Herpes zoster ophthalmicus (HZO) may cause a variety of ocular conditions including: dermatitis, corneal pseudo-dendrites, uveitis, retinitis, and cranial nerve palsies. Cranial nerve palsies caused by herpes zoster (HZ) are relatively uncommon clinical findings. Previous case reports have looked at this relationship, but the overall case studies are few. Other causes of nerve palsies typically need to be ruled out first with neuro-imaging and blood evaluations. However, when HZ is confirmed, treatment with oral antiviral therapy and/or oral corticosteroids along with monitoring the individual usually results in a self-limiting ophthalmoplegia that improves on its own over several months. We report a case of cranial nerve III palsy resulting from HZO.
El herpes zoster oftálmico (HZO) puede causar una serie de alteraciones oculares que incluyen: dermatitis, pseudo-dendritas corneales, uveítis, retinitis, y parálisis de los nervios craneales. Las parálisis de los nervios craneales causadas por herpes zoster son hallazgos relativamente infrecuentes. Los informes de casos previos han contemplado esta relación, pero los estudios de casos globales son escasos. Normalmente deben descartarse primeramente otras causas de parálisis nerviosas mediante neuro-imagen y pruebas sanguíneas. Sin embargo, al confirmarse el herpes zoster, el tratamiento con terapia antivírica oral y/o corticosteroides orales, junto con la supervisión del paciente, deriva normalmente en una oftalmoplejía auto-limitante que mejora por sí misma transcurridos varios meses. Reportamos el caso de una parálisis del III nervio craneal, causada por HZO.
