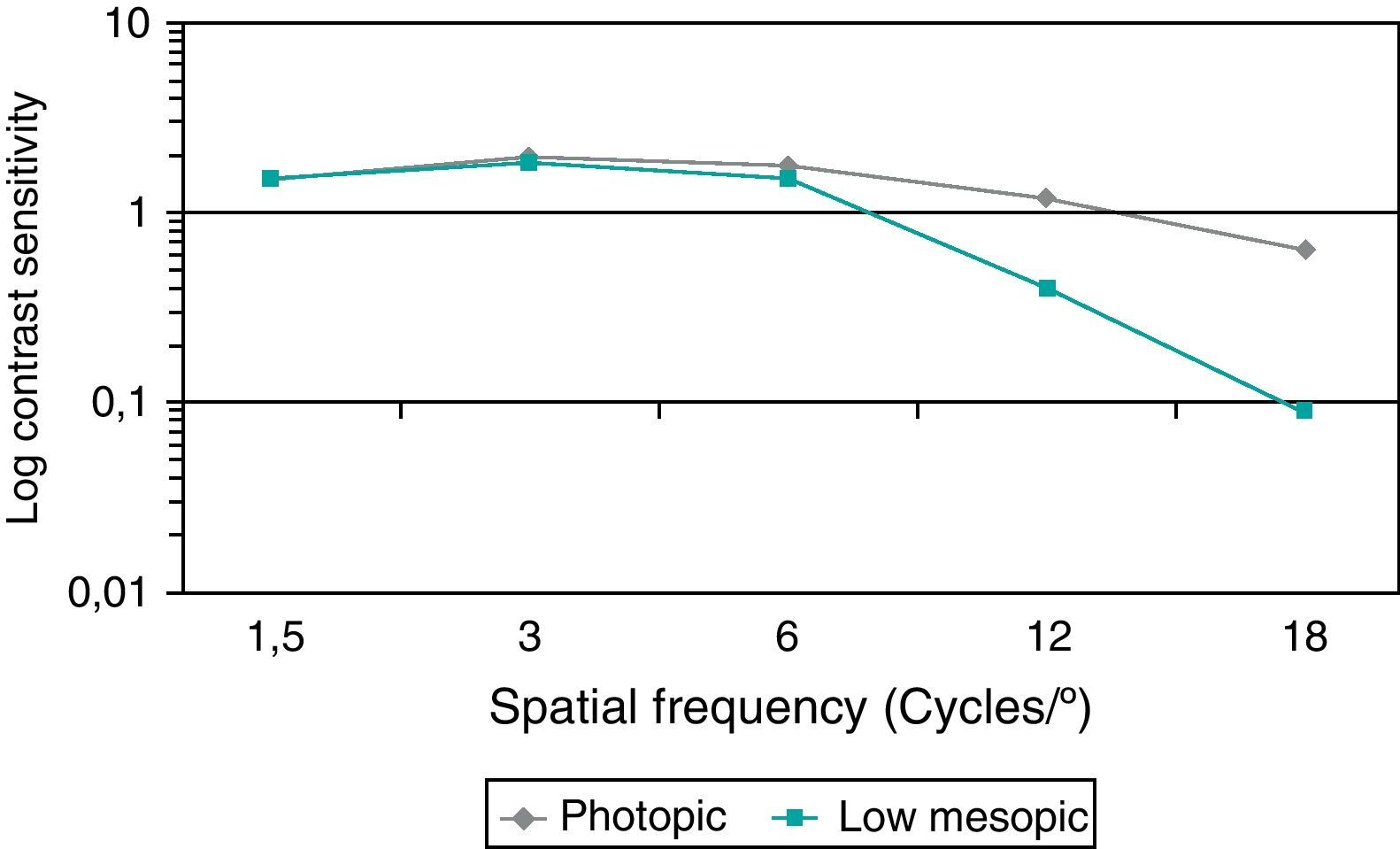
Postoperatively, patients were examined the day after surgery and then were scheduled to come back to the hospital 1, 3 and 6 weeks after the surgery. Regular examinations every six months were recommended during the first year and then every year. These visits included a complete ophthalmologic examination with the following tests: visual acuity, manifest refraction, slit-lamp biomicroscopy, applanation tonometry and fundus examination. In all cases, ocular aberrometry, CS and pIOL position were evaluated as well at least 12 months after the surgery (mean follow-up period: 47 months; standard deviation: 25 months; range: 12–80 months). Ocular aberrations were examined by means of the iTrace system 3.1 (Tracey Technologies Corp., Houston, TX, USA) under pharmacologically induced pupillary dilation (phenylephrine 10%). The following aberrometric parameters were calculated and analyzed for a 5-mm pupil: higher order root mean square (RMS), primary coma RMS (computed for the Zernike terms Z3±1), trefoil RMS (computed for the Zernike terms Z3±3), and the Zernike term corresponding to the primary spherical aberration (Z40) with its sign. Postoperative CS was measured with the Functional Acuity Contrast Test (FACT, Stereo Optical Co, Inc., Chicago, USA). This system allows presentation of sine-wave gratings of different spatial frequencies (1.5, 3, 6, 12 and 18 cycles per degree) with a contrast-level change step corresponding to 0.15 logarithmic units of CS. Measurements were performed monocularly under photopic (85cd/m2) and low mesopic conditions (5cd/m2).
