To evaluate the efficacy and tolerability of a reduced rate of bimatoprost administration.
MethodsDiurnal intraocular pressure (IOP) was recorded at 10 a.m., 2 p.m. and 6 p.m. in 40 eyes of twenty Caucasian patients (age range 56-75 years), with ocular hypertension or primary open angle glaucoma, at baseline and four weeks later. The right eye received daily bimatoprost, and in the left eye bimatoprost was administered every 48hours at 8 p.m. Bulbar conjunctival hyperaemia was assessed by direct observation by a masked observer at baseline and at week 4 and was graded as none, minimal, mild, or moderate or severe (0 to 4).
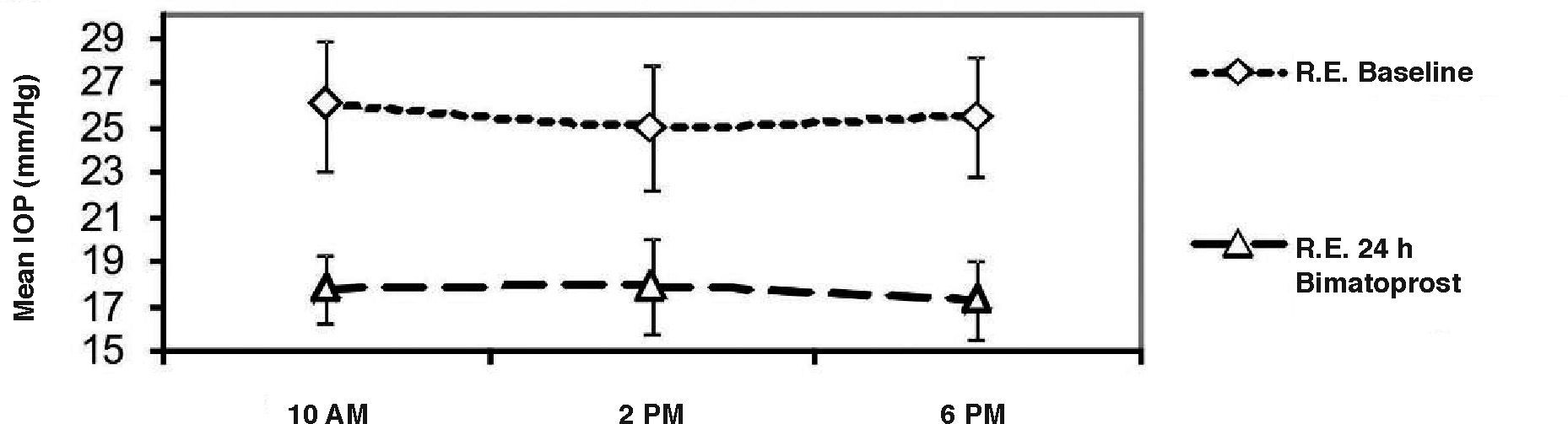
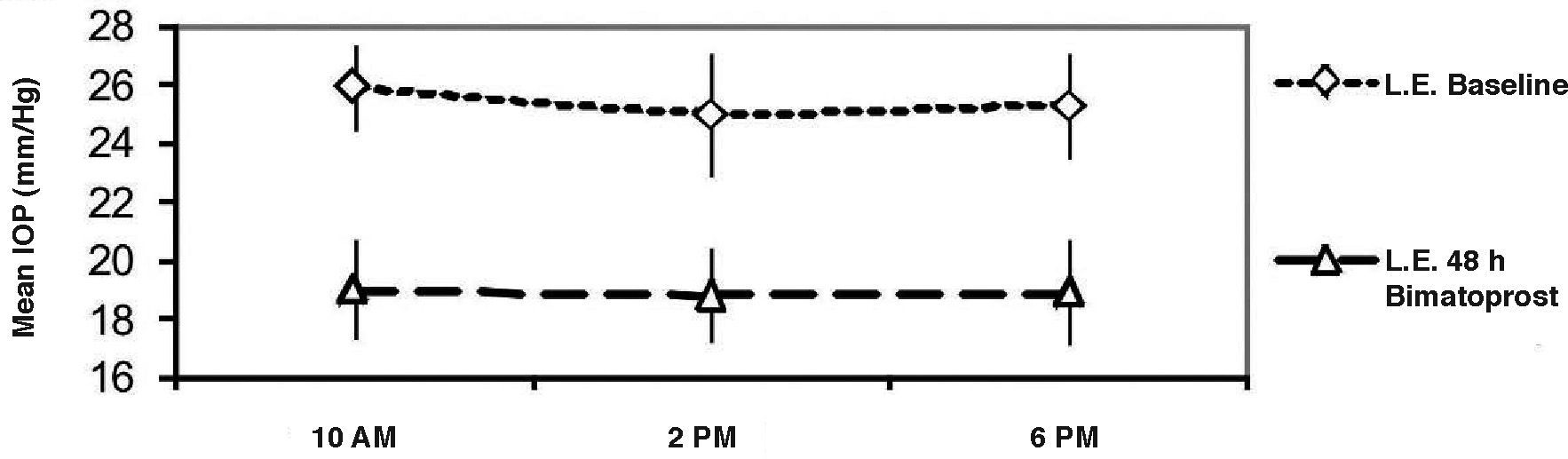
ResultsBaseline mean diurnal IOP in the right eye significantly (P<0.001) decreased from 25.4±2.8 to 17.8±1.8mmHg 14 to 22hours after daily bimatoprost. Baseline mean diurnal IOP in the left eye significantly (P<0.001) decreased from 25.2±2.0 to 19.0±1.7mmHg 38 to 46hours post-dose. The reduction in the right eye was significantly greater than in the left (P=0.02). The average conjunctival hyperaemia after bimatoprost every 24 and 48hours was graded as 2.4±1.0 and 1.8±0.8 respectively and this difference was significant (P<0.01).
ConclusionBimatoprost every 48hours was less effective than daily bimatoprost (24.6 vs. 30% IOP decrease), but caused less short term conjunctival hyperaemia. This off label dose schedule may be proposed to patients complaining of ocular redness especially in the first weeks of treatment.
