To compare the efficacy of epithelium-off corneal collagen crosslinking (CXL) with transepithelial CXL in patients with progressive keratoconus with a follow-up of 3 years, taking into account the patients’ age and the location of the corneal ectasia.
MethodsIn this prospective study participated 64 eyes with progressive keratoconus were included in this long-term study, of which 31 eyes were treated by epithelium-off CXL and 33 by transepithelial CXL. All of the patients with a follow-up of 36 months were evaluated for visual variables (corrected distance visual acuity (CDVA), corneal aberrations, and corneal densitometry), structure variables (astigmatism, keratometry, corneal asphericity, maximum posterior elevation, corneal thickness, and corneal volume), and keratoconus index variables.
ResultsAfter corneal CXL, CDVA improved significantly in both central and paracentral keratoconus, with greater improvement in the centrals (p = 0.001), asphericity at 6 mm improved in central keratoconus (p = 0.047). In the epi-off group, there was a significant improvement in coma-like (p = 0.038), higher-order aberrations (p = 0.036), asphericity at 8 mm (p = 0.049), asphericity at 10 mm (p = 0.049), and index of surface variance (p = 0.049).
ConclusionAlthough both techniques halted and stabilized the progression of keratoconus, epithelium-off CXL was more effective. In addition, after the corneal CXL, there was a greater degree of regularization of the corneal surface and, therefore, a greater improvement in the CDVA with central keratoconus than with paracentral keratoconus.
Keratoconus (KC) is a bilateral and generally asymmetric ectopic corneal disorder that induces a progressive thinning of the cornea that gives rise to its protrusion. This results in irregular astigmatism and, in the late stages, corneal fibrosis with substantial visual impairment. It is usually diagnosed during puberty, with a very variable and unpredictable progression rate between patients, progressing for up two decades following presentation.1
Currently, non-surgical treatments for KC range from corrective glasses for the most incipient grades, to contact lenses for moderate and advanced stages.2 There are, however, other therapeutic options for progressive KC, either involving the addition of tissue, as is the case with intrastromal ring implants, or photochemical treatments such as corneal collagen cross-linking (CXL) with riboflavin (RF) and ultraviolet A (UVA) radiation. The main objective of the latter treatment option is to halt or reduce the progression of ectasia, thereby avoiding or delaying the need for a corneal transplant.3
In 2003, Wollensak et al. introduced corneal CXL, which is one of the most promising procedures for halting progression of this disease, as it strengthens the cornea by increasing the cross-linking of collagen fibers, which in turn increases corneal rigidity, thereby reducing the morbidity of the disease and decreasing or delaying the need for a corneal transplant.3
Standard corneal cross-linking or epithelium-off CXL (epi-off CXL) requires removal of the corneal epithelium prior to impregnation of the cornea with RF and its irradiation for 30 min with ultraviolet A light (UV-A). Numerous studies have documented its safety and efficiency,4 and there have been publications reporting outcomes using this technique at more than 10 years of follow-up.5
However, this technique includes removal of the epithelium, which can result in postoperative pain and act as a potential source of complications (e.g., infectious keratitis, sterile infiltrates, and corneal haze).6
With the introduction of transepithelial corneal cross-linking (TE-CXL), removal of the corneal epithelium can be avoided and, therefore, also the associated inconveniences and complications. There has been an increasing number of published studies evaluating the therapeutic effects of TE-CXL. Recent studies have suggested that TE-CXL has ample potential to slow the progression of KC,7 and studies have compared both techniques in terms of the effects of the treatment.8–12
However, there have been few publications to date that compared both techniques taking into account the location of the cone. The initial corneal shape may be important for the final effect of the treatment since CXL increases the stability of the corneal structure and modifies the intrinsic biomechanical properties of corneal collagen.
Our work sought to compare both treatments after 3 years of follow-up, taking into account the patients’ age and the location of the corneal ectasia.
Materials and methodsThis was a comparative prospective study. Both the epi-off CXL and the TE-CXL collagen corneal cross-linking technique were evaluated. The patients were recruited at the Novovision Clinic (Madrid, Spain) and treated by the same medical team using the same surgical and post-surgical protocol. Before starting the study, the risks and benefits of the treatment were explained to the patients, and informed consent was obtained from all of the subjects. The patients were free to leave the study at any time. The study was conducted in compliance with good clinical practice guidelines, the institutional review board regulations, and the tenets of the Declaration of Helsinki revised in 2013.13 Moreover, the study was approved by the ethics committee of the Novovision Clinic.
I- Preoperative:
The following inclusion criteria were taken into account: all of the patients exhibited progressive KC according to topographic data (with progression defined as an increase of ≥ 1 diopter (D) in the manifest cylinder and/or an increase of ≥ 1 D in the maximum keratometry observed in three consecutive corneal topographies in the six months prior to the study8) that were grade I and II according to the Amsler-Krumeich classification (central keratometry less than 53 D, pachymetry greater than 400 μm, and myopia and/or astigmatism of 8 D or less1) and all of the patients were older than 12 years of age. This study also excluded patients with collagen or autoimmune diseases, severe atopy, or other ocular diseases, as well as individuals who were pregnant or lactating.
Patients who used contact lenses were instructed to discontinue their use 7–10 days prior to treatment. The location of the cone was taken into account, including patients with central and paracentral KC.