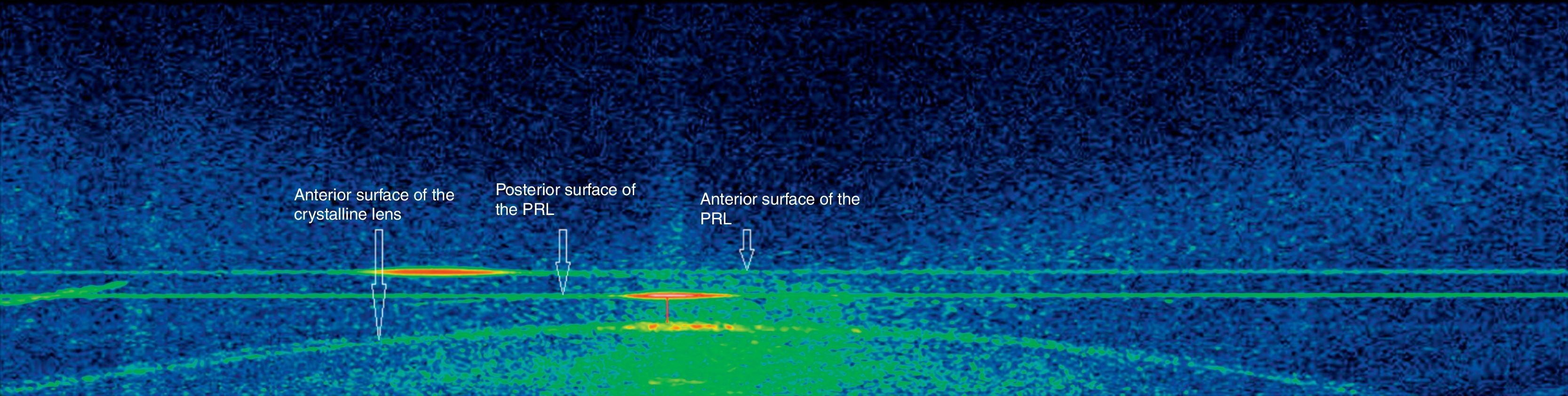
Positional analysis of the pIOL by spectral-domain AS-OCTAS-OCT is a noncontact imaging technology based on the use of light of a 1310-nm wavelength. It allows a greater penetration through highly reflective tissues as the limbus and the sclera and makes possible a proper visualization of angular structures.21 Two different AS-OCT devices have been developed and introduced in the clinical practice, the time-domain and spectral-domain OCT devices. Both technologies have been shown to be useful for anterior segment evaluation,21,22 but the spectral domain OCT provides higher resolution and allows a faster image acquisition.23 As a limitation of AS-OCT techniques, it should be mentioned that the iris pigment epithelium prevents OCT light radiation from penetrating into the eye and allowing the visualization of structures behind the iris. Specifically, in the current study, the Fourier-domain OCT 3D-1000 system (Topcon) was used to evaluate the position of the PRL into the eye as well as the relation of the PRL with the crystalline lens. AS-OCT technology has been shown to be adequate and useful to evaluate the in vivo position of pIOLs.18–20 Measurements were performed in a dim light room without using pharmacological agents in order to avoid artificial conditions. The following anatomical parameters were obtained and analyzed in the horizontals scans obtained in each eye: central vault (CENV), vault measured at 1.5mm nasally (NASV), and vault measured at 1.5mm temporally (TEMV) (Fig. 1). Temporal and nasal positions were determined with respect to the light reflex arising from the corneal apex (fixed reference point) (Fig. 1).
Surgical techniqueOne week prior to the surgical procedure two basal peripheral iridotomies were performed to avoid a potential blockage by the pIOL haptics. In the current study, all pIOLs were implanted through a 2.75mm clear corneal tunnel incision made with a diamond knife on the steepest meridian to minimize post-surgical astigmatism. In all cases, peribulbar anesthesia was administered and pupillary dilation was induced with a combination of tropicamide and phenylephrine 10% every 15min half an hour previous to the procedure. Iodine solution 5% was instilled on the eye ten minutes before the operation. A paracentesis was made 60–90° clockwise from the main incision and the anterior chamber was filled with 1% sodium hyaluronate lifting the pupillary border to ease the proper settlement of the lens. In all cases the folding forceps provided by the manufacturer was used to introduce the lens. The lens haptics were introduced into the posterior chamber with care by means of a spatula. Finally, the surgeon proceeded to retrieve the viscoelastic material using the irrigation-aspiration system. The pupil was constricted with acetylcholine and the procedure finished with the injection of cephuroxime into the anterior chamber. A combination of topical steroid and antibiotic (Tobradex, Alcon, Fort Worth, Texas, USA) was prescribed to be applied four times daily for a week after the surgery and three times daily the second postoperative week. In addition, non-steroidal anti-inflammatory drops (Dicloabak, Laboratorios Thea, Barcelona, Spain) were also prescribed to be applied three times daily during 3 weeks after surgery.
