To evaluate the safety and efficacy of a new multipurpose disinfecting solution (MPDS) with a formulation that includes aloe vera on its composition.
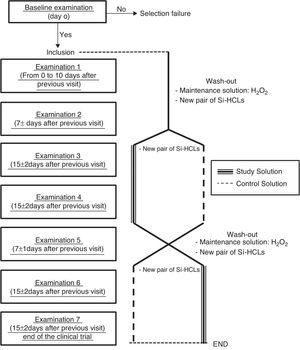
MethodsThis is a prospective, randomized, double-masked clinical trial with a crossover design that included seven examinations. Two different MPDSs, Avizor Alvera® (study solution) and All Clean Soft® (control solution), each were used for 1 month. Comfilcon A silicone hydrogel contact lenses were used during the trial. The main outcome variables were corneal staining and deposits on the surfaces of the contact lenses. Other parameters including ocular surface response, contact lens wettability, user satisfaction, and adverse events, were analyzed according to the International Organization for Standardization (ISO) 11980:2010 guidance for clinical investigation.
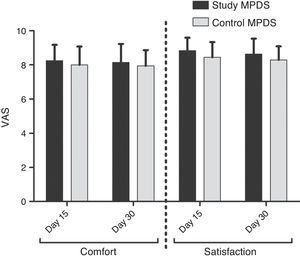
ResultsTwenty subjects (10 women, 10 men) (mean age, 27.7±5.6 years; range, 20–41) were included. No differences between both MPDSs were found in the percentage of subjects with corneal staining >0 at day 30 (study: 35%, control: 50%; p=0.46); neither in the percentage of subjects with deposits on the surface of the contact lens >0 at day 30 (study: 26.32%, control: 52.63%; p=0.18). The study MPDS received higher rates in comfort (study: 8.14±1.09, control: 7.94±0.92; p=0.56) and satisfaction at day 30 (study: 8.63±0.91, control: 8.29±0.80; p=0.19), however the scores were not significantly different with the control MPDS.
ConclusionsThe clinical trial showed that the study MPDS is safe, efficient, and has acceptable physiologic tolerance, according to the ISO 11980:2010 guidance for clinical investigation.
Evaluar la seguridad y la eficacia de una nueva solución única desinfectante (MPDS), con una formulación que incluye aloe vera en su composición.
MétodosEl estudio consistió en un ensayo clínico prospectivo, aleatorio y de doble ciego, con un diseño cruzado que incluyó siete exámenes. Se utilizaron durante un mes dos MPDS diferentes, Avizor Alvera® (solución en estudio) y All Clean Soft® (solución de control). Durante el ensayo se utilizaron lentes de contacto de hidrogel de silicona Comfilcon A. Las principales variables evaluadas fueron la tinción corneal y los depósitos sobre la superficie de las lentes de contacto. Se analizaron otros parámetros, que incluían la respuesta de la superficie ocular, la humectabilidad de las lentes de contacto, y los eventos adversos, de acuerdo con la normativa 11980:2010 de la Organización Internacional de Normalización (ISO) para investigación clínica.
ResultadosEl estudio incluyó a veinte sujetos (10 mujeres, 10 hombres) (edad media, 27,7±5,6 años; rango, 20-41). No se hallaron diferencias entre ambas MPDS en cuanto al porcentaje de sujetos con tinción corneal>0 al mes (estudio: 35%, control: 50%; p=0,46), ni el porcentaje de sujetos con depósitos en la superficie de las lentes de contacto>0 al mes (estudio: 26,32%, control: 52,63%; p=0,18). La MPDS en estudio obtuvo valoraciones superiores en cuanto a confort (estudio: 8,14±1,09, control: 7,94±0,92; p=0,56) y satisfacción (estudio: 8,63±0,91, control: 8,29±0,80; p=0,19); sin embargo, las puntuaciones no fueron significativamente diferentes en relación a la MPDS de control.