HZO is a reactivation of the childhood chicken pox virus (varicella zoster) along the ophthalmic division of the 5th cranial nerve (CN V1) which typically reveals itself as a vesicular rash or dermatitis.2–4 Varicella and HZO differ only in the location they occur in the body, not in mechanism or type of virus. The varicella zoster virus (VZV) typically lies latent in the dorsal root ganglion of nerves and, if reactivated, travels down the associated dermatome of the nerve involved. If VZV affects the ophthalmic division of cranial nerve V then it is termed HZO. If it affects another nerve it is referred to as shingles or just generic HZ.1–4 HZ typically affects older populations of individuals who were exposed to VZV as a child. A study has shown that over 90% of 12-year olds were seropositive for VZV and over 99% of adults ≥40 years old were seropositive for VZV in the United States.5 Severity is related to the age of the patient with older patients (≥60 years old) being affected much more severely than younger patients.6 In fact, the lifetime risk of HZ is estimated to be approximately 25% in the general population, and increases to 50% in patients more than 85 years old.5
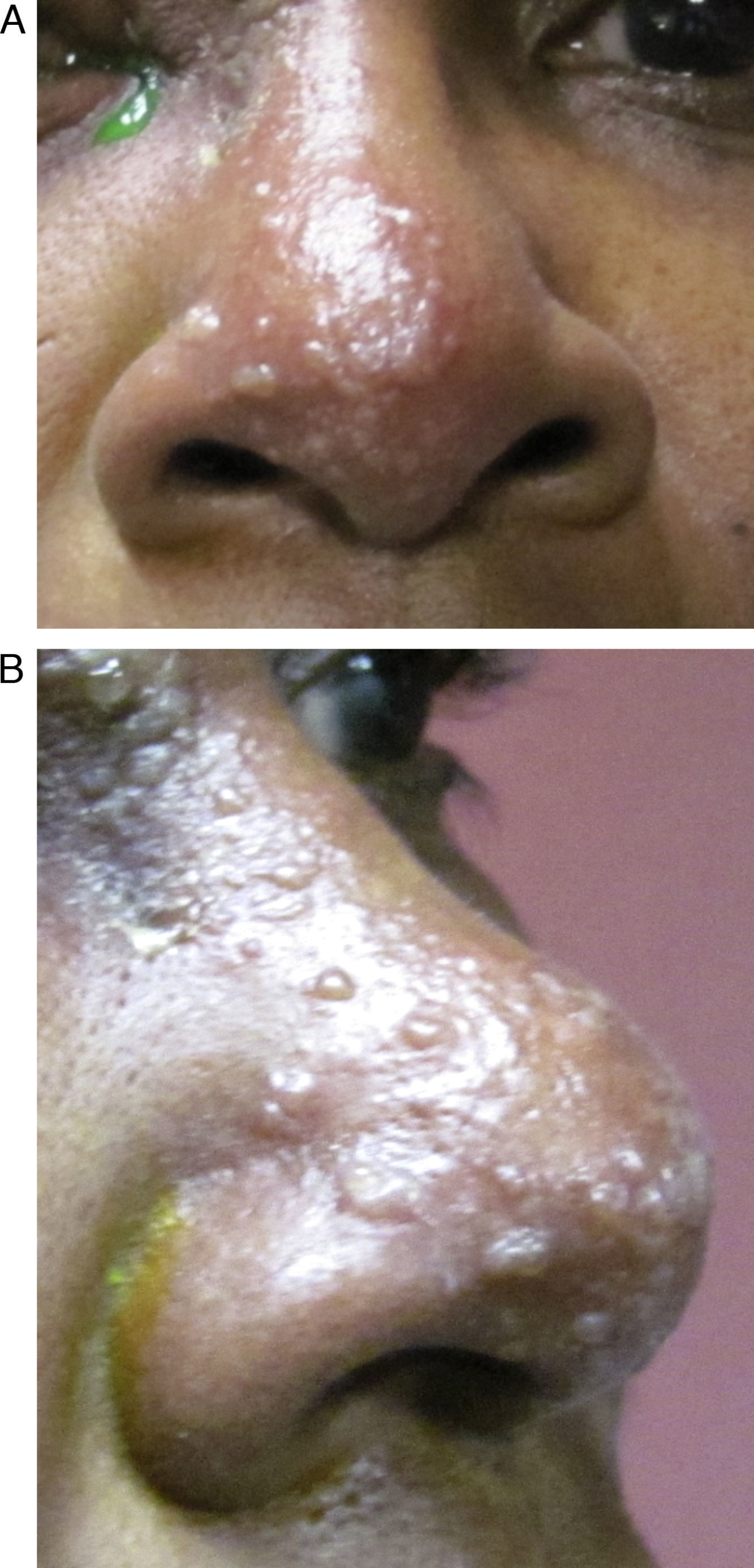
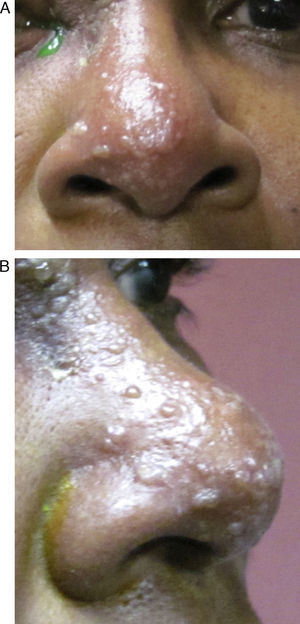
HZO occurs in 20% of all HZ outbreaks. Patients typically present with an acute onset of a unilateral vesicular dermatitis along the CN V1 dermatome which respects the midline. Pseudo-dendrites of the cornea are fairly common and one has a higher risk of ocular complications if the rash spreads to involve the tip of the nose, also known as a positive “Hutchinson's sign.” This signals nasociliary nerve involvement (Fig. 1A and B).2–4 Rarer ocular complications such as iridocyclitis, glaucoma, retinitis, and cranial nerve palsies may also occur in Zoster patients.2–4 The following case report discusses a patient who developed a complete oculomotor nerve (CN III) palsy with pupillary involvement after being diagnosed with HZO one week prior.
