Age related macular degeneration (AMD) is the leading cause of blindness in individuals older than 65 years of age. It is a multifactorial disorder and identification of risk factors enables individuals to make lifestyle choices that may reduce the risk of disease. Collaboration between geneticists, ophthalmologists, and optometrists suggests that genetic risk factors play a more significant role in AMD than previously thought. The most important genes are associated with immune system modulation and the complement system, e.g., complement factor H (CFH), factor B (CFB), factor C3, and serpin peptidase inhibitor (SERPING1). Genes associated with membrane transport, e.g., ATP-binding cassette protein (ABCR) and voltage-dependent calcium channel gamma 3 (CACNG3), the vascular system, e.g., fibroblast growth factor 2 (FGF2), fibulin-5, lysyl oxidase-like gene (LOXL1) and selectin-P (SELP), and with lipid metabolism, e.g., apolipoprotein E (APOE) and hepatic lipase (LIPC) have also been implicated. In addition, several other genes exhibit some statistical association with AMD, e.g., age-related maculopathy susceptibility protein 2 (ARMS2) and DNA excision repair protein gene (ERCC6) but more research is needed to establish their significance. Modifiable risk factors for AMD should be discussed with patients whose lifestyle and/or family history place them in an increased risk category. Furthermore, calculation of AMD risk using current models should be recommended as a tool for patient education. It is likely that AMD management in future will be increasingly influenced by assessment of genetic risk as such screening methods become more widely available.
La degeneración macular asociada a la edad (DMAE) constituye la causa principal de ceguera en personas mayores de 65 años. Se trata de un trastorno multifactorial, en el que la identificación de los factores de riesgo permite a las personas la elección de aquellos estilos de vida que pueden reducir el riesgo de la enfermedad. La colaboración entre genetistas, oftalmólogos y optometristas sugiere que los factores genéticos de riesgo juegan un papel más significativo en la DMAE que lo que se pensaba hasta ahora. Los genes más importantes están asociados a la modulación del sistema inmune y al sistema del complemento, como el factor H (CFH), el factor B (CFB) y el factor C3 del complemento, y el inhibidor de la peptidasa tipo serpina (SERPING1). También se ha implicado a los genes asociados al transporte a través de membranas, como las proteínas cassette de unión a ATP (ABC) y la subunidad gamma 3 de los canales del calcio dependientes de voltaje (CACNG3), al sistema vascular, como el factor 2 de crecimiento de fibroblastos (FGF2), la fibulina 5, los genes de tipo lisil oxidasa (LOXL1) y la selectina-P (SELP), y al metabolismo lipídico, como la apolipoproteína E (APOE) y la lipasa hepática (LIPC). Además, diversos otros genes muestran también cierta asociación estadística con la DMAE, como la proteína 2 de susceptibilidad a la maculopatía asociada a la edad (ARMS2) y el gen de la proteína de reparación de la escisión de ADN (ERCC6), aunque se precisa seguir investigando para establecer su significación. Los factores modificables del riesgo de DMAE deberán ser discutidos con los pacientes cuyo estilo de vida y/o historia familiar les sitúan en una categoría de riesgo incrementado. Además, debería recomendarse el cálculo del riesgo de DMAE utilizando los modelos actuales, como herramienta de educación al paciente. Es previsible que el tratamiento futuro de la DMAE se vea crecientemente influenciado por la evaluación del riesgo genético, y que por tanto estos métodos sean cada vez mas ampliamento aplicados.
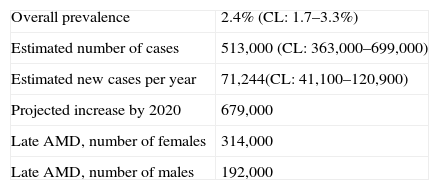
Age-related macular degeneration (AMD) is the most important cause of blindness in industrialized countries in individuals over 65 years of age. In the United Kingdom, for example, overall prevalence of late AMD is 2.4% of the population aged 50 years or more (Table 1) increasing to 4.8% in those 65 years of age or more and 12.2% in individuals greater than 80 years of age.1–4 As a consequence, there are estimated to be 513,000 individuals currently in the UK with the visual impairment characteristic of AMD suitable for registration as seriously visually impaired, the number of female cases being greater than males. This figure is predicted to rise to 679,000 by 20204 as the proportion of more elderly individuals in the population continues to increase, a phenomenon likely to be repeated in many countries.