Evaluar la eficacia y la tolerabilidad de bimatoprost cuando se reduce la frecuencia de administración.
MétodosEn 40 ojos pertenecientes a veinte pacientes de raza blanca con hipertensión ocular o con glaucoma primario de ángulo abierto, y con edades comprendidas entre 56 y 75 años, se midió la presión intraocular (PIO) durante el día al inicio del estudio y transcurridas 4 semanas. En el ojo derecho se administró bimatoprost una vez al día (cada 24horas), y en el ojo izquierdo se administró bimatoprost cada 48horas, a las 8 p.m. Se evaluó la hiperemia conjuntival bulbar mediante observación directa por parte de un observador (que desconocía la pauta administrada en cada ojo), tanto al inicio del estudio como en la 4ª semana del mismo. La escala utilizada (de 0 a 4) se corresponde con “ausencia de hiperemia”, “hiperemia mínima”, “hiperemia leve”, “hiperemia moderada” o “hiperemia grave”.
ResultadosRespecto al inicio del estudio, la PIO diurna media en el ojo derecho disminuyó significativamente (P<0,001), pasando de 25,4±2,8 a 17,8±1,8mmHg, transcurridas entre 14 y 22horas de la administración diaria de bimatoprost. Respecto al inicio del estudio, la PIO diurna media en el ojo izquierdo disminuyó significativamente (P<0,001), pasando de 25,2±2,0 a 19,0±1,7mmHg, transcurridas entre 38 y 46horas de la administración de la dosis. La disminución en el ojo derecho resultó ser significativamente mayor que la observada en el ojo izquierdo (P=0,02). La hiperemia conjuntival promedio, tras administrar bimatoprost cada 24 o cada 48horas, fue igual a 2,4±1,0 y a 1,8±0,8, respectivamente (P<0,01).