We classified the KC as being central if the distance between the point of least corneal thickness and the center of the cornea was 0.7 mm or less, while the KC was classified as paracentral if that distance was between 0.7 and 2.1 mm.14
Patients who met the inclusion criteria were assigned to one of the two treatment groups, i.e., the epi-off CXL or the TE-CXL group, in a consecutive manner starting with the TE-CXL until the required sample was reached. II- Intraoperative:
With both techniques, the same UVA emitter device, namely a CBM X-linker (CSO, Florence, Italy) was used with all of the patients.
With the epi-off CXL technique, the central 8 mm of corneal epithelium was removed with a scarifier. Drops of 0.1% RF solution were then instilled with 20% dextran (Ricrolin®, Florence, Italy) every 5 min for a total duration of 30 min to saturate the corneal stroma. Using a slit lamp, yellow-colored staining could then be seen throughout the corneal layer and in the anterior chamber. A UVA light source, at an intensity of 3 mW/cm2 and a wavelength of 370 nm (corresponding to the maximum absorption of the RF) was then applied over the course of 30 min until a total radiation exposure of 5.4 J/cm2 was achieved.3,4 During the treatment, the RF was instilled every 5 min and a topical anesthetic was administered every 15 min. At the end of the surgical procedure, a single drop of tobramycin and dexamethasone (Tobradex®, Barcelona, Spain) and a neutral therapeutic contact lens were applied.
For the TE-CXL technique, the procedure was performed in a similar manner as the CXL epi-off technique, although without removal of the corneal epithelium, using a TE-RF (Ricrolin TE®) composed of RF 0.1%, 15% dextran, trometamol, and ethylenediaminetetraacetic acid (EDTA). The remainder of the process was carried out in the same manner, and there was no need for a therapeutic contact lens.
III- Postoperative:
As postoperative treatment we recommended Tobramycin/Dexamethasone 1 mg/mL+3 mg/mL eye drops (tobradex®, Barcelona, Spain) every 6 h for one week and then Fluorometholone 0.1% eye drops (FML®, Madrid Spain) for 1 month in a de-escalating dose.
The two treatment groups were compared over time (6, 12, 24, and 36 months) taking into account the age of the patients and the location of the KC (central or paracentral). In all of the follow-up visits, an ophthalmological examination was performed, including assessment of the corrected distance visual acuity (CDVA), topography, and corneal aberrometry. The data analyzed in this work were obtained using a Pentacam (Oculus, Wetzlar, Germany) corneal topographer.
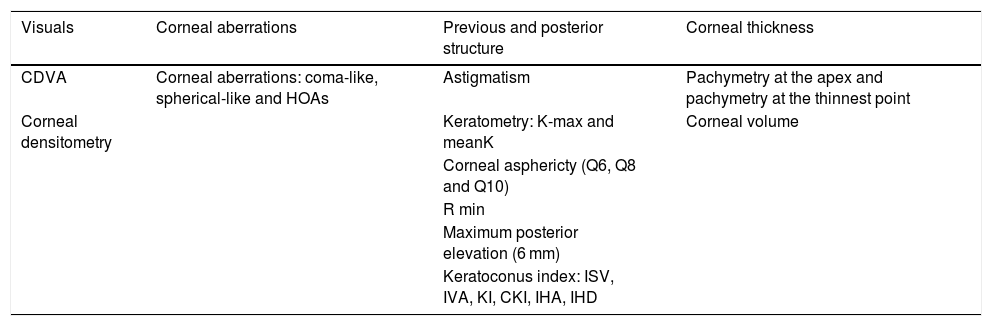
The variables evaluated in this study were divided into three groups, namely the visual quality, the corneal structure, and the KC index visuals variables, corneal aberrations, previous and posterior structure and corneal thickness (Table 1)
Variables and keratoconus index included in the study.
| Visuals | Corneal aberrations | Previous and posterior structure | Corneal thickness |
|---|---|---|---|
| CDVA | Corneal aberrations: coma-like, spherical-like and HOAs | Astigmatism | Pachymetry at the apex and pachymetry at the thinnest point |
| Corneal densitometry | Keratometry: K-max and meanK | Corneal volume | |
| Corneal asphericty (Q6, Q8 and Q10) | |||
| R min | |||
| Maximum posterior elevation (6 mm) | |||
| Keratoconus index: ISV, IVA, KI, CKI, IHA, IHD |
CDVA: corrected distance visual acuity; HOAs: higher-order aberrations; K-max: maximal keratometry; mean K: mean keratometry; Q6, Q8, Q10: asphericity at 6, 8, and 10 mm; ISV: index of surface variance; IVA: index of vertical asymmetry; KI: keratoconus index; CKI: central keratoconus index; IHA: index of height asymmetry; IHD: index of height decentration; R min: minimum radius of curvature.
The statistical analysis was performed using SPSS Statistics 23 software (IBM, Chicago, Illinois, USA). A descriptive analysis was carried out for all of the study variables, thus yielding frequency tables for the categorical variables (gender, eye, etc.) and tables with the descriptors of the continuous variables, which are presented as means and the standard deviation, and ranges. In all cases, the Kolmogorov-Smirnov normality test and the homogeneity test were carried out to determine the statistical approach to be used in each case.