ConclusionesEl ensayo clínico reflejó que la MPDS en estudio es segura y eficaz, mostrando una tolerancia fisiológica aceptable, de acuerdo con la normativa ISO 11980:2010 para investigación clínica.
About 76% of contact lens (CL) users clean and disinfect their CLs with chemical care systems, including multipurpose disinfecting solutions (MPDSs).1 For that reason, the MPDS formulas include an antimicrobial agent, a buffer system, and in some cases a surfactant agent.2 However, MPDSs can interfere with the integrity and function of the corneal epithelium which may cause clinically relevant signs and symptoms of discomfort with the CLs. Although surfactant agents also can cause irritation, antimicrobial or preserving agents are the most decisive factors that produce ocular surface irritation.3,4
The use of preserving and/or disinfectant agents in solutions that come into contact with the eyes (MPDS, lubricant drops, eyedrops) is not risk-free, particularly regarding the corneo-conjunctival surface, because their toxicity may affect the ocular surface cells. A study of several MPDSs that included different types of antimicrobial agents in their formulations reported abnormal exfoliation of the corneal epithelial layers.5
A new MPDS (Avizor Alvera® MPDS, Avizor S.A., Madrid, Spain) has been developed with a specific formulation that incorporates aloe vera, which protects the ocular surface epithelium.6 Aloe vera is a natural source of mannose-6-phosphate (M6P),7 this natural complex sugar can inactivate the action of certain inflammatory mediators by a competitive blocking of certain keratocytes receptors from which these mediators exert their action.8 Considering the negative effect of the antimicrobial agent in MPDSs on the corneal surface, it is of interest to identify an agent that prevents irritation caused by preserving and surfactant agents that can be incorporated into MPDSs without interfering with the cleaning and disinfecting processes of the MPDS.
The purpose of the current study was to determine the safety and efficacy of the Avizor Alvera MPDS compared with a control MPDS, All Clean Soft (Avizor S.A.) as a MPDS used to clean and disinfect silicone hydrogel CLs (Si-HCLs) for over 1 month.
MethodsDesignThe current study was a prospective, randomized, double-masked, crossover clinical trial with seven scheduled examinations. Fig. 1 shows a flow chart of the study for a better understanding of the design. The Valladolid Este Ethics Committee, Valladolid, Spain, and the Spanish National Health Authority (Spanish Drugs and Health Products Agency, registration number 2011-000604-18) approved the study. All subjects were treated according to the Declaration of Helsinki.
Inclusion/exclusion criteriaThe study included 20 hydrogel CLs wearers over 18 years of age with refraction between −10.00 diopters (D) and +8.00 D, astigmatism less than 2.25 D, and a minimum of 1 year of habitual CL wear (at least 5 days weekly for at least 5h daily). Both eyes were examined, but only data from one eye was chosen randomly for analysis. Subjects were excluded if they had an active ocular surface disease or a systemic disease that could affect ocular physiology or worsen with CL wear. The exclusion criteria included intolerance or sensitivity to the study products, ocular infection or a history of herpetic keratitis, severe inflammation during the 6 months before the study, use of an ocular topical medication in the previous 3 months (except artificial tears) or a systemic medication that could possibly affect the tear film, refractive surgery, corneal irregularities, pregnancy and breastfeeding, corneal edema, corneal neovascularisation or infiltrates graded 1 or higher, tarsal or conjunctival hyperemia graded 2 or higher, fluorescein corneal staining graded 2 or higher in any area, the sum of all the areas graded 4 or higher, or greater than 20% staining.9
CLs and solutionsFour pairs of silicone Si-HCLs (Biofinity™ and Biofinity Toric™, Comfilcon A, Cooper Vision Inc., Fairport, NY, USA, base curve: 8.60mm, diameter: 14.00mm, power range from +8.00 to −10.00, and cylinder up to 2.25 D) were dispensed to each subject during the trial: two pairs for the two washout periods (1 week each), and two pairs for the study or control solution periods (30 days each). Subjects were instructed not to sleep or nap while wearing the CLs during the study and to wear the provided CLs a minimum of 5h daily and 5 days weekly during the study.