Case reportAn 84-year-old American-American female presented to the Urgent Eye Care Service at the Illinois Eye Institute in Chicago, IL as a referral for an ocular examination following a prior diagnosis of HZO. The patient had been released from a local hospital the previous day. She presented with a complaint of soreness around her upper left eyelid and concomitant blurry vision in her left eye (OS). The patient denied diplopia and decrease in vision in her right eye (OD). Her ocular history was positive for cataracts in both eyes (OU), and she was scheduled for cataract extraction in 1–2 months. The patient's medical history was positive for hypertension, kidney problems, and a history of breast cancer. She reported taking carvedilol, nifedipine, furosemide, and a potassium pill for her hypertension and kidney issues. The patient had been prescribed oral acyclovir 800mg 5 times per day, acetaminophen/codeine as needed for pain, and erythromycin ointment for her left eye by the on-call physician at the hospital from the day before. She reported compliance with medications. The patient reported an unremarkable social history and denied any drug and/or medical allergies. She was oriented to time, place, and person.
Entering acuities with spectacle correction of +1.50−1.75×110 OD, +1.75−1.75×080 OS with a +3.00 add OU were 20/50+ OD and 20/60+ OS with no improvement with pinhole OU. The patient's pupils were equal, round, and reactive to light, with no sign of an afferent pupillary defect (APD) OU. Ocular motility showed full range of motion with no pain on eye movement OU. Confrontation visual fields were full-to-finger-count OD, with only a mild superior field defect OS secondary to her ptosis and mild edema of the upper eyelid.
Anterior segment examination revealed clear lids and lashes OD, but upper eyelid ptosis with mild edema and crusted-over vesicles were evident OS. The palpebral and bulbar conjunctivae were white and quiet OU. Corneal examination was clear OD, but pseudo-dendrites were noted superior temporally along the corneal limbus OS, which stained with sodium fluorescein (NaFl) but did not with lissamine green stain. The lenses were graded as having 2+ nuclear sclerosis with 1+ cortical changes. The patient was negative for Hutchinson's sign. All other findings were unremarkable. Goldmann tonometry was 15mmHg OD and 16mmHg OS. Fundus examination was also unremarkable. The patient was diagnosed with HZO of the left side and was educated to continue the oral acyclovir 800mg 5 times per day, as it is standard protocol for HZO reactivations, along with non-preserved artificial tears 4 times a day OU, and to return for follow-up in one week or sooner if her condition worsened.
Follow-up #1The patient presented one week later with complaints of fluctuating vision and tearing OS as well as an increased ptosis of the left eyelid. The patient felt her vision had decreased since the previous visit, and both she and her daughter reported excellent compliance with oral and topical medication therapy. Her entrance acuities with spectacle correction were 20/50+ OD and 20/200 OS, with no improvement with pinhole OU. Pupil examination revealed mild anisocoria with the left pupil being slightly larger than the right. The left pupil had a remarkably minimal response to light compared to the right and also showed a positive APD when a binocular indirect ophthalmoscope (BIO) was used to test pupils. The patient's ocular motility was normal OD, but was restricted in down gaze OS. Gross confrontation visual fields were full-to-finger-count OD, but the superior visual field defect was still present secondary to the increased lid ptosis OS. Slit lamp biomicroscopy was unremarkable OD except for moderate nuclear sclerosis and anterior cortical changes of the lens. Biomicroscopy OS revealed worsening lid swelling/ptosis of the left lid, 2–3+ conjunctival injection, 3–4+ diffuse corneal punctate epithelial erosions (staining with both lissamine and NaFl), dark and quiet anterior chamber, clear iris, and moderate nuclear sclerosis and anterior cortical changes to the lens. The patient was dilated with one drop of 1% tropicamide and one drop of 2.5% phenylephrine in both eyes. Her dilated fundus examination was performed with a 90D lens, and with BIO in conjunction with a 20D lens. The fundus examination was unremarkable OD, OS.
The patient was diagnosed with a partial oculomotor nerve (CN III) palsy with pupillary involvement OS, secondary to HZO. This condition is also referred to Herpes Zoster Ophthalmoplegia. Due to the cranial nerve and pupil involvement, she was referred her to her primary care physician for a magnetic resonance angiogram (MRA) and a magnetic resonance imaging (MRI) to rule out other etiologies of cranial nerve involvement such as an aneurysmal compression of the oculomotor nerve or space-occupying lesion.18 The patient was to continue the acyclovir 800mg 5 times per day in addition to non-preserved artificial tears 4 times per day and was to return for follow-up in four days after meeting her primary care physician to review the results of her imaging.