To calculate the sample size, the same procedure was followed as in the CXL study performed by Raiskup-Wolf et al.15 which was obtained by the difference between the expected average change of the maximum K after treatment (estimated at -1.46 D) and the acceptable average change of the maximum K value after treatment (estimated by Koller et al16 as +1 D). With this difference of -2.46 D, and taking into account a standard deviation of 3.7615 the sample size necessary for an alpha of 0.05 and a beta of 0.2 is 29 eyes in each group17 Taking into account a possible dropout rate during the study of 10%, the total number of eyes needed was estimated to be 32 per group.
With each variable, a repeated measures ANOVA (variance analysis) was performed to study the differences between both groups (epi-off CXL/TE-CXL) over time (6, 12, 24, and 36 months) according to the location of the KC cone (central or paracentral) and age as main effects, as well as all double interactions. A statistical significance of 0.05 was established (p < 0.05). The results are presented as means ± the standard deviation.
ResultsThe study included 64 eyes from 46 patients with progressive Amsler-Krumeich grade I and II KC, of which 31 eyes were treated with epi-off CXL and 33 with TE-CXL, with a mean age of 19.90 ± 5.7 years (17.82−21.99 years) and 25.33 ± 6.8 years (22.92–27.75 years), respectively.
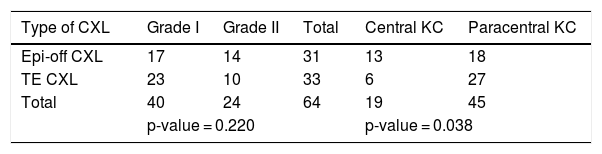
Table 2 shows the patient distribution according to the surgical technique, the degree of KC, and the location of the ectasia.
The number of eyes treated with CXL epi-off versus CXL TE according to the degree and the location of the KC.
| Type of CXL | Grade I | Grade II | Total | Central KC | Paracentral KC |
|---|---|---|---|---|---|
| Epi-off CXL | 17 | 14 | 31 | 13 | 18 |
| TE CXL | 23 | 10 | 33 | 6 | 27 |
| Total | 40 | 24 | 64 | 19 | 45 |
| p-value = 0.220 | p-value = 0.038 | ||||
CXL: corneal crosslinking; KC: keratoconus.
Chi-square test results of Grade I/II between two groups of treatment, p-value = 0.220.
Chi-square test results of central/paracentral kc between two groups of treatment, p-value =.
0.038.
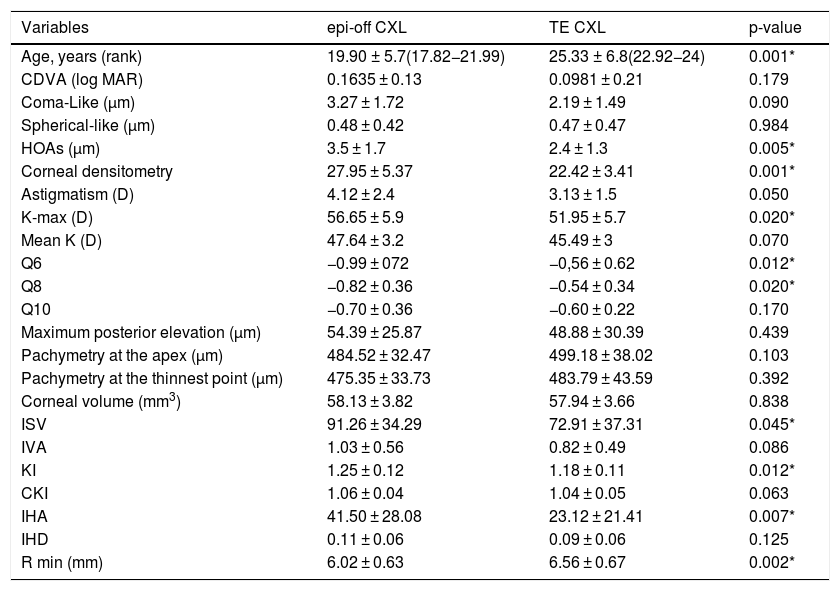
The baseline characteristics of each of the variables to be studied in both treatment groups and their degree of homogeneity are detailed in Table 3.
Baseline characteristics of the study groups.