Estimated prevalence and number of cases of late-stage age-related macular degeneration (AMD) in the UK, CL=95% confidence interval.1–4
| Overall prevalence | 2.4% (CL: 1.7–3.3%) |
| Estimated number of cases | 513,000 (CL: 363,000–699,000) |
| Estimated new cases per year | 71,244(CL: 41,100–120,900) |
| Projected increase by 2020 | 679,000 |
| Late AMD, number of females | 314,000 |
| Late AMD, number of males | 192,000 |
There are two forms of AMD, viz., the ‘atrophic’ or ‘dry’ form and the ‘exudative’ or ‘wet’ form. The dry form is characterized by degeneration of the macular pigment epithelium, choriocapillaris, and photoreceptor cells. Larger areas of degeneration result from the merging of small areas of atrophy and this state is often referred to as ‘geographic atrophy’. By contrast, in the wet form, new choroidal vessels are formed (‘neovascularisation’) and ultimately, retinal detachment, edema, and hemorrhage may occur resulting in degeneration of the macula.
In the last decade, collaboration between geneticists, ophthalmologists, and optometrists has revealed significant evidence that genetic factors are involved in AMD5–8 and play a significant role in the disease alongside other risk factors such as smoking,9–12 diet,13–18 and exposure to sunlight.19,20 Several genes have now been implicated as possible risk factors,21–26 the strongest case being made for genes involved in immune modulation and the complement system. Hence, genetic predisposition to AMD may increase susceptibility to other risk factors such as smoking, diet, and exposure to sunlight. The objectives of this review are to discuss the results of these interdisciplinary studies and most specifically to identify those genes that have been associated with an increased risk of AMD. These genes are discussed in five main categories: (1) immune modulation and the complement system, (2) membrane transport, (3) blood vessel development and vascular modulation, (4) lipid metabolism, and (5) miscellaneous genes. In addition, insights that these genes provide as to pathogenesis of AMD together with protective factors, modifiable risk factors, and risk factor assessment scores are also discussed. A list of abbreviation and acronyms used in this review is given in Table 2.
List of abbreviations used in the text.
| ABCR | Adenosine triphosphate (ATP)-binding cassette rim protein |
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| AMD | Age-related macular degeneration |
| APOE | Apolipoprotein E |
| AREDS | Age Related Eye Disease Study |
| ARMS2 | Age-related maculopathy susceptibility protein 2 |
| C3 | Complement factor 3 |
| CACNG3 | Voltage-dependent calcium channel gamma-3 |
| CFB | Complement factor B |
| CFH | Complement factor H |
| dGI | Dietary glycemic index |
| ERCC6 | DNA excision repair protein |
| FGF2 | Fibroblast growth factor 2 |
| HTRA1 | Serum peptidase 1 |
| IDL | Intermediate density lipoprotein |
| LDL | Low density lipoprotein |
| LIPC | Hepatic lipase |
| LOXL1 | Lysyl oxidase-like 1 |
| MtDNA | Mitochondrial DNA |
| p,q | Short and long arms of chromosome |
| ROS | Reactive oxygen species |
| SELP | Selectin-P |
| SNP | Single nucleotide polymorphism |
| Tr | Transferrin |
Many of the genes implicated as possible risk factors for AMD involve single nucleotide polymorphisms (SNP) in which one amino acid within the protein is substituted for another. Many substitutions commonly result from ‘missense mutations’, i.e., the alteration of one base within a codon. SNP may have varying consequences. Those mutations which give rise to the substitution of a similar amino acid within a protein, such as valine for alanine, result in little protein modification. By contrast, other substitutions may be of greater consequence, resulting in modified protein structure, through greater numbers of disulphide bridges, modified hydrophobicity and charge, which in turn, compromise protein function and interaction with other molecules. A further two types of substitution are less common but potentially more disruptive than missense mutations. First, a codon is modified by base substitution from one coding for an amino acid such as glutamate, to a stop or termination codon (‘null mutation’ or ‘nonsense mutation’) and frequently results in a truncated protein with little natural function. Second, ‘splice-site’ mutations, i.e., a substitution in a region separating an exon from an intron can result in the incorporation of intron DNA into protein synthesis, resulting in proteins markedly different from their natural counterparts. These genetic changes often occur as natural allelic variations within the population conferring either increased risk or protection against AMD. By contrast to SNP, copy number variation, i.e., variation in the number of copies of a specific DNA sequence within a gene appears to be less important, although a small number of such associations with AMD have been recently reported.27 An alphabetical list of the major genes currently associated with AMD, their functions, and locations within the genome together with their abbreviations is given in Table 3.