The study (Avizor Alvera, Avizor S.A.) and control (All Clean Soft, Avizor S.A.) solutions were randomly assigned. The use of the MPDS was randomized and double-masked during the study. Both solutions were masked by the manufacturer. The packaging of both solutions was identical. A 5-digit code was randomly assigned to each bottle. Two codes were assigned to each subject, one corresponding to the control solution and other one to the study solution. The solutions delivery and the evaluation of subjects were performed by different evaluators. Patients received written instructions regarding the use and maintenance of their Si-HCLs. The composition of both MPDSs is shown in Table 1. A hydrogen peroxide system was provided (Ever Clean, Avizor S.A.) for washout periods.
Composition of the multipurpose disinfecting solutions used in the study.
| Brand | Components | Other | ||
|---|---|---|---|---|
| Buffer | Preservative | Surfactant | ||
| Avizor Alvera | Borax/boric acid | EDTA, polyhexanide 0.0002% | Poloxamer | Polyvinylpyrrolidone aloe vera |
| All Clean Soft | Borax/boric acid | EDTA, polyhexanide 0.0002% | Poloxamer | |
| Ever Clean | Hydrogen peroxide 3% | Phosphates | ||
All subjects completed seven examinations with two washout periods. The study design followed the requirements of the International Organization for Standardization (ISO) 11980:2010 guidance for clinical investigation (Ophthalmic optics, CL, and CL care products).9
Baseline examinationThe subjects attended this examination without wearing CLs for at least 24h. The clinical trial procedures were explained to the subjects, and each subject provided informed consent and signed a data protection form.
Medical and CL histories were obtained and the subjects underwent an ocular examination. Subjective refraction and LogMAR visual acuity (LogMAR VA) with an ETDRS chart was measured; keratometry and slit-lamp evaluation of the ocular surface were performed to determine if they met the inclusion and exclusion criteria. Women of childbearing age who wished to participate in the study should perform a pregnancy test. All subjects who complied with the inclusion and exclusion criteria were scheduled for the next examination within the following 10 days after baseline examination.
Examination 1Subjects attended the examination without wearing CLs for at least 24h. Changes in health status or medication and possible adverse events since the previous examination were scored; if the medication change might affect the ocular surface the patient discontinued the study. A new Si-HCL with the subjects parameters was inserted, and after waiting 30min the following procedures were performed: measurement of the LogMAR VA, assessment of the Si-HCL fit (corneal coverage, position, and mobility), and evaluation of limbal and bulbar hyperemia. The subjects wore this Si-HCL for 1 week during the first washout period and received a hydrogen peroxide system (Ever Clean) for cleaning and maintaining the Si-HCLs.
Examination 2This examination was 7±1 days after examination 1. The subjects attended the clinic after having worn the Si-HCLs for at least 5h. Changes in health status or medication and possible adverse events since the previous examination were scored. Biomicroscopy signs evaluated were bulbar and limbal hyperemia, presence of papillae and follicles, epithelial and stromal edema, corneal infiltrates, endothelial regularity, corneal vascularization, anterior segment inflammation and bulbar and corneal staining. After the eyes were rinsed, a new pair of Si-HCLs was inserted and after 60min, the LogMAR VA, CL fit, bulbar and limbal hyperemia, and deposits on the anterior and posterior CL surfaces were evaluated. This pair of Si-HCL was worn for 30 days (at least 5h daily and 5 days weekly) using the assigned MPDS solution (study or control, randomly assigned) according to the oral and written instructions provided.
Examination 3This examination was 15±2 days after examination 2. At this visit the same procedure as in the previous visit was followed. Tests performed at this visit were: subject comfort and satisfaction, LogMAR VA, non-invasive break-up time (NIBUT), CL fit and grade of deposits and biomicroscopy signs.