ConclusionesBimatoprost cada 48horas resultó ser menos eficaz que bimatoprost administrado diariamente (disminución de la PIO de un 24,6% frente a un 30%), pero causa menos hiperemia conjuntival a corto plazo. Se podría proponer esta nueva pauta posológica, distinta de la que aparece en la ficha técnica del producto, a aquellos pacientes que se quejen de tener los ojos rojos (hiperemia conjuntival), especialmente durante las primeras semanas de tratamiento.
It is well known that people over the age of 65 are at higher risk for glaucoma, and that the prevalence of primary openangle glaucoma (POAG) increases with increasing age. POAG needs to be treated, and the randomized, prospective Ocular Hypertension Treatment Study documented the protective effect of the prophylactic lowering of intraocular pressure (IOP) in cases of ocular hypertension, which is the main risk factor for developing POAG.1
Bimatoprost is well known for lowering IOP.1 This agent, which is a synthetic molecule that is structurally and pharmacologically similar to prostaglandin F2 alpha,2 is typically dosed once daily, and provides sustained IOP lowering over long-term use.3 However, ocular adverse effects,4 such as conjunctival hyperaemia, and also burning, eyelash lengthening and periocular pigmentation,5 may affect compliance or cause drug discontinuation. Hyperaemia has been reported in 48% of patients treated with prostaglandin analogs and negatively affected continuation with therapy.6 Patient education can improve the acceptance of hyperaemia associated with bimatoprost therapy, which can potentially increase compliance.7
The purpose of our study was to evaluate the IOP and the conjunctival hyperaemia when bimatoprost was administered less frequently.
MethodsBaseline IOP was recorded at 10 a.m., 2 p.m., and 6 p.m. in 40 eyes of twenty Caucasian patients with a mean age of 64±8.1
years (range 56-75) who had been newly diagnosed with either ocular hypertension (OH, 12 patients) or POAG (8 patients). None of the patients (13 female and 7 male) had a history or signs of allergy or dry eye, as determined from tear-film function using Schirmer I and tear break-up time tests.OH patients met the following inclusion criteria: baseline IOP exceeding 21mmHg on at least two measurements on different days prior to inclusion in the study, normal appearance of the optic disc using slit-lamp binocular ophthalmoscopy, and normal Humphrey (HFA) perimetry, using the 24-2 Swedish Interactive Threshold Algorithm standard program (Carl Zeiss Meditec Dublin, CA, USA). Normal perimetry was defined as a mean deviation and pattern standard deviation (PSD) within 95% confidence limits and a Glaucoma Hemifield Test (GHT) result within normal limits.
The diagnostic criteria for PAOG were as follows: baseline IOP exceeding 22mmHg, glaucomatous optic disc (cup-to-disc ratio >0.4 with focal or diffuse thinning of the neuroretinal rim) and characteristic optic-nerve-related visual field loss (GHT result outside normal limits and/or PSD with P<0.05). All criteria were confirmed by at least two reliable (HFA 24-2) visual fields. Reliability was defined as 33% or fewer false-positive or false-negative results, and 20% or fewer fixation losses.
The study was in adherence to the tenets of the Declaration of Helsinki. Both the study and the data collection were carried out in conformity with Italian laws and approved by the local ethics committee.
All subjects provided informed consent to being treated with bimatoprost 0.03% every 24hours at 8 p.m. for four weeks in the right eye, and with bimatoprost 0.03% every 48hours at 8 p.m. for four weeks in the left eye. Diurnal curves were recorded at week 4 for both regimens on the first and on the second day after bimatoprost for the right and the left eye, respectively.
Bulbar conjunctival hyperaemia was evaluated by the same masked examiner, who was unaware of the study design, and prior to checking the IOP using direct observation slit-lamp biomicroscopy under diffuse white illumination. IOP measurements where performed at baseline, prior to bimatoprost administration, and at week 4 for both regimens, specifically on the first and on the second day after bimatoprost instillation for the right and left eye, respectively. Hyperaemia was graded (scale from 0 to 4) as none, minimal, mild, moderate or severe, following Stewart and coworkers’ classification.8
After verifying that the distribution of data was normal, we used the univariate procedure for the paired t-test and the frequency procedure for Mc Nemar's test from the SAS statistical software, version 9.1.3, 2004 (SAS Institute Inc, Cary, NC, USA).