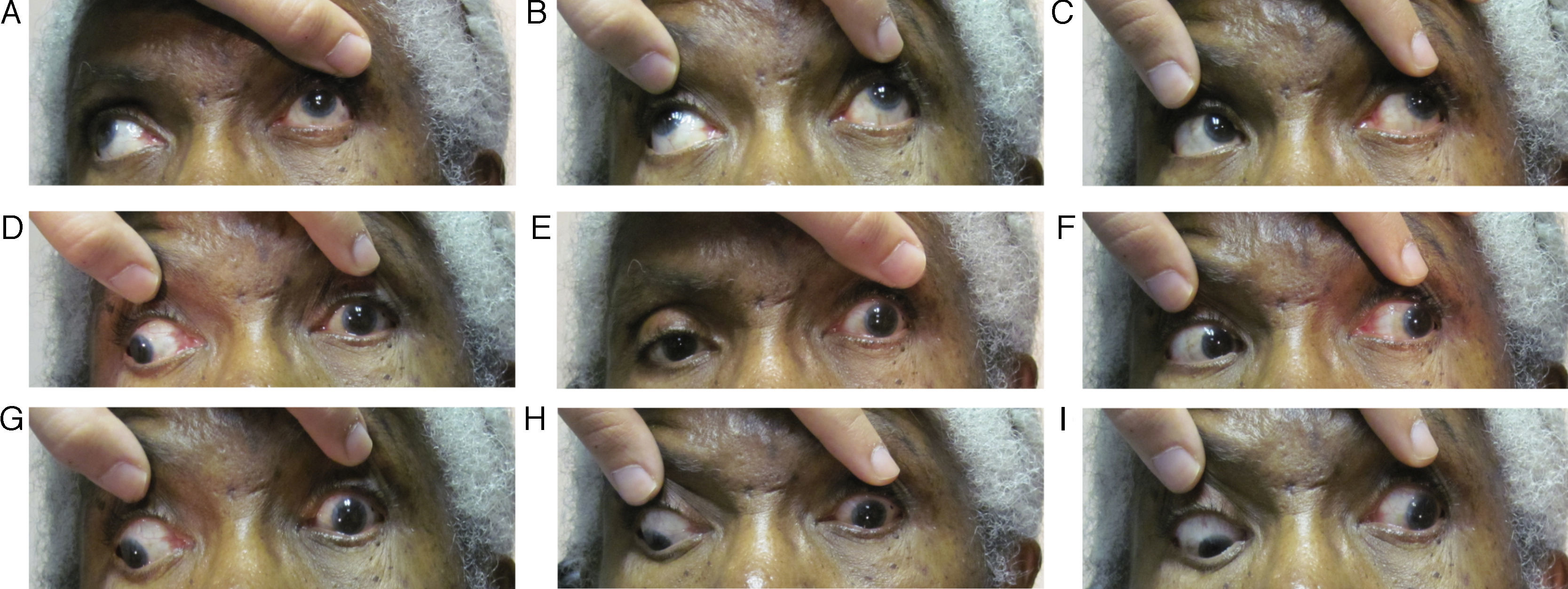
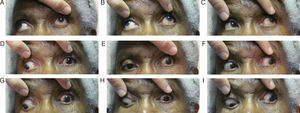
Follow-up #2The patient presented eleven days after her initial visit and reported undergoing the recommended imaging four days prior; however, the results were not scheduled to be discussed until she saw her primary care doctor the following day. The patient complained of worsening of her left lid ptosis as well as diplopia when she manually lifted her left lid to see (Fig. 2A–I). Entering acuities remained unchanged from the previous visit, 20/50 OD and 20/200 OS with no improvement with pinhole OU. Her pupils were anisocoric (OS larger) and were minimally reactive to light when tested with a BIO light source. External examination revealed a complete left lid ptosis. The patient's ocular motility now revealed a worsening/complete cranial nerve III palsy OS. Upon further questioning, the patient reported feeling slightly more ill than previously as well as having jaw pain and scalp tenderness along her left side. Based on these new findings and complaints, she was sent for work-up for both aneurysmal compression of the third cranial nerve and giant cell arteritis (GCA), which included erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) blood work, as well as treatment with intravenous steroids.