| Variables | epi-off CXL | TE CXL | p-value |
|---|---|---|---|
| Age, years (rank) | 19.90 ± 5.7(17.82−21.99) | 25.33 ± 6.8(22.92−24) | 0.001* |
| CDVA (log MAR) | 0.1635 ± 0.13 | 0.0981 ± 0.21 | 0.179 |
| Coma-Like (µm) | 3.27 ± 1.72 | 2.19 ± 1.49 | 0.090 |
| Spherical-like (µm) | 0.48 ± 0.42 | 0.47 ± 0.47 | 0.984 |
| HOAs (µm) | 3.5 ± 1.7 | 2.4 ± 1.3 | 0.005* |
| Corneal densitometry | 27.95 ± 5.37 | 22.42 ± 3.41 | 0.001* |
| Astigmatism (D) | 4.12 ± 2.4 | 3.13 ± 1.5 | 0.050 |
| K-max (D) | 56.65 ± 5.9 | 51.95 ± 5.7 | 0.020* |
| Mean K (D) | 47.64 ± 3.2 | 45.49 ± 3 | 0.070 |
| Q6 | −0.99 ± 072 | −0,56 ± 0.62 | 0.012* |
| Q8 | −0.82 ± 0.36 | −0.54 ± 0.34 | 0.020* |
| Q10 | −0.70 ± 0.36 | −0.60 ± 0.22 | 0.170 |
| Maximum posterior elevation (µm) | 54.39 ± 25.87 | 48.88 ± 30.39 | 0.439 |
| Pachymetry at the apex (µm) | 484.52 ± 32.47 | 499.18 ± 38.02 | 0.103 |
| Pachymetry at the thinnest point (µm) | 475.35 ± 33.73 | 483.79 ± 43.59 | 0.392 |
| Corneal volume (mm3) | 58.13 ± 3.82 | 57.94 ± 3.66 | 0.838 |
| ISV | 91.26 ± 34.29 | 72.91 ± 37.31 | 0.045* |
| IVA | 1.03 ± 0.56 | 0.82 ± 0.49 | 0.086 |
| KI | 1.25 ± 0.12 | 1.18 ± 0.11 | 0.012* |
| CKI | 1.06 ± 0.04 | 1.04 ± 0.05 | 0.063 |
| IHA | 41.50 ± 28.08 | 23.12 ± 21.41 | 0.007* |
| IHD | 0.11 ± 0.06 | 0.09 ± 0.06 | 0.125 |
| R min (mm) | 6.02 ± 0.63 | 6.56 ± 0.67 | 0.002* |
CDVA: corrected distance visual acuity; HOAs: higher-order aberrations; K-max: maximal keratometry; mean K: mean keratometry; Q6, Q8, Q10: asphericity at 6 mm,8 mm and 10 mm; ISV: index of surface variance; IVA: index of vertical asymmetry; KI: keratoconus index; CKI: central keratoconus index; IHA: index of height asymmetry; IHD: index of height decentration; R min: minimum radius of curvature. * p < 0.05; one-way ANOVA for independent measure.
The groups were not homogeneous in terms of age or the maximum K value, as the patients in the group being treated with epi-off CXL tended to be younger and have more advanced KC. Since age is an important factor that needs to be taken into account with this pathology, it was included as a covariate in the statistical method.
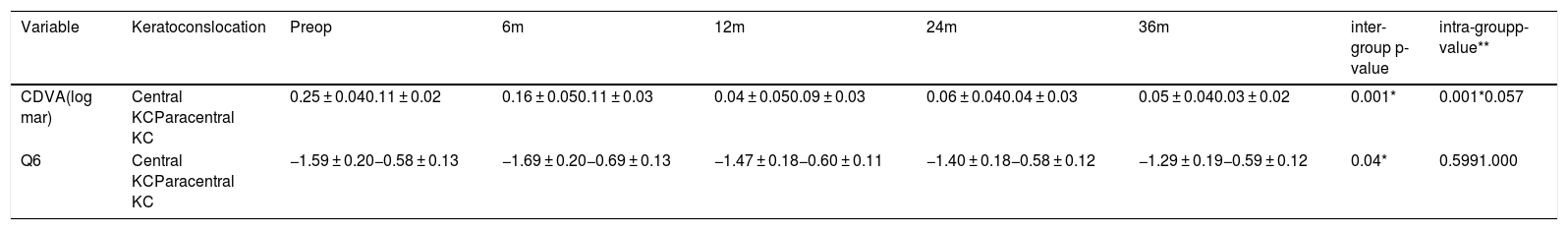
After the collagen corneal CXL procedure, significant changes in the corrected distance visual acuity (CDVA) and asphericity at 6 mm (Q6) were found over time that depended on the location of the cone. The CDVA improved both for those with a central KC (mean of -0.2 log MAR) and with a paracentral KC (mean of -0.07 log MAR) (p-value < 0.05; one-way ANOVA for repeated measures). The asphericity at 6 mm improved for those with a central KC, (p-value < 0.05; one-way ANOVA for repeated measures), while it remained stable with a paracentral KC (Table 4)
The values for the CDVA and Q6 after the corneal CXL taking into account the location of the cone over time.
| Variable | Keratoconslocation | Preop | 6m | 12m | 24m | 36m | inter-group p-value | intra-groupp-value** |
|---|---|---|---|---|---|---|---|---|
| CDVA(log mar) | Central KCParacentral KC | 0.25 ± 0.040.11 ± 0.02 | 0.16 ± 0.050.11 ± 0.03 | 0.04 ± 0.050.09 ± 0.03 | 0.06 ± 0.040.04 ± 0.03 | 0.05 ± 0.040.03 ± 0.02 | 0.001* | 0.001*0.057 |
| Q6 | Central KCParacentral KC | −1.59 ± 0.20−0.58 ± 0.13 | −1.69 ± 0.20−0.69 ± 0.13 | −1.47 ± 0.18−0.60 ± 0.11 | −1.40 ± 0.18−0.58 ± 0.12 | −1.29 ± 0.19−0.59 ± 0.12 | 0.04* | 0.5991.000 |
CDVA: corrected distance visual acuity; Q6: asphericity at 6 mm; p: statistical change at month 36 compared with preoperative data; one-way ANOVA for repeated measures.