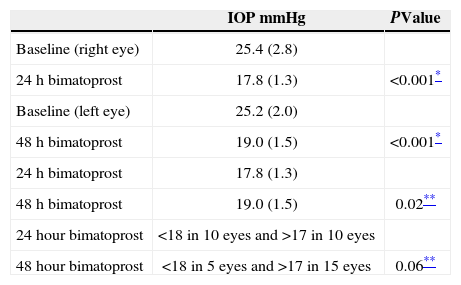
ResultsMean (±SD) baseline diurnal IOP in the right eye significantly (P<0.001) decreased from 25.4±2.8 (range 22-28) to 17.8±1.3 (range 16-20) mmHg (means of the three measurements) 14 to 22hours after the daily bimatoprost dose. As regards every-other-day bimatoprost administration, baseline mean diurnal IOP in the left eye significantly (P<0.001) decreased from 25.2± 2.0 (range 23-27) to 19.0±1.5 (range 17-22) mmHg, as measured 38 to 46hours post-dose. There was a significant (P=0.02, paired t-test) difference of IOP between the two dosing regimens (see Figure 1, Table 1).The mean IOP drop was -7.6 (30% decrease) and -6.2mmHg (24.6% decrease) following daily and every-other-day bimatoprost instillation, respectively. In 10 right (50%) and 5 left (25%) eyes the IOP was below 18mmHg; 10 (50%) and 15 (75%) of the right and left eyes, respectively, had an IOP greater than 17mmHg (P=0.06 from Mc Nemar's test) (see Table 1).
Average (SD) IOP measured in twenty patients, both at baseline and 4 weeks after bimatoprost instillation every 24 or 48hours
| IOP mmHg | PValue | |
| Baseline (right eye) | 25.4 (2.8) | |
| 24 h bimatoprost | 17.8 (1.3) | <0.001* |
| Baseline (left eye) | 25.2 (2.0) | |
| 48 h bimatoprost | 19.0 (1.5) | <0.001* |
| 24 h bimatoprost | 17.8 (1.3) | |
| 48 h bimatoprost | 19.0 (1.5) | 0.02** |
| 24hour bimatoprost | <18 in 10 eyes and >17 in 10 eyes | |
| 48hour bimatoprost | <18 in 5 eyes and >17 in 15 eyes | 0.06** |
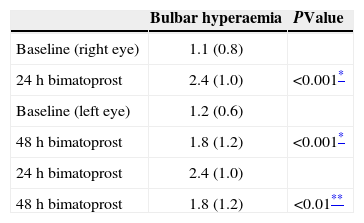
The baseline bulbar conjunctival hyperaemia evaluated by means of direct observation with a slit-lamp (1.1±0.8 for the right eyes and 1.2±0.6 for the left eyes) increased (P<0.001) as a result of bimatoprost administrations with both the 24-hour (2.4±1.0) and the 48-hour (1.8±1.2) schedules, but the difference between the hyperaemia for the two administration rates was found to be statistically significant (P<0.01) (see Table 2). Six (30%) right eyes and two (10%) left eyes developed moderate conjunctival hyperaemia under the 24- and the 48-hour bimatoprost protocol, respectively. All patients completed the study; but three patients with moderate conjunctival hyperaemia were switched to another IOP-lowering therapy in the follow-up, because they no longer tolerated the conjunctival hyperaemia.