Calculation of pIOL power was performed considering the anatomical parameters obtained with the IOL-Master biometer as well as the refractive error. In almost all cases (14 eyes, 77.8%), horizontal white to white distance was above 11.3mm and therefore the model selected to implant was PRL™ 101 according to the manufacturer guidelines. The PRL™ 100 was implanted in the other 4 cases. The mean power of the PRL lens implanted in the current series was −8.82D (standard deviation, SD: 2.23D; range: −5 to −12D). Target postoperative refraction was emmetropia in all eyes.
Refraction notationThe spherocylindrical refractions obtained before and after surgery were converted to vectorial notation using the power vector method described by Thibos and Horner.24 Using this procedure, any spherocylindrical refractive error can be expressed by 3 dioptric powers: M, J0 and J45, being M a spherical lens equal to the spherical equivalent of the given refractive error, and J0 and J45 two Jackson crossed cylinders equivalent to the conventional cylinder. These numbers are the coordinates of a point in a three-dimensional dioptric space (M, J0, J45). The length of this vector is a measure of the overall blurring strength B of a spherocylindrical refractive error.
According to the power vector method, manifest refractions in conventional script notation (S [sphere], C [cylinder×φ [axis]) were converted to power vector coordinates and overall blurring strength (B) by the following formulas: M=(S+C)/2; J0=(−C/2)cos(2φ); J45=(−C/2)sin(2φ); and B=(M2+J02+J452)1/2.
Statistical analysisData analysis was performed using the software SPSS for Windows version 15.0 (SPSS Inc., Chicago, USA). Normality of data samples was evaluated by means of the Shapiro–Wilk test. When parametric analysis was possible, the Student t-test for paired data was used for comparisons between the preoperative and postoperative data and between postoperative contrast sensitivity data in photopic and low mesopic conditions. When parametric analysis was not possible, the