Inter-group p-value (preoperative inter-group difference: 36 months).
intra-group p-value ** (preoperative intra-group difference: 36 months).
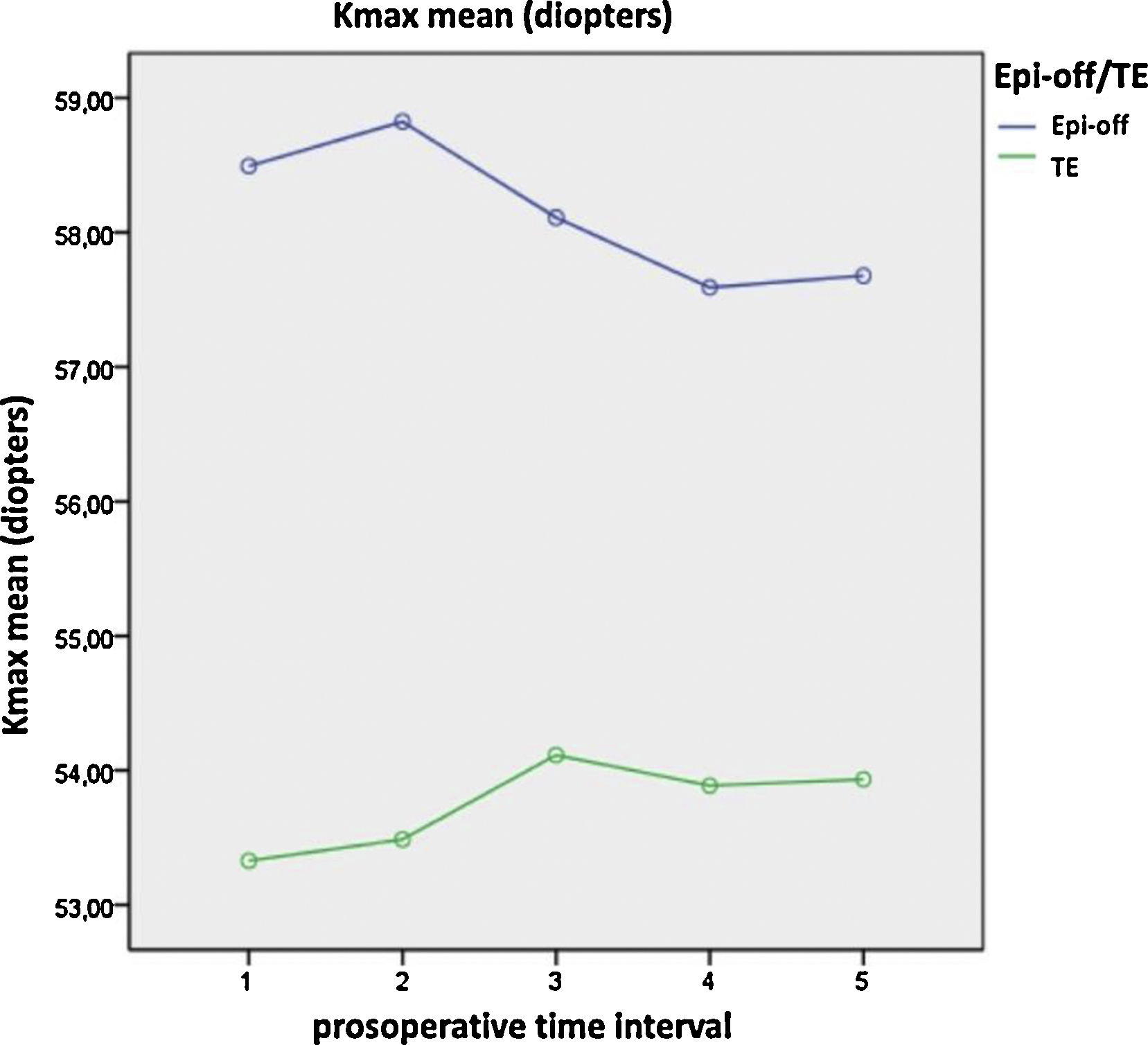
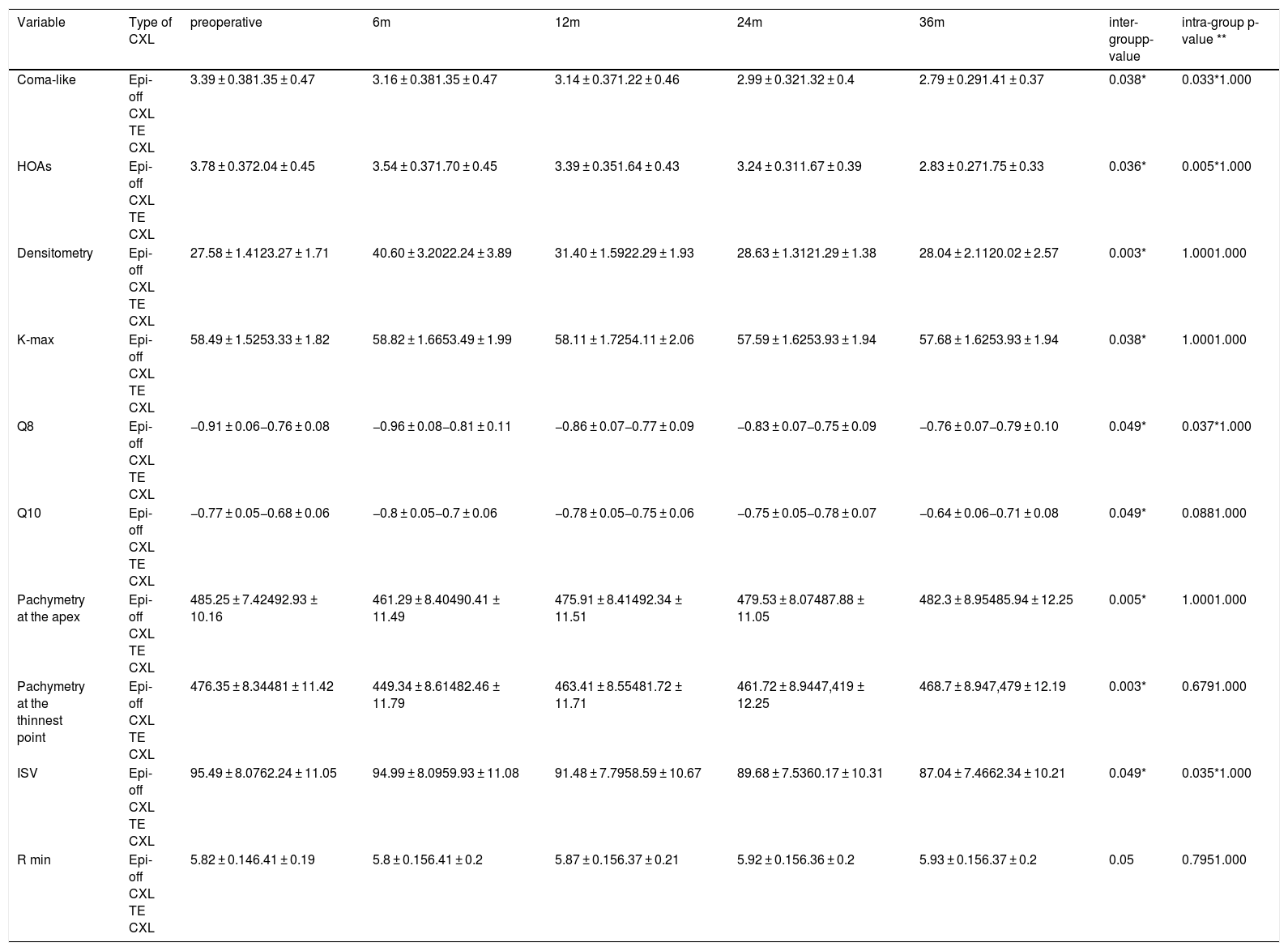
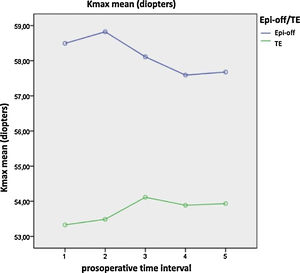
Significant differences were found between both treatments over time in terms of the coma-like aberration, higher-order aberrations (HOAs), densitometry, maximum K (Fig. 1), asphericity at 8 mm and 10 mm (Q8, Q10), pachymetry at the apex, pachymetry at the thinnest point, index of surface variance (ISV), and the minimum radius of curvature (R min) (p-value < 0.05; one-way ANOVA for repeated measures). After epi-off CXL, there was improvement in the coma-like aberration, HOAs, Q8, Q10, and ISV values, while after TE-CXL, these variables remained stable. Additionally, the changes in densitometry and corneal pachymetry were significant after epi-off CXL, remaining stable after TE-CXL, and the variables of maximum K and the anterior minimum radius remained stable with both treatments (Table 5).
The values for coma-like, HOAs, densitometry, K-max, Q8, Q10, pachymetry at the apex, pachymetry at the thinnest point, ISV, and anterior minimum radius taking into account both techniques over time.