Average (SD) bulbar conjunctival hyperaemia rating after bimatoprost instillation every 24 or 48hours
Our results, based on a small series of patients with limited follow up, indicate that mean IOP can be effectively lowered by bimatoprost administered either every 24 or 48hours. The IOP-lowering action of bimatoprost, monitored in our study for up to 46hours after the last dose, parallels the effect of travoprost 12 and 44hours post dose, with a reported IOP reduction of 9.1 and 7.7mmHg, respectively.9
The average 24.6% IOP decrease 38 to 46hours postdose in our series was significantly different (P=0.02), compared with the average 30% IOP decrease 14 to 22hours post-dose. Thus, on average, a significantly better IOP control was achieved in the right eye relative to that attained in the left eye, even though we did not establish target IOP for each patient. However, an analysis of the lowest and highest IOP values in both treated eyes, with a cut-off IOP at 17mmHg, reached borderline significance only (P=0.06).
It cannot be ruled out that the daily dosing on the right eye could have affected the left eye's IOP. The mean IOP change in the fellow eye was –0.64±3.05mmHg in a monocular trial where 59% of subjects received a prostaglandin analog in one eye only.10 However, latanoprost, and reasonably any prostaglandin analog, has little (if any) contralateral IOP effect, due to its rapid systemic metabolism.11
A possible waning effect of bimatoprost closer to the 48hours, immediately pre-dosing, and nocturnal fluctuation in IOP were not checked. According to a recent meta-analysis, bimatoprost and travoprost showed the greatest 24-hour IOP reduction among monotherapy treatments (prostaglandins, betablockers, carbonic anhydrase inhibitors) for POAG/OH patients. Both bimatoprost and travoprost did not demonstrate lower nighttime pressure values than daytime ones.12 Last but not least, it is worthwhile to note that the cost per treatment success in the US, especially at target pressures <15mmHg, is generally lower for bimatoprost than for either timolol, latanoprost or timolol/dorzolamide.13
The ocular redness found in two different studies after the fixed combination of bimatoprost 0.03%+0.5%14-15 was similar to that observed in our patients at the reduced rate of administration of bimatoprost (8.5% vs. 10% of conjunctival hyperaemia and 1.62 vs. 1.8 of hyperaemia grade for the fixed combination and for bimatoprost every 48hours, respectively). However, there is evidence that in healthy subjects, the duration of conjunctival hyperaemia may be significantly shorter with latanoprost (lasting five days), compared with bimatoprost or travoprost.5 The findings of a meta-analysis in POAG/OH patients suggest a greater efficacy of bimatoprost, compared with that of latanoprost and travoprost, although the incidence of hyperemia was lower with the latter two agents.16 As conjunctival hyperaemia with bimatoprost is highest during the first weeks and then diminishes by three months17, a reduced dose of bimatoprost may be better tolerated in the first weeks, whereas the standard regimen could be recommended later. We are aware that the method of subjectively grading ocular hyperaemia from direct observation18 or colour photographs8 is less sensitive and reliable than objective image analysis,19 which requires appropriate software and instrumentation unavailable to us.
Adherence and persistence is likely to be worse with prescriptions other than daily dosing. On the other hand, this study indicates that sporadic omission of a daily dose would not lead to clinically relevant IOP fluctuations. Eyelash growth and increased pigmentation of the iris and periocular skin were not noticed in our short-term study but it is reasonable to suppose that less frequent administration of bimatoprost should reduce these late side effects, as well as the effects of cumulative exposure of the ocular surface to cytotoxic preservatives such as benzalkonium chloride.20
Limitations of the study included: a limited number of patients, the subjective grading of ocular hyperaemia from direct observation, and the absence of investigation about the long-term effects on hyperaemia or other adverse effects due to both regimens of administration.
To summarize, bimatoprost every 48hours was moderately less effective (on the second day) than daily bimatoprost, but caused less short-term conjunctival hyperaemia. Since bimatoprost-induced conjunctival hyperaemia is reported to be higher in the first month, this reduced dosing schedule may be proposed, especially in the first weeks of treatment to selected patients; those who start on a daily dose of bimatoprost 0.03%, show satisfactory IOP decrease but complain of ocular redness.
Financial disclosure: Financial support or relationships that may pose conflicts of interest: none.