Genes associated with age-related macular degeneration (AMD).
| Gene | Function | Location | Association with AMD |
| Apolipoprotein E (APOE) | Lipid transport | 19q13.2 | Controversial, possibly with early-onset AMD55–57 |
| ATP-binding cassette rim protein (ABCR) | Membrane transport | 1p22-p22.1 | Controversial, possibly with wet AMD35–40 |
| Age-related maculopathy susceptibility protein 2 (ARMS2) | Unknown | 10q26.13 | Wet AMD, less with dry AMD48,50,58,59 |
| ATP synthase (MT-ATP6) | Produces ATP from ADP | MtDNA | Implicated in AMD60 |
| Complement factor H (CFH) | Inhibition of inflammatory response | 1q32 | Bilateral dry, wet AMD26,28–31,34 |
| Complement Factor B (CFB) | Component of alternative pathway | 6p21.3 | AMD32 |
| Complement factor 3 (C3) | Mediates inflammatory response | 19p13.3-p13.2 | Wet AMD22,33 |
| DNA excision repair protein (ERCC6) | Promotes complex formation at repair sites | 10q11.23 | Possible association with AMD61 |
| Fibroblast growth factor 2 (FGF2) | Diverse biological functions | 4q26 | Dry AMD48 |
| Fibulin 5 (FBLN5) | Polymerization of Elastin | 14q32.1 | AMD49 |
| Hepatic lipase (LIPC | Converts IDL toLDL | 15q21 | Reduced risk of wet and dry AMD55 |
| Lysyl oxidase-like 1 (LOXL1) | Connective tissue development | 15q24.1 | Wet AMD24,50 |
| Selectin P (SELP) | Pl atel et/l eucocyte Interactions | 1q22-q25 | Dry AMD51 |
| Serpin peptidase inhibitor (SERPING1) | Regulates complement | 11q12-q13.1 | AMD23 |
| Serum peptidase 1 (HTRA1) | Regulates cell growth | 10q25.2-q26.2 | Bilateral wet AMD21,24,25 |
| Transferrin (Tf) | Iron binding protein | 3q29 | AMD in smokers62,63 |
| Voltage-dependent calcium channel 3 (CACNG3) | L-type calcium channel protein | 16p12 | AMD47 |
Genes for the complement system protein factor H (CFH),26,28–31factor B (CFB),32 and factor 3 (C3)22,33 appear to exhibit the strongest associations with the risk of developing AMD.34 A higher risk of wet AMD is associated with polymorphisms of the CFH gene and with lower visual acuity after26 A strong association between CFH and bilateral dry AMD has also been demonstrated.34CFH is involved in the inhibition of the inflammatory response mediated via C3b by acting both as a cofactor for cleavage of C3b to its inactive form, C3bi, and by weakening the active complex that forms between C3b and factor B. C-reactive protein and polyanionic surface markers such as glycosaminoglycans normally enhance the ability of CFH to inhibit complement. Specific mutations in CFH (e.g., Tyr402His) reduce the affinity of CFH for C3f and may also alter the ability of CFH to recognize specific glycosaminoglycans. This change results in reduced ability of CFH to regulate the activity of complement proteins on critical surfaces, such as the retina, and may lead to an increased inflammatory response at the macula. By contrast, CFB is a component of the alternative pathway of complement activation. When cleaved, an active subunit is formed which is involved in the proliferation of pre-activated B-lymphocytes.
Serpin peptidase inhibitor (SERPING1)Genetic variants of the serpin peptidase inhibitor (SERPING1) gene, which regulates the activity of complement proteins, have recently been shown to be associated with AMD23; the protein inhibits the first components of the complement cascade and thus regulates complement activation.