| Variable | Type of CXL | preoperative | 6m | 12m | 24m | 36m | inter-groupp-value | intra-group p-value ** |
|---|---|---|---|---|---|---|---|---|
| Coma-like | Epi-off CXL TE CXL | 3.39 ± 0.381.35 ± 0.47 | 3.16 ± 0.381.35 ± 0.47 | 3.14 ± 0.371.22 ± 0.46 | 2.99 ± 0.321.32 ± 0.4 | 2.79 ± 0.291.41 ± 0.37 | 0.038* | 0.033*1.000 |
| HOAs | Epi-off CXL TE CXL | 3.78 ± 0.372.04 ± 0.45 | 3.54 ± 0.371.70 ± 0.45 | 3.39 ± 0.351.64 ± 0.43 | 3.24 ± 0.311.67 ± 0.39 | 2.83 ± 0.271.75 ± 0.33 | 0.036* | 0.005*1.000 |
| Densitometry | Epi-off CXL TE CXL | 27.58 ± 1.4123.27 ± 1.71 | 40.60 ± 3.2022.24 ± 3.89 | 31.40 ± 1.5922.29 ± 1.93 | 28.63 ± 1.3121.29 ± 1.38 | 28.04 ± 2.1120.02 ± 2.57 | 0.003* | 1.0001.000 |
| K-max | Epi-off CXL TE CXL | 58.49 ± 1.5253.33 ± 1.82 | 58.82 ± 1.6653.49 ± 1.99 | 58.11 ± 1.7254.11 ± 2.06 | 57.59 ± 1.6253.93 ± 1.94 | 57.68 ± 1.6253.93 ± 1.94 | 0.038* | 1.0001.000 |
| Q8 | Epi-off CXL TE CXL | −0.91 ± 0.06−0.76 ± 0.08 | −0.96 ± 0.08−0.81 ± 0.11 | −0.86 ± 0.07−0.77 ± 0.09 | −0.83 ± 0.07−0.75 ± 0.09 | −0.76 ± 0.07−0.79 ± 0.10 | 0.049* | 0.037*1.000 |
| Q10 | Epi-off CXL TE CXL | −0.77 ± 0.05−0.68 ± 0.06 | −0.8 ± 0.05−0.7 ± 0.06 | −0.78 ± 0.05−0.75 ± 0.06 | −0.75 ± 0.05−0.78 ± 0.07 | −0.64 ± 0.06−0.71 ± 0.08 | 0.049* | 0.0881.000 |
| Pachymetry at the apex | Epi-off CXL TE CXL | 485.25 ± 7.42492.93 ± 10.16 | 461.29 ± 8.40490.41 ± 11.49 | 475.91 ± 8.41492.34 ± 11.51 | 479.53 ± 8.07487.88 ± 11.05 | 482.3 ± 8.95485.94 ± 12.25 | 0.005* | 1.0001.000 |
| Pachymetry at the thinnest point | Epi-off CXL TE CXL | 476.35 ± 8.34481 ± 11.42 | 449.34 ± 8.61482.46 ± 11.79 | 463.41 ± 8.55481.72 ± 11.71 | 461.72 ± 8.9447,419 ± 12.25 | 468.7 ± 8.947,479 ± 12.19 | 0.003* | 0.6791.000 |
| ISV | Epi-off CXL TE CXL | 95.49 ± 8.0762.24 ± 11.05 | 94.99 ± 8.0959.93 ± 11.08 | 91.48 ± 7.7958.59 ± 10.67 | 89.68 ± 7.5360.17 ± 10.31 | 87.04 ± 7.4662.34 ± 10.21 | 0.049* | 0.035*1.000 |
| R min | Epi-off CXL TE CXL | 5.82 ± 0.146.41 ± 0.19 | 5.8 ± 0.156.41 ± 0.2 | 5.87 ± 0.156.37 ± 0.21 | 5.92 ± 0.156.36 ± 0.2 | 5.93 ± 0.156.37 ± 0.2 | 0.05 | 0.7951.000 |
HOAs: higher-order aberrations; K-max: maximal keratometry; Q8, Q10: asphericity at 8 mm,10 mm; ISV: index of surface variance; R min: minimum radius of curvature; P: statistical change at month 36 compared with preoperative data. One-way ANOVA for repeated measures.
Inter-group p-value (preoperative inter-group difference: 36 months).
Intra-group p-value ** (preoperative intra-group difference: 36 months).
We did not have any complications with any of the techniques.
DiscussionSince Wollesak introduced the crosslinking of corneal collagen (CXL) in the treatment of QC in 2003,3 it has been shown to be effective in slowing down or stopping the progression of QC.4,5 However, the removal of the corneal epithelium remains controversial.6,17,18 On the other hand, TE-CXL is developed to avoid the adverse effects caused by the removal of the corneal epithelium.7 And in recent years, several clinical trials have been designed to compare the therapeutic effects between the two methods.8–12
Our study sought to compare both treatments over a three-year period, taking into account the age of the patients and the location of the corneal ectasia.
One of the drawbacks of our study was the lack of homogeneity in the groups of patients treated with the different surgical techniques, since the baseline characteristics of patients treated with TE-CXL comprised less advanced KC than was the case for patients treated with epi-off CXL. Certain variables present clinically different values in the preoperative period. This is justified by the allocation criteria for each epi-off CXL or TE-CXL group. As this was not a clinical trial, the study was not randomized and it followed the protocol for the assigned allocation of the private clinic in which the study was conducted. As can be seen in the tables, the values of the variables that exhibited significant differences in the preoperative period were higher than those performed with epi-off CXL. This indicates a greater degree of development of keratoconus in those undergoing this procedure.
Age did not influence the variables studied, except for the index of height asymmetry (IHA).
Both of the surgical techniques resulted in stabilization of the CDVA during the 36-month follow-up, which is similar to the results obtained in a number of other studies,19 although most of the comparative studies to date have reported improvement with both techniques.8–12
CDVA improved in patients with central and paracentral KC treated with corneal CXL, with significant differences between them over time, with a greater increase in central KC.