Follow-up #3Upon discharge from the hospital the patient returned to our clinic five days later for follow-up (two and a half weeks after initial presentation). She reported feeling better and noticed slight reduction in the degree of her left ptosis. The patient brought her emergency visit records with her for us to review as well. Her ESR was reported at 21 and the CRP was <0.3, which is within normal limits for her age and suggested GCA was not the cause of the CN III palsy. The complete blood count (CBC) with differential proved virtually normal as well. The patient's glucose levels ranged from 168 to 225mg/dl while on the intravenous steroids. The patient's rapid plasma reagin (RPR) and human immune-deficiency virus (HIV) testing were negative. However, her CD3 and CD4 white blood cell counts were low indicating a certain degree of immunosuppression. Her MRI/CT scans revealed possible enhancement within the posterior aspect of the clivus, which was suspicious for neoplastic disease. Thus, the hospital physicians scheduled her for consultation with a neurologist secondary to her history of breast cancer.
Entering acuities at this visit with spectacle correction improved to 20/40-2 OD and 20/70 OS with no improvement with pinhole OD, but pinhole improved the OS to 20/60. Gross confrontation visual fields were full-to-finger-count in the OD, while the superior visual field defect remained secondary to the lid ptosis OS. Her pupils were equal, but minimally reactive to light with no signs of APD OU. Her ocular motility was still full OD, but reduced in nasal, inferior, and superior gazes OS. Her slit lamp examination revealed fully resolved corneal issues in the OS and otherwise normal anterior segments OU. At this time, pending the neurology consult, we diagnosed our patient with HZO-induced ophthalmoplegia of the oculomotor/CN III nerve. The patient was directed to continue her oral prednisone and follow-up with the neurologist for findings on the MRI suggestive of possible metastatic/neoplastic disease.
Follow-ups #4 and 5Four weeks after her initial presentation, our patient reported improvement in her signs and symptoms. Her vision was back to pre-HZO levels of 20/50+ OU. Her confrontation visual fields were unchanged from previous visits. Her ocular motility was still full OD and restricted OS, but definitely improving from previous visits. Adnexal examination revealed the on-going ptosis OS, but the remaining components of the slit lamp examination were normal OU with the exception of the lenticular changes previously noted. The patient was released and asked to return for follow-up after her neurology consult in 2 weeks.