Genes involved in membrane transportATP-binding cassette rim protein (ABCR)Earlier studies suggested that a protein found in retinal rod cells, viz., adenosine triphosphate (ATP)-binding cassette rim protein (ABCR), may be involved in a number of ocular disorders including Stargardt's macular dystrophy, retinitis pigmentosa (RP), cone-rod dystrophy, and AMD.35–38 The ABCR protein comprises two structurally related halves, each of which contains a section which spans the rod membrane, and a section which can bind to ATP, and is abundant in the outer segments of rod cells but absent from cone cells.39 Within the rod outer segment, the protein is localized within the rim region of the rod membranes and aids the movement of all-trans retinal within the membrane.40
The ABCR gene is 100 KB long and consists of 50 exons ranging in size from 33 to 406 nucleotides.41 A full length transcript of the gene sequence was cloned in 199842,43 allowing the gene to be located to the short arm of chromosome 1 near to the centromere (1p21-p22.1). A large number of mutations of the ABCR gene have now been identified indicating that ABCR is an especially ‘polymorphic’ gene.44 In five early studies, however, there was little agreement concerning the importance of the ABCR gene in AMD. A study of dry AMD in Japan concluded that there was no relationship between allelic variation in ABCR and the disorder38 and the study of De la Paz et al.36 concluded that ABCR was not a major risk factor. By contrast, a study of wet AMD37 concluded that 4% of familial cases of AMD may be related to ABCR. In addition, other studies have concluded that possession of some variants of the ABCR gene may increase susceptibility to the disease.45 Recent studies have also been controversial. Hence, the presence of three significant mutations in ABCR was examined in eight cases of wet AMD,46 the analysis failing to identify the presence of disease-causing mutations in the cases examined. However, ABCR mutations causing the protein to accumulate in the inner segment of rod cells have been reported to be frequent in patients with severe retinal dystrophies, including a small number of AMD cases.40
Hence, ABCR may influence the active transport of a wide range of drugs, metabolites, peptides, and lipids and may also be involved in the transport of retinal derivatives, phospholipids, peptides, or other endogenous substrates across the disk membrane. Hence, disturbed transport across the membranes of retinal cells is one possible, albeit rarer, route to AMD.
Voltage-dependent calcium channel gamma-3(CACNG3)Voltage-dependent calcium channel gamma-3 (CACNG3) is a protein subunit of the L-type calcium channel located in the cell membrane and regulates trafficking and channel gating. Recent genetic analysis has suggested there is a gene associated with AMD on chromosome 16 (16p12).47 At least five candidate genes occur in this region of the chromosome but the most likely to be associated with AMD is CACNG3. These data suggest a possible link between calcium ion channel modulators in the photoreceptors and AMD but further research is necessary to establish the significance of this gene.
Genes involved in blood vessel development and vascular modulationA number of genes linked to the development of blood vessels or modulation of vascular processes have been implicated in AMD and these genes are interesting because of the involvement of the vasculature in wet AMD (neovascularization).
Fibroblast growth factor 2 (FGF2)Fibroblast growth factor 2 (FGF2) is a protein involved in various biological processes including mitosis, the development of blood vessels, wound healing, and tumor growth. In a Spanish population, Brion et al.48 has shown that although the principal genetic risk factors are ARMS2 and CFH, FGF2 may also be associated with some cases of dry AMD.
Fibulin-5Rare forms of AMD, exhibiting an autosomal dominant pattern of inheritance, may be related to the fibulin-5 gene. Fibulin-5 is an excreted extracellular matrix protein and is expressed in basement membranes of epithelial cells and developing blood vessels and is essential for the polymerization of elastin. AMD patients were screened and a statistically significant correlation observed between mutations of fibulin-5 and AMD.49
Lysyl oxidase-like 1 (LOXL1)The Lysyl oxidase-like 1 gene (LOXL1) codes for an enzyme involved in the development of connective tissue, including that of blood vessels, and has been shown to be significantly associated with wet AMD in a Japanese50 and a Korean population although the biological basis for this association is unclear.24
Selectin-P (SELP)The activity of macrophages and endothelial cell activation in the pathogenesis of AMD may also be involved in AMD.51 The selectin gene group function as cell adhesion molecules on the surface of activated endothelial cells and platelets and is involved specifically in the interactions between platelets and leucocytes. No single SNP of SELP is highly correlated with AMD, but a polymorphism of the gene has been found to be statistically associated with dry AMD.51 Platelet and leukocyte interactions promote thrombus formation and are active at the sites of inflammation but how these processes may contribute to dry AMD, is unclear.