Wilcoxon rank sum test was applied to assess the significance of such comparisons. Differences were considered to be statistically significant when the associated p-value was <0.05. Correlation coefficients (Pearson or Spearman depending if normality condition could be assumed) were used to assess the correlation between different variables. It should be noted that sample size calculation to reach a proper statistical power was not performed since the aim of this preliminary study was to detect trends and correlations to be confirmed with future larger samples.
ResultsMean patient age of the analyzed sample was 37.8 years (SD: 6.7; range: 29–49 years). 44.4% of patients were male and 55.6% female. Mean axial length of the evaluated sample was 27.24mm (SD: 1.13; range: 25.58–29.46mm) and mean anterior chamber depth was 3.76mm (SD: 0.43; range: 3.10–4.52mm). Mean preoperative flattest keratometric reading, steepest keratometric reading and central pachymetry were 43.79D (SD: 1.23; range: 42.00–46.50D), 44.84D (SD: 1.70; range: 42.37–47.37D), and 541.17μm (SD: 28.67; range: 506–595μm), respectively. Mean preoperative scotopic pupil size was 6.36mm (SD: 1.16; range: 4.00–7.00mm).
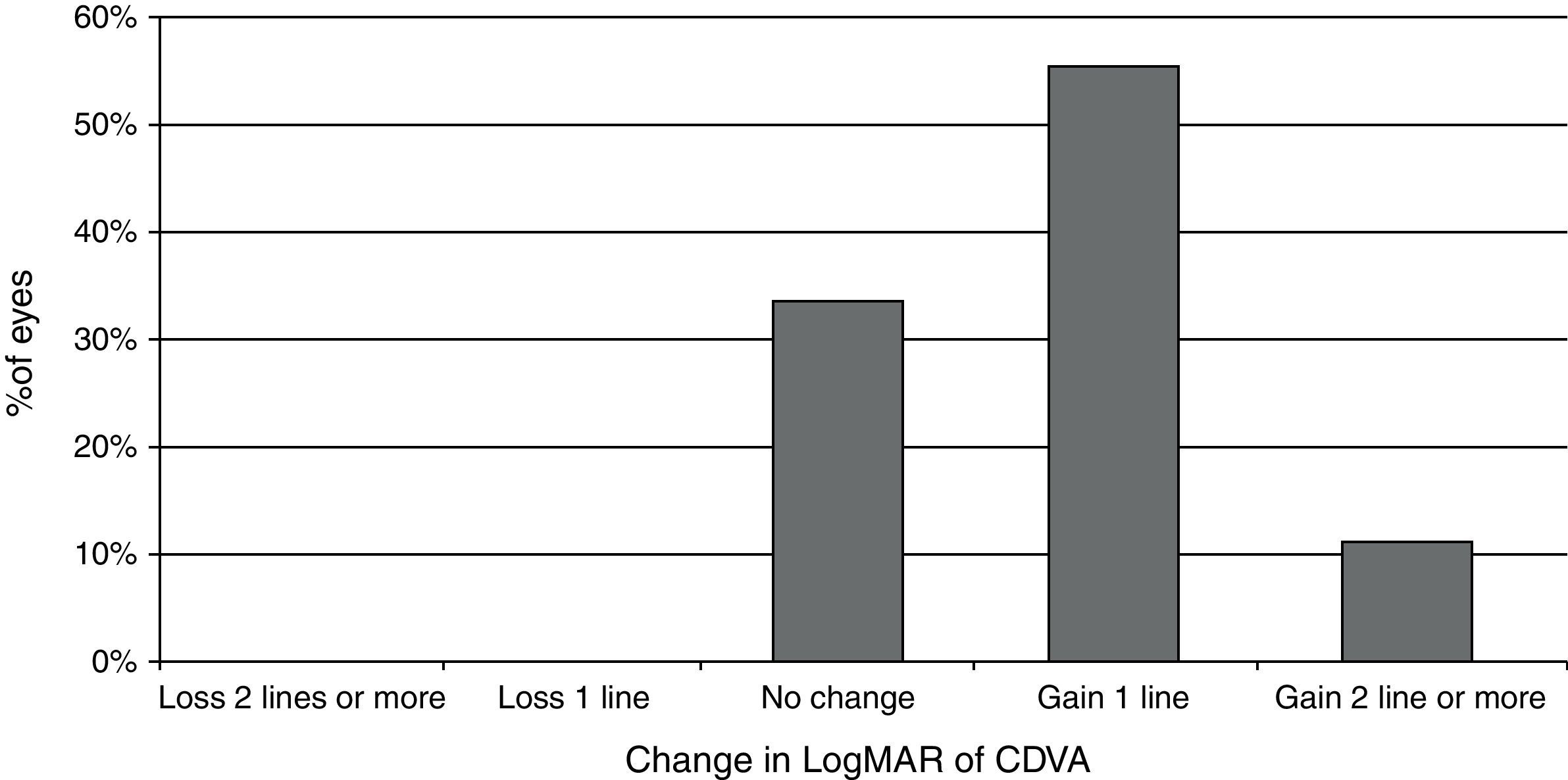
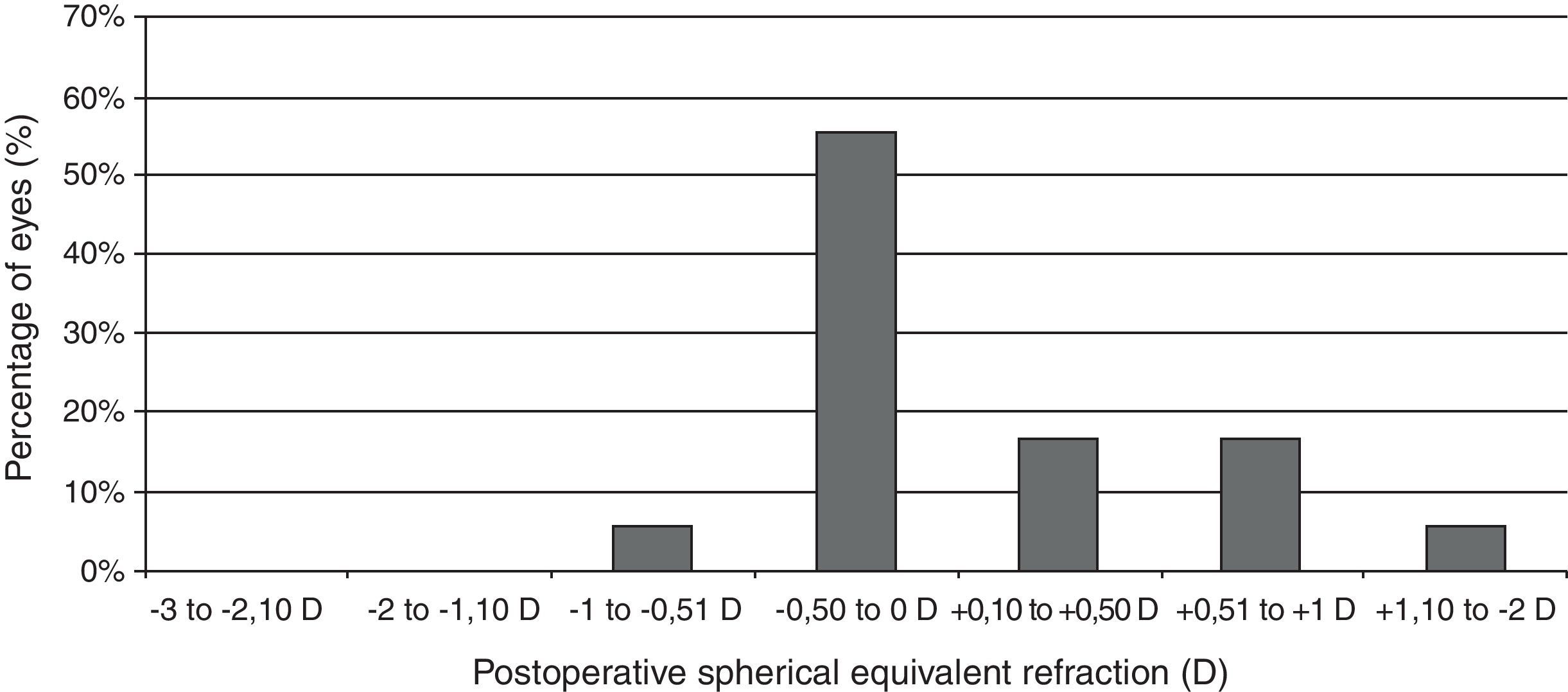
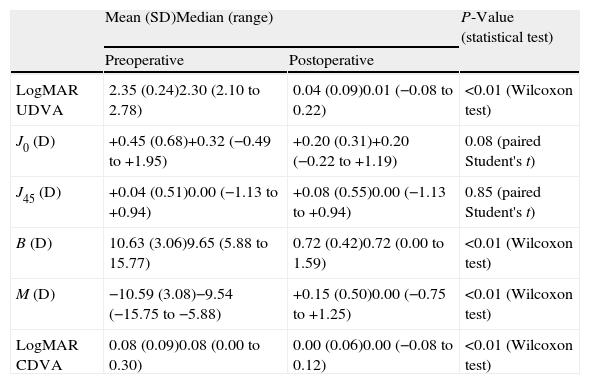
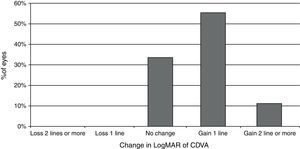
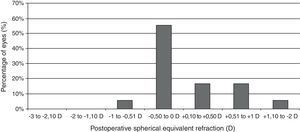
Visual and refractive outcomesTable 1 summarizes the preoperative and postoperative visual and refractive outcomes obtained in the analyzed sample. As shown, a statistically significant improvement in LogMAR UDVA and CDVA was observed postoperatively (Wilcoxon test, p<0.01). At the end of the follow-up, postoperative LogMAR UCVA was 0.3 (about 20/40) or better in all eyes (100%) and 0.1 (about 20/25) or better in 15 eyes (83.3%). Postoperative LogMAR BCVA was 0.1 or better in all cases (100%). No eye of the current sample lost lines of CDVA (Fig. 2). In contrast, gains of lines of CDVA were detected in a total of 12 eyes (66.7%) (Fig. 