Examination 4This examination was 30±2 days after examination 3. The same process as examination 3 was performed. The Si-HCLs and MPDSs for this part of the trial were collected, and a new pair of Si-HCLs was inserted. After 30min the LogMAR VA, SI-HCL fit, and bulbar and limbal hyperemia were evaluated. This pair of lenses was worn for 1 week, corresponding to the second washout period. The care solution for this phase was the same hydrogen peroxide system used in the first washout period between examinations 1 and 2.
Examinations 5, 6, and 7The same procedures and same schedule were followed as for examinations 2, 3, and 4, respectively. The subjects received the second randomized study or control solution.
Clinical measurementsThe main safety variable was defined as the percentage of subjects without corneal fluorescein staining or a maximum of grade 1 staining at the end of the month using the study MPDS versus the control MPDS.
To assess the corneal fluorescein staining, 5¿l of 2% sodium fluorescein were instilled in the lower conjunctival cul-de-sac with a micropipette (Finnpipette® 0.5–10¿l; Vantaa, Finland). Two minutes later, the anterior corneal surface was examined by slit-lamp with a 16× magnification lens and the cobalt blue filter and through a Wratten #12 yellow filter. The cornea was evaluated using a grid with five areas (central, temporal, nasal, superior, and inferior). The intensity of the corneal fluorescein staining was evaluated in each area and scored on a scale from 0 to 4.9
The main efficacy variable was defined as the difference in the percentage of subjects without deposits on the anterior or posterior CL surfaces or maximum of grade 2 at the end of the month using the study MPDS versus the control MPDS. Deposits were evaluated by slit-lamp with 20× magnification and white light, evaluating the CL anterior and posterior surfaces while the CL was on the eye, and the deposits were graded on a scale of 0–4.9
Secondary variables evaluated were: subject comfort and satisfaction, CL anterior surface wettability (NIBUT), lens fitting characteristics, and ocular surface health status evaluated by slit-lamp.
Patient comfort and satisfaction were evaluated using a visual analog scale,10 which consisted of a 10-centimeter-long vertical line on which each centimeter was marked with a horizontal line and numbered from bottom to top with 0 to 10. With a horizontal line, the subjects indicated the degree of comfort and satisfaction achieved with their CLs at the time of the evaluation. Scores at the lower end indicated extreme discomfort or extreme dissatisfaction with the CLs; scores at the upper end indicated extreme comfort or extreme satisfaction with the CLs. The evaluation was performed by measuring in millimeters the space from the bottom (0) up to the top (10). User comfort also was analyzed by assessing the use of artificial tears and the hours of daily CL use.
To analyze the CL anterior surface wettability, the NIBUT was measured using TearScope Plus® (Keeler, Windsor, Berkshire, England, UK) by projecting its light grid on the anterior CL surface.11 The subjects were instructed to blink three times and then keep their eyes open until instructed to blink again. The time in seconds that elapsed was recorded from when the subject stopped blinking until the reflected image was deformed, indicating tear film discontinuity. This measurement was performed three times, and the average of the three measurements was recorded.
The characteristics of lens fitting (corneal coverage, position, and mobility) were assessed according to the standardized scale.9 With a slit-lamp it was determined if the CL completely covered the cornea. The scale used to assess the position ranged from 0 to 2, with 0 indicating a suitable and centered position, 1 a slightly off-centered position, and 2 an excessively off-centered position. Regarding mobility, the scale used ranged from −2 to +2, with negative values indicating lack of mobility, 0 suitable mobility, and positive values excessive CL mobility.
Ocular surface health was evaluated at the slit-lamp. All signs were evaluated on a scale of 0 to 4.9
The same experienced practitioner examined all patients.