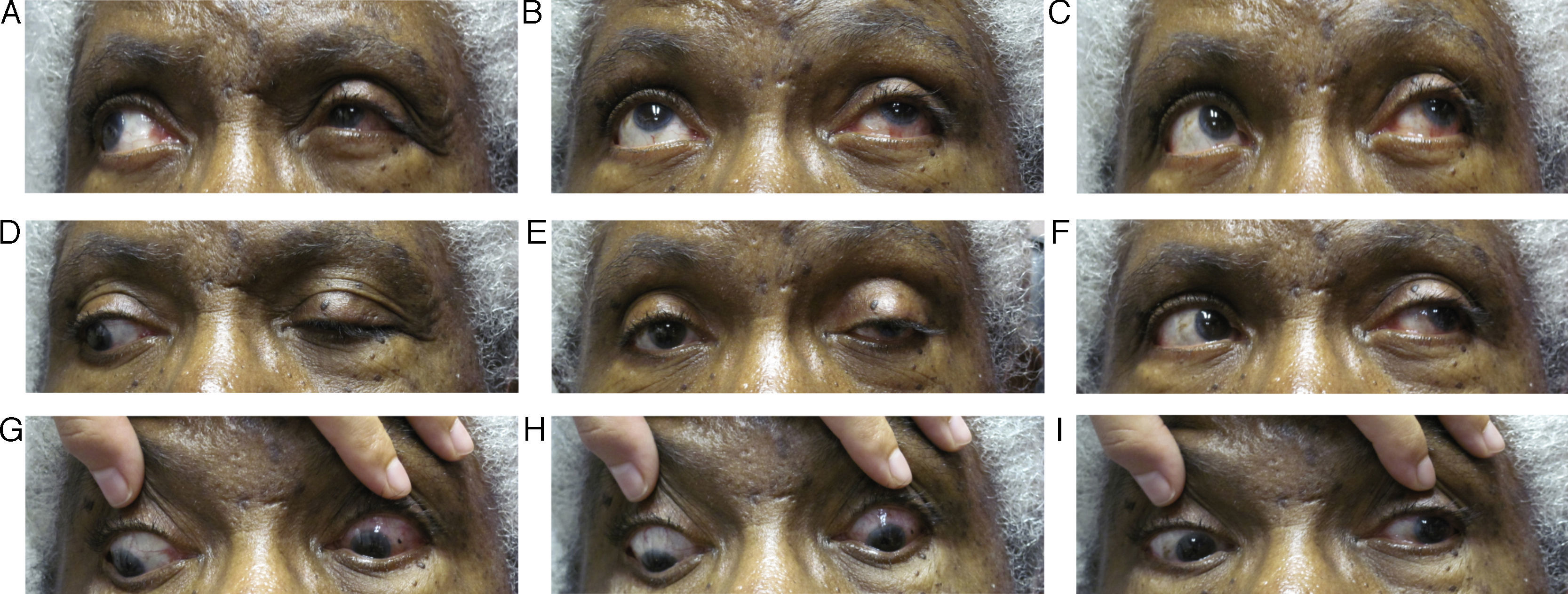
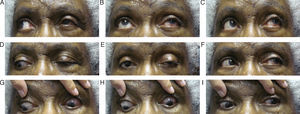
Follow up #6The patient returned 2 weeks after her consultation with the neurologist (seven weeks after her initial presentation). The neurologist disagreed with the possible metastatic/neoplastic disease suggestions of the emergency physicians at this time, but wanted to monitor the patient and repeat the MRI/CT within 2–4 weeks to rule this out and monitor for changes. The neurologist agreed with our diagnosis of oculomotor nerve palsy most likely induced by the HZO outbreak. At this visit, the patient's acuities were stable at 20/50+ OD and 20/40+ OS. Her confrontation visual fields were full-to-finger count OU and her pupils were equal and reactive to light without an APD OU. Her ocular motility had dramatically improved, yet a residual ptosis was still noted OS, though it had improved from previous visits (Fig. 3A–I). Again, her slit lamp examination was within normal limits except for the on-going lenticular changes OU. The patient was told to keep her follow-up appointment with her neurologist and to follow up with us in 4–6 weeks to monitor for anticipated continuing improvement in ocular motilities. Six months after the patient's initial presentation, her ptosis OS had resolved and she regained full ocular motilities. She is currently being followed every six months and denies ocular problems other than persistent irritation and tingling around her left eye.
DiscussionA cranial nerve III palsy results in adduction and vertical ocular motility deficits when the entire nerve is implicated and the pupil can be involved as the pupillary fibers course through cranial nerve III.7,8 Partial palsies can result in varying degrees of involvement of directions of eye movements. Causes are varied and can include space-occupying lesions, microvascular infarctions, aneurysmal compressions, inflammation, infections, and trauma. Our patient initially showed anisocoria, ptosis, and restricted down gaze, which was suggestive of a partial cranial nerve III palsy. Two weeks after her initial presentation, she showed a positive APD, increased ptosis, and restricted extraocular motilities suggestive of a complete CN III palsy. The patient's ophthalmoplegia and ptosis continued after the resolution of her cutaneous lesions and keratitis. Secondary to the ophthalmoplegia, the patient was treated with intravenous and oral steroids. Over the course of six months, the patient's signs and symptoms improved markedly. Six months after the onset of HZO, she had fully recovered from the ophthalmoplegia.