2). Regarding manifest refraction, a large and statistically significant reduction in B and M value were found postoperatively (Wilcoxon test, p<0.01). Almost all eyes (17 eyes, 94.4%) had a postoperative value of M (spherical equivalent) within ±1.00D of emmetropia, whereas 13 eyes (72.2%) had a postoperative M value within ±0.50D of the targeted refraction (Fig. 3). In addition, no significant changes were detected postoperatively in the power vector components of manifest astigmatism, J0 (paired Student's t test, p=0.08) and J45 (paired Student's t-test, p=0.85). It should be noted that a slight decrease in the J0 component of astigmatism was obtained after PRL implantation (Table 1), because surgeries were performed with incisions in the steep meridian to achieve this reduction, but this change was not statistically significant given the low amount of preoperative astigmatism in our sample.
Summary of the preoperative and postoperative visual and refractive outcomes in the current series.
| Mean (SD)Median (range) | P-Value (statistical test) | ||
| Preoperative | Postoperative | ||
| LogMAR UDVA | 2.35 (0.24)2.30 (2.10 to 2.78) | 0.04 (0.09)0.01 (−0.08 to 0.22) | <0.01 (Wilcoxon test) |
| J0 (D) | +0.45 (0.68)+0.32 (−0.49 to +1.95) | +0.20 (0.31)+0.20 (−0.22 to +1.19) | 0.08 (paired Student's t) |
| J45 (D) | +0.04 (0.51)0.00 (−1.13 to +0.94) | +0.08 (0.55)0.00 (−1.13 to +0.94) | 0.85 (paired Student's t) |
| B (D) | 10.63 (3.06)9.65 (5.88 to 15.77) | 0.72 (0.42)0.72 (0.00 to 1.59) | <0.01 (Wilcoxon test) |
| M (D) | −10.59 (3.08)−9.54 (−15.75 to −5.88) | +0.15 (0.50)0.00 (−0.75 to +1.25) | <0.01 (Wilcoxon test) |
| LogMAR CDVA | 0.08 (0.09)0.08 (0.00 to 0.30) | 0.00 (0.06)0.00 (−0.08 to 0.12) | <0.01 (Wilcoxon test) |
Abbreviations: SD: standard deviation; UDVA: uncorrected distance visual acuity; J0 and J45: power vector components of manifest cylinder; M: spherical equivalent; B: overall blurring strength of the manifest spherocylindrical error; CDVA: corrected distance visual acuity; D: diopters.