Genes involved in lipid metabolismApolipoprotein E (APOE)Apolipoprotein E (APOE) is a lipid transport protein essential for the catabolism of triglyceride-rich lipoproteins. It transports lipoproteins, fat-soluble vitamins, and cholesterol to the lymph system and then to the blood. APOE is a polymorphic gene with three major isoforms, viz., β2, β3, and β4. These alelic forms differ only slightly but are sufficient to alter APOE structure and function. A pooled analysis of 15 studies52 found associations between APOE alleles β4 and β2 with late AMD but there was no interaction with gender or smoking behavior. By contrast, Adams et al.53 also found an association between possession of APOE allele β2 and early onset AMD, an association that was present in non or previous smokers but not in current smokers suggesting an interaction between genetics and smoking. More recently, a meta-analysis of early onset AMD has suggested an association with APOE.54
Hepatic lipase (LIPC)The hepatic lipase (LIPC) gene codes for the enzyme hepatic triglyceride lipase which is expressed in liver and the adrenal glands. The principle function of the enzyme is conversion of intermediate density lipoproteins (IDL) to low density lipoproteins (LDL). There may be an association between the proportion of different lipoproteins and AMD in that a specific genotype of LIPC has been linked with a reduced risk of both dry and wet forms of the disease.55 Increased incidence of early AMD has been observed in patients with raised C-reactive protein,56 which is associated with increased consumption of lard and solid fat.57 High triglyceride levels, by contrast, were associated with a lower risk and therefore, certain types of lipid could be protective against AMD. In a prospective study, association between AMD and fat intake was examined by Cho et al.13 Total fat intake of those patients who were the highest consumers was associated with increased risk of AMD. In addition, linolenic acid consumption was associated with AMD whereas docosahexaenic acid (DHA) had a modest inverse relationship with AMD. Hence genes which affect lipid metabolism may alter the balance between different types of fat in the body thus influencing risk of AMD.
Miscellaneous genesIn addition to the above groups of genes, a number of genes of varied or unknown function show some statistical association with AMD. Their significance is more problematic and further research will be necessary to establish their significance.
Age-related maculopathy susceptibility protein 2 (ARMS2)Age-related maculopathy susceptibility protein 2 (ARMS2) is a mitochondrial protein, currently of unknown function, which may play a role in various diseases of the elderly including AMD.58,59 SNP of the ARMS2 gene are especially associated with wet AMD and the frequency of progression from dry to wet forms of the disease8 but are less associated with dry AMD.50 In a Spanish population, ARMS2 has been reported to be one of the principal risk factors for AMD.48
ATP synthaseATP synthase is a mitochondrial enzyme important in providing energy to the cell via ATP. Mutations of the ATP synthase gene have been related to RP and this gene may also be implicated in some cases of AMD.60
DNA excision repair protein (ERCC6)The DNA excision repair protein gene (ERCC6) interacts with several transcription and excision repair proteins. Excision repair proteins remove mutations resulting from UV damage and result in the removal of a short single-stranded segment of DNA containing the mutation. A new complimentary strand is then synthesized using the remaining strand as template. Disease can result from genetic mutations which damage DNA repair mechanisms such as ‘Cockayne's syndrome’, a disorder characterized by photosensitivity and a pigment retinopathy.61 A study has also suggested an association between SNP of ERCC6 and AMD61 indicating that UV damage to genes which is unrepaired could be a factor.
Serum peptidase 1 (HTRA1)The serum peptidase 1 (HTRA1) gene encodes for a secreted serine protease.21,24,25 The protein is a secreted enzyme proposed to regulate the availability of insulin-like growth factor (IGF) by cleaving IGF-binding protein. IGF binds to insulin-like growth receptor thus initiating intracellular signaling and subsequently, stimulating cell growth and inhibiting apoptosis (programmed cell death). Hence, HTRA1 may inhibit cell death in the retina and mutations in this gene result in increased cell losses. Tong et al.25 emphasized the importance of gene interactions; HTRA1 plays a significant role in AMD when it is found in combination with ARMS2.