Adverse ocular events and severe adverse eventsAdverse ocular events were defined as reduction of at least one line of LogMAR VA that could not be improved by the instillation of artificial tears, the presence of corneal edema, corneal infiltrates, corneal vascularization, ulcers, any corneal event resulting in permanent opacity, or any severe adverse ophthalmic event. A severe adverse event was defined as any event resulting in death or threat to the life of the subject, permanent disability, required an extended hospital stay, involved cancer and congenital abnormalities, or was the result of an overdose (administration of a dose higher than prescribed).12
Statistical analysisStatistical analyses were performed using SPSS 18.0 statistical software for Windows (IBM Corporation, Armonk, NY, USA) and R version 3.1.0. The Shapiro–Wilk test was used to check the normality of distribution. p≤0.05 was considered statistically significant.
Quantitative variables were described using means and standard deviations (SD). Qualitative ones were summarized by percentages.
For the primary safety and efficacy variables, the differences in the percentage of subjects were evaluated by equality proportion test for paired groups. The same statistical method was used to compare differences in qualitative variables between control and study groups. Mean differences for quantitative variables were checked by t-test for paired samples or its non-parametric alternative (Wilcoxon test) when the normality assumption could not be assumed.
ResultsTwenty subjects (10 men, 10 women) were included in the study. The average patient age was 27.7±5.6 years (range, 20–41 years). All subjects met the inclusion criteria, and no subjects discontinued the study. All CL were exhibiting good fit throughout the study.
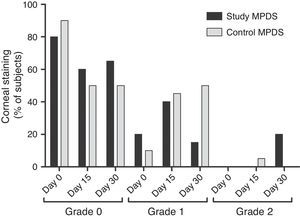
Primary safety variablesMost subjects had fluorescein staining values of grade 0 or a maximum of grade 1 staining, except for one subject with grade 2 staining after 15 days using the control MPDS and four subjects with grade 2 staining after 1 month using the study MPDS (Fig. 2). These differences were not significant. Staining higher than grade 2 did not occur during the study.
By assessing the percentage of subjects with corneal staining exceeding grade 0 with both MPDSs and for the three examinations, we observed a higher percentage after 15 and 30 days of use of the control MPDS (50% in both cases) compared with the test MPDS (40% and 35%, respectively). These differences were not significant.
No adverse events were detected that were either related directly to the study or control MPDS or the CL used, and no severe adverse events developed throughout the study.
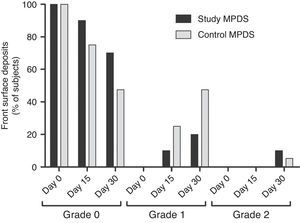
Primary efficacy variableMost subjects had anterior surface deposits that were grade 0 or grade 1 maximally, except for one subject with grade 2 deposits after 1 month using the control MPDS and two subjects with grade 2 deposits after 1 month using the study MPDS (Fig. 3). Regarding posterior surface deposits, one subject had grade 1 deposits after 1 month using the study MPDS. These differences were not significant.
Deposits on the front surface of the hydrogel contact lens (Si-HCL) (primary efficacy variable). The data show the percentage of subjects with deposits on the front surface of the Si-HCL on 0, 15, and 30 days of use of each multipurpose disinfecting solution (MPDS) (study and control). No staining higher than grade 2 occurred throughout the study.
Regarding the percentage of subjects with deposits on the anterior surface exceeding grade 0 with both MPDS and for three examinations, we observed higher percentages after 15 and 30 days of use of the control MPDS (25% and 52.63%, respectively) than when using the study MPDS (10% and 26.32%, respectively). These differences were not significant.
Secondary variablesSubject comfort and satisfaction were assessed after 15 and 30 days of use of both MPDSs. We observed higher average values of comfort and satisfaction for the study MPDS than for the control MPDS throughout the study; however, these differences were not significant (Fig. 4).
No subject reported the need for artificial tears while wearing the CLs throughout the study. No differences were found in the hours or days of CL use between the MPDSs (hours of use/week, study MPDS, 50.3±14.93; control MPDS, 58.16±12.33, p=0.2453).