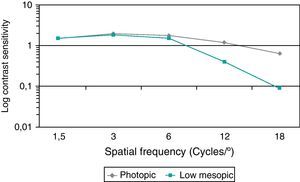
Fig. 4 summarizes the monocular contrast sensitivity outcomes obtained in the analyzed sample under photopic and low mesopic conditions. As shown, the lightning conditions of measurements had a significant influence on CS for almost all spatial frequencies. Specifically, significant differences in CS between photopic and low mesopic conditions were found for the spatial frequencies of 3 (Wilcoxon test, p=0.02), 6 (Wilcoxon test, p=0.01), 12 (Wilcoxon test, p<0.01) and 18cpd (Wilcoxon test, p=0.01). No significant differences in photopic and low mesopic CS was detected for the lowest spatial frequency evaluated (Wilcoxon test, p=0.63).
Regarding HOA analysis, mean postoperative higher order, primary coma, and trefoil RMS values were 1.55μm (SD: 0.79; range: 0.29–2.65μm), 1.01μm (SD: 0.66; range: 0.04–2.00μm), and 0.54μm (SD: 0.30; range: 0.03–0.91μm). Mean magnitude of the Zernike term corresponding to the primary spherical aberration was +0.46μm (SD: 0.33; range: +0.01 to +0.99μm).
Positional analysis of the pIOL by spectral-domain AS-OCTMean postoperative CENV, NASV, and TEMV were 157.88μm (SD: 69.93; range: 66–306μm), 168.29μm (SD: 65.02; range: 82–323μm), and 188.59μm (SD: 55.99; range: 91–307μm). Mean difference between NASV and TEMV was −20.29μm (SD: 57.29), ranging from −127 to 100μm. This difference was statistically significant (paired Student's t test, p=0.01).
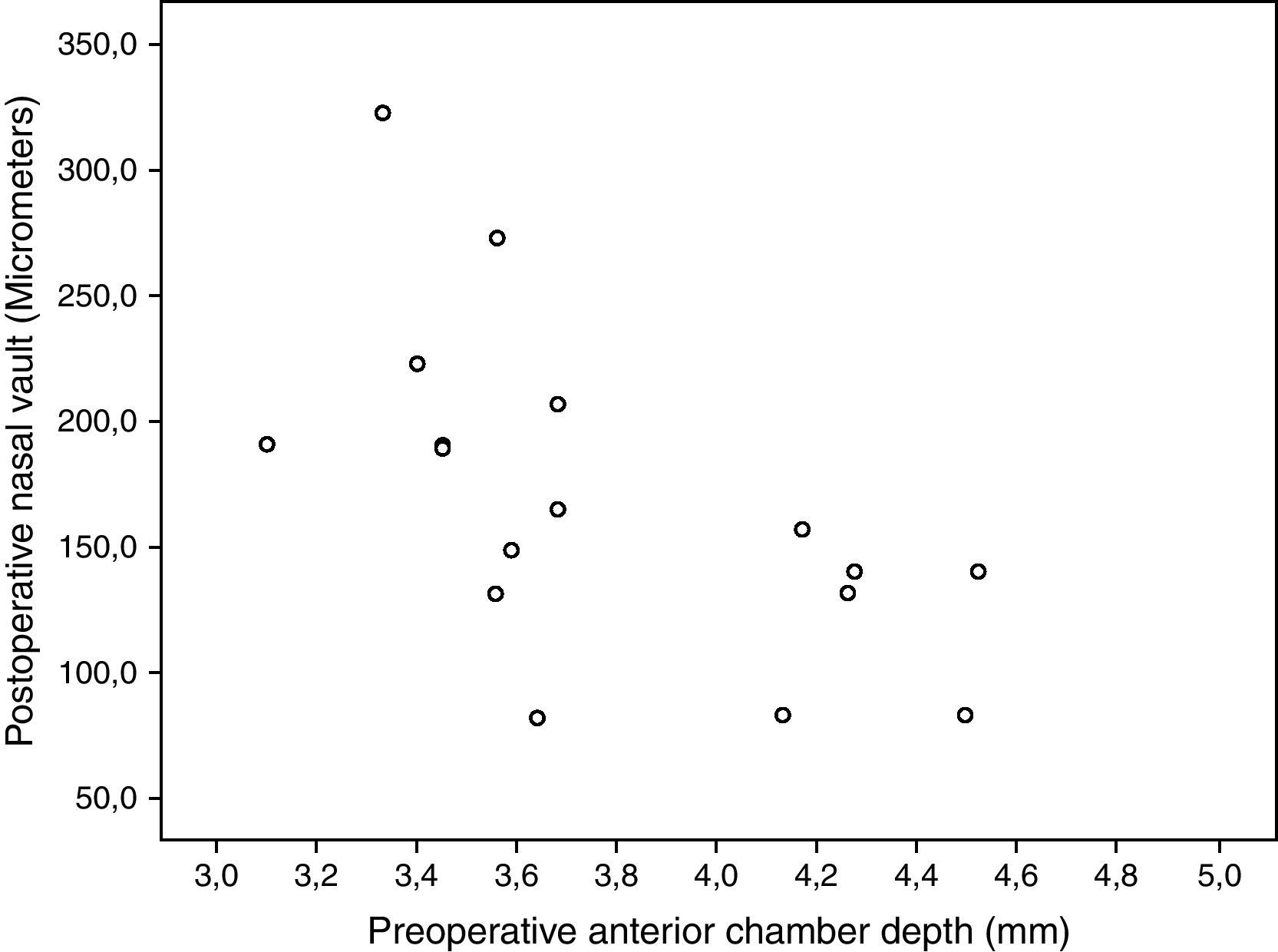
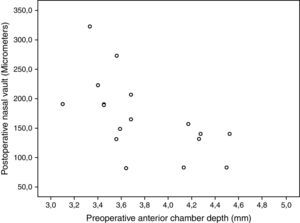
Correlation between pIOL position and clinical dataNo statistically significant correlations were found between ocular aberrometric and OCT anatomical parameters (−0.45≤r≤0.23, p≥0.12). Photopic CS for the 6-cpd spatial frequency was found to correlate significantly with CENV (r=0.76, p<0.01), NASV (r=0.65, p=0.02), and TEMV (r=0.76, p<0.01). Likewise, low mesopic CS for the 18-cpd spatial frequency was found to correlate significantly with CENV (r=0.63, p=0.02), NASV (r=0.63, p=0.02), and TEMV (r=0.57, p=0.04). Furthermore, NASV was also found to correlate significantly with postoperative UDVA (r=−0.61, p=0.01), postoperative B (r=−0.50, p=0.04), and the anterior chamber depth (r=−0.65, p=0.01) (Fig. 5).