Transferrin (Tr)Transferrin (Tr) is an iron-binding blood plasma glycoprotein which controls the level of free iron in biological fluids.62 Oxidative stress is regarded as a major risk factor for AMD and increased levels of reactive oxygen species (ROS) are produced by reactions catalyzed by iron in the retina. Hence, changes in iron metabolism genes may be involved in AMD. Recently, SNP in the Tr gene have been shown to be significantly associated with AMD especially in smokers63 emphasizing the interactions that may occur between genetic susceptibility and environmental risk factors.
Genes and the pathogenesis of AMDThe most important genes implicated in AMD appear to be those involved in immune modulation and the complement system such as CFH,26,28–31CFB,32C3,22,33 and SERPING123 strongly suggesting that inflammatory processed are important in AMD. It has been concluded that SNP in CFH, C3, HTRA1, and SERPING1 together could account for 45% of the risk of developing AMD.23 Genes associated with membrane transport, e.g., ABCR35–38 and CACNG47, the vascular system, e.g., FGF2,22LOXL121,30 and SELP,31 and with lipid metabolism, e.g., APOE52–54 and LIPC55 are also involved. Of these, the vascular genes are of particular interest because of the involvement in neovascularization. In addition, a series of other putative genes of variable or unknown function could be involved in AMD including ARMS2,58,59ATP synthase,60ERCC6,61 and HTRA121,24,35 and it is likely that many further genes will be identified.
Hence, there are likely to be multiple pathways leading to the same ultimate pathology in AMD, i.e., loss of retinal photoreceptors. Nevertheless, it is possible that carriers of high-risk genotypes may show detectable and specific visual changes before overt pathology is present. Feigle et al.64 assessed the critical fusion frequency (CFF) in individuals with normal vision who were carriers of high-risk variants of genes such as CFH, LOC, and HTRA1. CFF mediated by rods and cones was significantly reduced in gene variant-positive rather than gene variant-negative individuals although CFF mediated by cones alone was unaffected. This was the first study to relate early AMD risk genotypes to visual function suggesting that there may be many other gene/vision relationships to be identified in AMD.
Protective factorsVarious nutritional factors may be involved in the pathogenesis of AMD including anti-oxidants and anti-oxidant co-factors, a number of vitamins, zinc, and anti-free radicals such as lutein, zeaxanthin and components of the membrane of photoreceptor cells.65 Consumption of omega-3 fatty acids in combination with anti-oxidants has been suggested as a preventative strategy66 but no fully developed double blind studies have been carried out to date to test this hypothesis. Eating more cold-water fish rather than red meat and adding nuts to the diet have all been suggested to reduce the risk.64 For example, Chua et al.14 suggested that a 40% reduction in early AMD could be achieved by changing to a diet with significantly increased fish consumption.67 In addition, there is evidence that supplementing the intake of DHA and eicosapentaemic acid (EPA) and reducing dietary glycemic index (dGI) may be protective against AMD in the ‘Age Related Eye Disease Study (AREDS) trial.17
A review of all randomized controlled trials comparing anti-oxidant vitamins concluded, however, that there was little benefit of any anti-oxidant combination in AMD.68 They also concluded that there was accumulating evidence that neither vitamin E nor β-carotene in themselves could prevent or delay the onset of AMD. In addition, Delcourt et al.69 studied the relationship between nutrition and AMD and found that individuals taking the antioxidants, lutein, zeaxanthin, and omega-3 poly unsaturated fatty acids had a lower risk of AMD. A similar result was reported by Lecerf and Desmettre.70 By contrast, Chong et al.71 using meta-analysis, studied the effect of dietary anti-oxidants in nine prospective cohorts and three random clinical trials. Pooled results suggested that vitamins A, C, and E, zinc, lutein, zeaxanthin, carotene, P-cryptoxanthin, and lycopene had little or no effect on preventing early AMD. However, there was evidence that antioxidant supplements containing zinc could prevent visual loss in individuals with early AMD.72