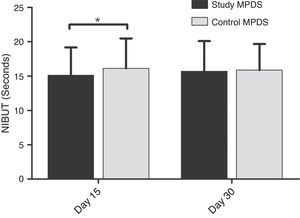
Higher NIBUT values for the control MPDS were observed after 15 and 30 days, with a significant (p=0.0481) difference at 15 days and a difference between the MPDSs of 1.62±3.436seconds. This difference decreased after 30 days and was no longer significant (0.75±3.54seconds; p=0.3934) (Fig. 5).
Anterior surface wettability (NIBUT) of the hydrogel contact lenses with both multipurpose disinfecting solutions (MPDSs) (study and control). The data show the average NIBUT values on days 15 and 30 for each MPDS. The error bars represent the standard deviations. * indicates a significant (p<0.05) difference between the control and study MPDSs on day 15.
Epithelial edema, infiltrates, endothelial regularity, follicles and anterior segment inflammation did not change throughout the study; all subjects had grade 0 at all examinations.
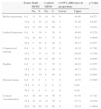
Analysis of the data obtained for bulbar hyperemia, limbal hyperemia, conjunctival staining, papillae, stromal edema, and corneal vascularization showed no significant differences in any variables at any examinations between the MPDSs (Table 2). Nevertheless, we observed trends in the percentage of subjects with values exceeding grade 0. We observed fewer subjects with conjunctival (bulbar) hyperemia and staining higher than grade 0 after 15 and 30 days with the study MPDS than with the control MPDS. Regarding limbal hyperemia, the number of subjects with a score higher than grade 0 after 15 days using the study MPDS tended to increase, a trend that reversed after 30 days. The trend toward an increase was maintained throughout the examinations for the control MPDS.
Subjects with biomicroscopy signs exceeding grade 0 during the study.
| Exam | Study MPDS | Control MPDS | CI 95% difference in proportions | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | Lower | Upper | |||
| Bulbar hyperemia | 0 d | 11 | 55 | 10 | 50 | 0 | 49.86 | 0.8271 |
| 15 d | 11 | 55 | 12 | 60 | 0 | 51.95 | 0.8347 | |
| 30 d | 12 | 60 | 16 | 80 | 0 | 71.11 | 0.4431 | |
| Limbal hyperemia | 0 d | 6 | 30 | 4 | 20 | 0 | 40.68 | 0.5229 |
| 15 d | 10 | 50 | 7 | 35 | 0 | 54.87 | 0.4609 | |
| 30 d | 5 | 25 | 8 | 40 | 0 | 49.72 | 0.3971 | |
| Conjunctival staining | 0 d | 4 | 20 | 5 | 25 | 0 | 34.32 | 0.7382 |
| 15 d | 7 | 35 | 11 | 55 | 0 | 60.64 | 0.3348 | |
| 30 d | 10 | 50 | 12 | 60 | 0 | 55.76 | 0.6684 | |
| Papillae | 0 d | 4 | 20 | 7 | 35 | 0 | 46.83 | 0.3557 |
| 15 d | 5 | 25 | 8 | 40 | 0 | 49.72 | 0.3971 | |
| 30 d | 6 | 30 | 8 | 40 | 0 | 46.41 | 0.5903 | |
| Stromal edema | 0 d | 0 | 0 | 1 | 5 | 0 | 14.55 | 0.3049 |
| 15 d | 0 | 0 | 0 | 0 | 0 | 0.00 | ||
| 30 d | 0 | 0 | 0 | 0 | 0 | 0.00 | ||
| Corneal vascularization | 0 d | 16 | 80 | 14 | 70 | 0 | 63.50 | 0.7141 |
| 15 d | 15 | 75 | 14 | 70 | 0 | 57.73 | 0.8526 | |
| 30 d | 16 | 80 | 16 | 80 | 0 | 55.44 | 1.0000 | |
MPDS: multipurpose disinfecting solution; CI: confidence interval; Exam: examination; No.: number of subjects who met the criteria; %: percentage of subjects who met the criteria. 0 d: day 0, 1 hour of Si-HCL use (examination 2 or 5); 15 d: 15 days of Si-HCL use (examination 3 or 6); 30 d: 30 days of Si-HCL use (examination 4 or 7).