The two most significant indicators of improvement in vision after corneal CXL were the preoperative values of low CDVA (≤ 20/40) and high K-max values (≥ 55 D).1,8,20,21 In the current study, the central KCs exhibited lower average values for the preoperative CDVA, with higher K-max values (0.25 ± 0.04 log MAR and 56.6 D) compared to paracentral KC (0.1 ± 0.2 log MAR and 53.84 D), which could explain these results. The greater improvement in the CDVA in the central cones is in accordance with what has been published by other authors,22,23 and this may be due to the fact that the central area is closer to the radiation of the CXL, so the intensity of radiation is higher than in the paracentral region, plus the depth does not appear to be homogeneous within the treatment area, exhibiting a decrease towards the periphery of the cornea.24
The coma-like and HOA values remained stable after TE-CXL, while there was a significant improvement after epi-off CXL despiste the fact that patients treated with epi-off CXL had a more advanced KC. This may be explained by a greater degree of regularization of the corneal surface due to more compaction of the collagen fibers following this technique. The spherical aberration and coma values remained stable with both techniques in the comparative study by Rossi et al.25 The random controlled trial by Stojanovic et al.26 did find significant changes in HOAs during the 12-month follow-up in all of the groups and between them.
The densitometry readings remained stable after TE-CXL, while after epi-off CXL there was a significant increase after 6 months, going from an initial average of 27.6 ± 1.4–40.6 ± 3.2, only to decrease at each of the subsequent checkup examinations, with no significant change with respect to the preoperative values from 12 months to the end of the study. Similar results were obtained by Greenstein et al.6 The increases may be associated with the depth of CXL in the stroma, as well as with the amount of keratocyte loss, activated keratocytes, inflammation of the stroma due to changes in pressure, interactions of proteoglycans-collagen, hydration of glycosaminoglycans, and an increase in the diameter of collagen fibers of 12.2% (3.96 nm).27
The K-max is an indicator of the stability of the KC after CXL. Progression of the KC is generally defined as an increase in the K-max of 1 D or more at 6,8 12,28 or 2429 months. With both techniques, the K-max values remained stable, thus halting further KC progression and taking into account that the patients with more advanced KC were treated with epi-off CXL, which is similar to what was noted by Stojanovic et al.26 and Cifariello et al.11 Most of the studies to date have reported a decrease or stabilization of the K-max with both techniques.10,19,25 However, Kocak et al.30 obtained an improvement in the K-max after epi-off CXL and a worsening after TE-CXL.
One simple method to quantitatively describe the corneal shape is the corneal asphericity. The Q6 values of the central KC became less negative and the Q8 and Q10 values became less negative after epi-off CXL in this study. Therefore, epi-off CXL regularized the corneal surface more than TE-CXL, and it also improved more in central KC than in paracentral KC. This improvement in the Q values following CXL may be due to compaction of the collagen fibers that renders the cornea surface more regular. Sedaghat et al.31 reported stabilization of corneal asphericity values following epi-off CXL after 12 months of follow-up.
The groups were homogeneous both in terms of pachymetry at the corneal apex and at the thinnest point, decreasing in the first few months and then increasing from 12 months to the end of the study following epi-off CXL while remaining stable after TE- CXL. This is similar to what has been found in a number of studies,6although other publications,19,25 have reported stability with both techniques. The reduction in corneal thickness following epi-off CXL could be a consequence of several factors such as collagen fiber compaction, stromal dehydration, cell death due to apoptosis of keratocytes after exposure to UVA light, alterations in the scarring and the epithelial distribution, and its subsequent increase could be a consequence of the repopulation of keratocytes that takes place after the sixth month.32 Failure to find these thickness changes following TE-CXL could indicate less of a structural effect.8
Following epi-off CXL, the ISV improved significantly, while remaining stable following TE-CXL, with a statistically significant difference with both treatments over the time period that was studied. With both techniques, the previous minimum radius remained stable during the study period, although there was a significant difference with both techniques over time. Magli et al.19 observed a statistically significant decrease in the IHA with both techniques, without finding differences between them. Çerman et al.8 found a significant decrease in the ISV and IVA (vertical asymmetry index) with both treatments. The KI (KC index) decreased after epi-off CXL, while it remained stable after TE-CXL. The CKI (central KC index) remained stable in both groups. The IHA did not change after epi-off CXL, although it increased following TE-CXL. The IHD (index of height decentration) increased in both groups. Today, to assess the evolution of keratoconus without surgery, several indices could be used, such as the D index, the index for height symmetry or the keratoconus progression index (KPI), which have been shown to be effective.33 Likewise, the analysis of topography and epithelial thickness could be of interest to assess the effects that the different types of CXL have on this corneal layer and their responsibility for the changes found after the different types of treatment
In our study, no significant differences were found in terms of astigmatism, posterior elevation, or corneal volume.
Even though the most frequent postoperative complications after the epi-off CXL technique are infection,34 persistent corneal edema35 and the need for keratoplasty,36 our study did not reveal a difference between the group treated with epi-off CXL and treatment with transepithelial CXL, as in other works.8,25 This may be due to the fact that patients were treated in the early stages of keratoconus and the sample size was not too big, which suggests that a larger number of subjects may be needed to be able to compare the rate of postoperative complications between CXL techniques
In conclusion, epi off-CXL was more effective from the point of view of the aberometric and morphological properties of the cornea although both techniques halted and stabilized the progression of KC, epi-off CXL was more effective. Additionally, following the corneal CXL technique, there was a greater degree of regularization of the corneal surface and, therefore, a greater degree of improvement in the visual acuity that was corrected much more in central KC than in paracentral KC.
Financial disclosureNone of the authors has a financial or proprietary interest in any material or method mentioned.
Conflict of interestThe authors declare that there is no conflict of interest.