Modifiable risk factorsSeveral studies have proposed additional lifestyle changes to reduce the lifetime risk of AMD that should be considered in patients in high genetic risk categories. Hence, high fat intake may be associated with an increased risk of AMD in both women and men. Reducing fat intake to this level means reducing the consumption of red meat and dairy products such as whole milk, cheese, and butter which are high in saturated fats. Higher red meat intake has been positively associated with early AMD whereas consumption of chicken may be negatively associated with AMD.73 There is considerable evidence that the incidence of AMD varies with both ethnicity and geographical location which could be attributed in part to varying diet. Hence, the risk of AMD varies among Americans of different Asian origin74, overall, 5.4% of Asian Americans having dry and 0.49% wet AMD, Chinese and Pakistani Americans having a significantly increased risk of dry AMD compared with non-hispanic whites. By contrast, Japanese Americans have a 29% decreased risk of dry AMD.74 There were no significant differences in the risk of wet AMD in Asian Americans of any ethnicity compared with white Americans. Outside the US, a Japanese study at Funagata75 found that the prevalence of early AMD in individuals greater than 35 years of age was 3.5%, and of late AMD 0.5%, while the age-standardised prevalence in the right eye was 4.1%. In rural China, prevalence of wet and dry AMD is 1.45% and 1.55% respectively, increasing with age.76 An overview of epidemiological studies from around the world72 suggests prevalence of early AMD is similar in the white, black, and hispanic populations, but that whites have a greater prevalence of late AMD. Hence, prevalence studies provide considerable evidence that environmental, and lifestyle differences among populations as well as genetic variation are likely to be involved in determining the risk of AMD.
Risk factor assessment scoresThe identification of specific genetic defects leads to the possibility of more widespread screening for AMD especially in affected families and hence, the possibility of identifying asymptomatic carriers of the disease. A review of research to date suggests that smoking is the single most important modifiable risk factor for AMD.9–12 Studies taken collectively suggest that long-term smoking is associated with approximately a doubling of the risk of late-onset AMD. Hence, it is particularly important that patients with a familial history of the disease should cease smoking. A risk assessment model has been developed for advanced AMD and is available for online use.7 Based on data from 2846 AMD patients followed over 9.3 years, Cox proportional hazard analysis was used to assess the significance of demographic, environmental, phenotypic, and genetic covariates. The final model incorporates age, smoking behavior, family history, and genetic variation (especially of CFH and ARMS2) to predict the risk of AMD.7 Grassman et al.77 have constructed a genetic risk score for AMD based on 13 ‘at risk’ variations of eight individual genes. AMD patients younger than 75 years of age had a significantly higher score compared with those patients older than 75. In addition, Seddon et al.6 have proposed a prediction model for the risk of advanced AMD based on genetic, demographic and environmental variables. This model included several genetic variants including CFH, LOC, C2, CFB, and C3, all of which were related to the prevalence of advanced AMD, and all, with the exception of CFB, significantly related to the progression to advanced AMD. In addition, smoking was independently related to AMD with a multiplicative joint effect with genotype. These advances have also resulted in the development of specific screening tests for AMD, such as the ‘macula risk test’ which involves taking a sample of cells from the cheek of the patient for genetic analysis.78 The results of the test include a risk estimate based on the genetic polymorphisms identified and genetic counselling support. The test has five categories of risk determined by an algorithm that takes into account genetics and also smoking history. Hence, risk category 1 is associated with a lifetime risk of 0.65–4.6% whereas risk category 4 is associated with a 55–94% lifetime risk.
ConclusionModifiable risk factors for AMD should be discussed with patients whose lifestyle and/or family history place them in an elevated risk category. Protective modifications include: cessation of smoking, sunglass protection under conditions of elevated light intensity, dietary modifications to reduce intake of saturated fats and increase omega 3 fatty acids. Calculation of AMD risk using current models should be recommended as a tool for patient education. Furthermore, AMD management frequency may be influenced by an assessment of genetic risk, as such provisions are likely to become more widely available. Interactions between different genes and between genes and the environment also play a prominent role in the development of AMD and are likely to be profitable areas of future research.