To guarantee the safety and efficacy of new CL care solutions before they become commercially available it is necessary to perform a clinical trial. The main purpose of this paper is to show the results of this clinical trial as is required by the clinical trials regulations.
Aloe vera is well known for its protection effect on the epithelium against potentially toxic substances.13 It also has bactericidal and bacteriostatic properties and can absorb ultraviolet light (UV) radiation.14 These properties are ideal as components of a solution for cleaning and maintaining CLs. Aloe vera is also a component that is added in different ophthalmic formulations.6,13–16
The effect of aloe vera on ocular health has been studied because of its humectant properties. Burgalassi et al.15 concluded that ophthalmic solutions containing aloe vera were suitable to relieve dry eye symptoms. Ji and Jia16 found in an in vitro experiment that by incorporating aloin and polysaccharides (found in aloe vera extracts) into a binary solution, eyes were protected from bacterial infection and UV radiation. This implies that aloe vera may reduce the toxic effect of irritants, thereby preserving the viability of corneal epithelium cells against potential long-term toxicity caused by continuous exposure of the ocular surface to MPDSs. This effect is highest in aloe vera concentrations close to 1% (Avizor S.A.).
This is a crossover study in which each subject used two MPDSs (study and control). Each solution was used for 1 month with the same type of Si-HCL (Comfilcon A). Only one material was used to avoid interference resulting from changes in material, and this guaranteed that all differences were related to the MPDSs. Also, we compared the average hours of weekly use of the Si-HCLs with each of the MPDSs evaluated. Small differences were seen that were possibly related to different habits of each subject. In any case, these differences were not significant, and this factor did not affect the results obtained for the others variables studied.
The new MPDS was not associated with adverse ocular effects for any of the safety variables included in the ISO 11980:2010 guidance for clinical investigation recommendations.9 No significant differences were detected between the study MPDS and the control MPDS, which confirmed the safety of the new MPDS.
No significant differences were found between both solutions in the evaluation of lens performance and acceptance. Results showed no interference in the cleaning ability of the MPDS when aloe vera was included in the formulation. The higher percentage of subjects with deposits exceeding grade 0 on the front and back surfaces of the CLs after 15 and 30 days of use of the control MPDS could have been due to a more beneficial effect of the study MPDS. The increased deposits found between days 15 and 30 for both solutions could have been due to deposits over the CL surface that increased with time of wear and CL age. These results agreed with previous reports.17–19
In the current study, no differences in symptoms were found between the two MPDSs. Higher NIBUT values for the control MPDS was observed at 15 days, but these differences may not be considered clinically relevant, and disappeared at 30 days of use. No differences were found in the ocular surface slit-lamp evaluation, indicating that both MPDSs had the same effect on the ocular surface. No variations were found throughout the study in the fitting characteristics indicating that the study MPDS did not alter CLs parameters. No significant differences in comfort or satisfaction were found between the MPDSs; however, the study MPDS obtained higher averages than the control MPDS for both parameters on days 15 and 30 of the study.
This study has some limitations like the small sample size, although according to the statistical calculation. Furthermore, some items, like the mechanism of action of aloe vera or the advantages of adding aloe vera in the MPDSs, cannot be discussed using the results of this clinical trial cause it has been designed exclusively to study the security and efficiency of the new MPDS. For that reason more studies to prove the advantages of adding aloe vera in the MPDS are warranted.
In conclusion, the study MPDS is safe, has an acceptable physiologic tolerance, and is efficient according to the ISO 11980:2010 guidance for clinical investigation.9
Conflicts of interestRubén Urbano-Rodríguez works for the company that has supported the study: Avizor. The rest of the authors don’t have any conflicts of interest to declare.
This study was supported financially by Avizor S.A. (Spain). This randomized controlled trial was approved by the Spanish National Health Department (Drugs National Agency – AEMPS) with EUDRA number 2011-000604-18.