ComplicationsNo intraoperative complications occurred. One case (5.6%) of acute IOP increase in the immediate postoperative period was detected, which resolved with systemic and topical treatment. One case (5.6%) of decentration of the PRL visible at the slit lamp examination was observed during the follow-up. Furthermore, a case of subcapsular opacification of the crystalline lens was detected during the follow-up (5.6%) which affected slightly CS but did not require cataract surgery.
DiscussionAs with any type of pIOL, an adequate behavior of the PRL into the anterior segment is determinant to avoid potential complications. One of the most frightened complications is the development of a secondary cataract. This fact may occur as a consequence of the contact between the anterior surface of the crystalline lens and the posterior surface of the PRL or because of an alteration in the circulatory dynamics of the aqueous humor between both structures that results in inflammation and cataract. Specifically, anterior subcapsular cataract in eyes implanted with posterior chamber pIOLs has been shown to be related with a low vaulting distance leading to fibrous metaplasia of the anterior lens epithelial cells.25 Likewise, this vaulting has been shown to decrease with time which increases the risk for cataract development.26 When the incidence of secondary cataract reported in the peer-reviewed literature is compared for the two models of posterior chamber pIOL (PRL vs. ICL) that are currently available, it is found to be markedly lower after PRL implantation.26–30 One of the factors attributed to this fact is the potential protective effect of the rotating design of the pIOL, avoiding the continuous contact between the pIOL and any specific area of the anterior surface of the crystalline lens. This is achieved due to the theoretical stabilization of the PRL over the zonule that has also some potential risks. The continuous contact of the haptics of this pIOL with the zonule may cause a progressive weakening of this structure and induce one of the most frightened complications with this modality of pIOL, its spontaneous dislocation toward the vitreous cavity.8,13,15 The current study was aimed at analyzing by means of spectral-domain optical coherence tomography the anatomical relationship of the PRL with the anterior surface of the crystalline lens in a sample of high to moderate myopic eyes implanted with this modality of pIOL. The correlation of the anatomical references evaluated with the clinical outcomes obtained was also investigated.
As in previous series,1–9,11 we found in our sample of eyes that the evaluated pIOL provided an efficient, predictable and safe correction of the spherocylindrical error, with a great percentage of eyes gaining lines of CDVA probably due to the change in the retinal image magnification and ocular aberrations31 and no eyes losing lines of CDVA. The visual improvement and the refractive correction achieved with this specific type of pIOL are comparable to that reported with other anterior and posterior chamber pIOLs,28,32 such as the ICL (Implantable Contact Lens, Staar). Regarding refractive correction, an excellent outcome was obtained (72.2% of eyes within ±0.50D), with a slight trend to hyperopic results after implantation of the pIOL (Fig. 3). Besides the IOL power calculation formula used, the final position of the pIOL into the posterior chamber after implantation may have accounted for this phenomenon. In addition, no significant changes were observed in the astigmatic vector components after surgery. It should be noted that preoperatively the magnitude of astigmatism was limited in all cases because other types of pIOL with the option of including toricity were preferred in those eyes with higher amounts of astigmatism. The PRL is a spherical pIOL not providing astigmatic correction and therefore only a limited astigmatic corrective effect could be achieved by means of the corneal incision which was placed on the steepest corneal meridian.