There are various ocular sequelae with HZO, some more common than others. Typically in HZO, the virus causes a dermatitis/vesicular rash of the ophthalmic branch of CN V. Corneal involvement is a relatively common ocular finding and can lead to pseudo-dendritic keratitis which can result in decreased corneal sensitivity, neurotrophic ulcers, and/or decreased vision secondary to scarring.9 If the tip of the nose is involved with the vesicular rash this is termed “Hutchinson's sign” and suggests there is an increased risk of corneal/ocular sequelae secondary to nasociliary nerve involvement.2–4,19 One of the most common complications of any HZ outbreak tends to be post-herpetic neuralgia (PHN) which can cause very intense episodes of pain affecting the same areas as the vesicular rash.21
Overall, ocular complications occur in 50% of HZO cases and typically fall into four categories: keratitis, iritis, muscle palsies, and optic neuritis.10 However, HZO uncommonly results in ophthalmoplegia. In one study, HZO was diagnosed in 10% of all cases of HZ/shingles, and of those HZO cases, 11–29% went on to develop ophthalmoplegia secondary to the HZ reactivation.10 This means only 1.1–2.9% of all HZ/shingles attacks will result in ophthalmoplegia of some form, demonstrating how rarely a case like ours occurs. The total incidence of extraocular muscle palsy following HZO was reported by Edgerton11 to be 13%. The oculomotor nerve (CN III) is most commonly involved (47% of the time) followed by the abducens nerve (CN VI) (23% of the time), and then the trochlear nerve (CN IV) (10% of the time).10–12 However, there have been documented cases of complete ophthalmoplegia (CN III, CN IV, and CN VI palsies occurring concurrently) in patients following an outbreak of HZO,1,10 estimated to occur 20% of the time by Edgerton.11
On an average, HZO-induced ophthalmoplegia occurs 9.5 days after the onset of the HZO rash in 75% of cases with a range of 2–42 days.1 Our patient's experience is in accordance with this estimate as she first had symptoms/signs of ophthalmoplegia eight days after the onset of her HZO. Ophthalmoplegia has been reported to occur concurrently with the HZO outbreak, although this is not the normal presentation.1 Typically, the ophthalmoplegia is self-limiting and results in complete or near-complete resolution on its own in 4.4 months on an average in 65–76.5% of afflicted individuals.1 However, duration varies and can range from 2 weeks to 1.5 years.1 In some patients, residual ptosis and/or limited ocular motility can last indefinitely.20
Treatment of HZO has historically been with oral antiviral agents and consists of: 800mg of acyclovir 5 times a day, 1000mg of valacyclovir 3 times a day, or 500mg famciclovir 3 times a day for 7–10 days.4 However, with HZO-induced ocular motor palsies the treatment is controversial, because the overall pathophysiology of ophthalmoplegia associated with HZO is unclear. One study by Theil et al. looked at cranial nerve nuclei in five brains to determine where and how Herpes Simplex-1 virus, VZV, and human Herpes Virus type 6 reactivate to cause cranial nerve palsies and found that VZV DNA was absent in all the brains studied. This finding suggests that the triggering of the nerve palsies is in the cranial nerve nuclei and most likely occurs further downstream in the nerve conduction pathway.13 This would be consistent with the current theory of reactivation of VZV in the dorsal root ganglia. In general, the pathophysiology of HZO is believed to be derived from different components: viral infection, inflammatory and immune reactions, vascular and neural inflammation, and tissue scarring.6 As a result one study has suggested benefits of concomitant oral and/or intravenous antiviral and oral corticosteroid use to decrease both viral load and the resulting neural inflammation; however, conclusive statistical evidence remains elusive.1 Our patient was treated with oral steroids after thorough discussion with her neurologist to prevent occlusive vasculitic events. The patient's blood sugar was monitored regularly as elevated blood sugar is a known side effect of systemic steroids and has been reported to be a common cause of drug-induced diabetes mellitus.14 If such a patient experiences unwanted diplopia, occlusion or prismatic correction might be beneficial. However, for our patient, it was not needed due to the ptosis preventing diplopia.
One of the most common complications of any herpes zoster outbreak is PHN and treatment success remains moderate at best. PHN has no clear definition but is loosely defined as “clinically significant pain or painful abnormal sensations (allodynia or itch) that persist 3 months or more after rash onset”.15,21 One study reported the presence of PHN in 15% of patients being treated with antiviral drugs compared to 35% of patients not being treated with any antiviral agents at all.5 PHN is much more common in elderly populations than in younger patients. However, PHN does not respond very well to treatment and can lead to depression as the PHN can drastically affect patients’ quality of life.22 As a result, tricyclic antidepressants and selective serotonin-reuptake inhibitors are a mainstay of treatment for both pain and depression. Topical agents such as lidocaine patches or capsaicin creams have been shown to have some efficacy.9,16 Anticonvulsants such as gabapentin have also been shown to relieve the pain of PHN.9,16,20,21 Lastly, NSAIDs, nonsteroidal anti-inflammatory drugs, have a very small effect on PHN and instead, opioids such as tramadol, oxycodone, and morphine are used more often.9,16 A combination of many pharmaceutical agents is typically employed to maximally relieve a patient's pain as no single agent drastically reduces the effects of PHN.9,16 Since our patient presented with symptoms similar to those described in GCA, but had normal CRP and ESR values, we can assume the pain/discomfort our patient experienced was most likely from PHN affecting CN V and its branches. We will continue to monitor our patient for PHN, along with her neurologist, and if any lingering pain occurs we plan to refer her to either the neurologist or a pain specialist for PHN therapy.
A newer treatment to reduce the risk of HZ is via vaccination with a drug known as Zostavax. It is an FDA-approved, live attenuated zoster vaccine used to boost the cell-mediated immunity against HZ in adults ≥50 years old.17 It is delivered via a single subcutaneous injection. This drug was developed based on the Shingles Prevention Study which found individuals who were vaccinated had a 51% lower incidence of herpes zoster, a 67% reduction in PHN, a 61% lower burden of illness, and a 73% reduction in the number of cases with severe and long-lasting pain when compared to placebo.17 Current data shows continued immunity to HZ through 7 years post-vaccination with ongoing research out to 10 years post-vaccination in place. Due to the success of this vaccination, medical professionals may recommend Zostavax for patients older than 50 years of age. Vaccination is not necessary for those individuals who have already experienced a HZ outbreak as that event parallels exactly what the vaccine would do by boosting the immune system. Re-activation of a second HZ event is very unlikely for this same reason.17 Thus, Zostavax would not necessarily be required for our patient, but could be a consideration which can be discussed on a patient-by-patient basis by weighing the benefits and risks of vaccination.
ConclusionAs with any condition that can have many causes, timely referral, imaging, and blood work are important when a cranial nerve palsy is diagnosed, to rule out possible life-threatening conditions. In our case, MRI/MRA ruled out a posterior communicating artery aneurysm and/or space occupying lesion, and the appropriate blood work helped us to rule out GCA. Once these conditions were ruled out then we could be more confident that our patient's signs and symptoms were secondary to HZO-induced ophthalmoplegia, for which our patient fits the typical timeline, signs, and symptoms. The jaw pain and scalp tenderness that our patient complained of (often associated with GCA), was most likely related to PHN. PHN can affect the branches of the trigeminal nerve that are sensory to the same areas (CN V1, V2, V3) and is the most common complication for individuals who have experienced HZO attacks. Blood work revealed lowered CD3 and CD4 counts in our patient signaling a certain degree of immunosuppression, which was most likely the inciting event of the original HZO outbreak. Immunosuppression is also a very common finding in individuals suffering from HZ outbreak. This case demonstrates the relatively uncommon ophthalmoplegia induced by HZO, and the importance of co-management between health care practitioners.
Conflicts of interestThe authors have no conflicts of interest to declare.