Regarding CS, the values obtained postoperatively under photopic conditions in the current series were similar to those reported with other types of anterior and posterior chamber pIOLs.33–36 The visual performance obtained with the evaluated pIOL was excellent in conditions of pupillary constriction, with values of CS within the physiological range.37 However, CS under low mesopic conditions was significantly lower when compared with the values obtained under photopic conditions. This reveals the relevance that pupil size and then the limited optical zone has in the visual performance obtained with this pIOL. This same trend has been also reported with other modalities of pIOL, although with better CS values for the highest spatial frequencies.35 Yu et al.38 performed a comparative study to evaluate the effect on CS of three different types of pIOL, an angle-supported pIOL (Phakic 6-H), an iris-fixated pIOL (Verisyse), and the PRL. These authors found that angle-supported and iris-fixated PIOLs provided better postoperative photopic and mesopic CS for some spatial frequencies when compared to the PRL.9 This potential limitation in CS with the PRL, especially in mesopic conditions, may be in relation with the aberrometric profile and the ocular scattering induced by the pIOL. In the current series, we also evaluated the postoperative ocular aberrometric profile. The mean postoperative higher order and primary coma RMS values obtained with this pIOL were higher than those reported for other types of anterior and posterior chamber pIOLs using for the aberrometric calculation the same pupil aperture as us.39–42 Several factors may have accounted for this finding: differences in the population analyzed, differences in the instruments used in each of the referenced works (Hart-Shack versus ray-tracing aberrometers), and a more limited optical performance of the PRL due to its specific design. In addition, mean primary spherical aberration of the eye implanted with the evaluated pIOL was found to be positive and of half of a micron, as occurs with the iris-fixated pIOL Artisan.40,41 This finding suggests that the surfaces of these pIOLs (PRL and Artisan) did not present an aspheric design, something that may be overcome in the future with more optimized designs.
The spectral-domain AS-OCT analysis revealed the presence of a vaulting for the evaluated PRL with a mean central value similar or even smaller to that reported for the other type of posterior chamber pIOL, the ICL.12,43–47 Indeed, Pitault et al.12 found in a comparative study of the PRL and ICL using ultrasonography that the central vault between the implant and the crystalline lens was greater in eyes with ICL (ICL, 402±194μm; PRL, 256±187μm). This supports the idea that the rotating design of the PRL may be the main reason for a less incidence of secondary cataract with this pIOL. According to the criteria defined by Alfonso et al.,47 vault level 0 was only observed in one case of our series, with most of eyes showing a vault between the levels 1 and 2. It should be mentioned that the mean values for vaulting reported in different studies should be compared with caution because different devices have been used in each study and even different references have been used for measuring the vault distance.13,43–47 One additional and interesting finding of the current study is the significant difference found between the nasal and temporal vault. This reveals the presence of a small decentration toward the nasal position or tilt of the PRL in almost all cases. Possibly, this is due to the configuration of the zonule in this kind of eyes (high to moderate myopes), although this is something that should be studied in detail in further studies. Indeed, the only case of significant decentration observed in this case series was observed nasally. The potential of this trend to decentration or tilt of being related to the risk of zonular dehiscence and dislocation of the PRL into the vitreous cavity should be studied further in order to find the most optimum configuration of the pIOL for avoiding this frightened complication.
Correlations between clinical outcomes and pIOL position into the anterior chamber were also investigated. The magnitude of ocular higher order aberrations did not correlate significantly with the OCT anatomical parameters. Therefore, it seems that the trend to minimal nasal decentration or tilt of this pIOL has a minimal effect on visual quality. Indeed, our research group has recently reported a case of minimization of ocular higher order aberrations with a decentration of a PRL.9 However, contrast sensitivity was significantly correlated to the central, nasal and temporal vaulting distances. Specifically, the better the contrast sensitivity (logarithmic values), the larger were the vaults. Therefore, a decentration or tilt of a pIOL close to the crystalline lens seems to have a greater impact on visual performance than the same magnitude of decentration associated to large vaulting distances. Several factors may have accounted for this fact, such as the change in the ocular optical effect of the pIOL as a function of the distance with the crystalline lens or even the potential impact on the transparency of this ocular element of a pIOL very close to it. Furthermore, an inverse correlation of the nasal vault with the anterior chamber depth was found. The deeper the anterior chamber, the lower was the nasal vault, with a clearer trend to nasal decentration. There is some scientific evidence showing a more anterior position of the ciliary body in eyes with a narrow anterior chamber.48,49 Possibly, in eyes with a deep anterior chamber, the ciliary body presents a more posterior position, especially in the nasal area. This would lead to a more significant level of misalignment of the crystalline lens with respect to the cornea. This is something that should be studied further in order to optimize the design of the pIOL. Therefore, the zonule configuration may be the main factor defining the final position of a PRL, zonular or sulcus. In addition, the nasal vault was found to have a limited but significant impact on refraction and UDVA, mainly due to changes in manifest astigmatism.
In conclusion, the correction of moderate to high myopia by means of PRL implantation is effective and predictable, with a safe position of the pIOL into the anterior chamber. However, this modality of posterior chamber pIOL shows a trend to nasal decentration or tilt with respect to the anterior surface crystalline lens that has an impact on the refractive and visual performance. This trend seems to be more significant in eyes with a deep anterior chamber depth. A more comprehensive study of the zonular configuration in moderate to high myopes should be performed in order to optimize the design of the PRL for such cases. The ability of the anterior chamber depth as a risk factor for complications related to PRL position should be studied. The sample size was the main limitation of the current study but it should be considered that the aim of this preliminary study was to detect trends and correlations to be confirmed with future larger samples.