To evaluate skin biocompatibility of a nighttime hydrating eyelid gel and possible ocular surface effects in contact lens users (CLU) and non-contact lens users (NCLU). The formulation is registered as a medical device as Tridocosahexaenoine-AOX® (TDHA-AOX) (a concentrated DHA triglyceride), containing also hyaluronic acid (HA).
MethodsA prospective, randomized, masked clinical trial was performed with 62 participants of both sexes, aged 20–70 years, split into: (1) CLU (n = 30) and (2) NCLU (n = 32). All participants were instructed to apply a single dose of the moisturizing gel (containing TDHA-AOX and HA) nightly to the upper and inner eyelids of their right eye (RE) only, and during 2 consecutive weeks. Personal interviews, questionnaires, ophthalmic examinations and reflex tear collection were performed. Ophthalmological parameters included ocular surface response and contact lens status. Levels of satisfaction/adverse events were also recorded. Biochemical parameters included basal and final determination of pro-inflammatory mediator molecules in tear samples by multiplex analyses. Statistics were done by the SPSS 24.0 program.
ResultsThe CLU group had higher OS dysfunction than NCLU, but overall clinical parameters (corneal staining, and Schirmer/FBUT tests) and OSDI scores showed significant improvement in CLU individuals as compared to the NCLU participants, at the end of study. CLDEQ-8 scores pinpointed significant amelioration in initial risk of developing DEs by applying eyelid gel. Multiplex analyses demonstrated significantly lower VEGF expression levels (p < 0,05) in tears among the CLU compared to NCLU after nightly application of eyelid gel.
ConclusionsEyelid gel appeared to safely and efficiently provide hydration and decongestion of the skin and amelioration of the ocular surface during sleep.
Dry eyes (DEs) result from a wide variety of changes occurring in one or more ocular surface (OS) components. Ocular discomfort and visual disability occurs in varying degrees through the disease.1,2According to etiology and clinical manifestations, the aqueous-deficient and evaporative DE forms need to be considered.1–4 Recognized pathogenic mechanisms are increased tear osmolarity and OS inflammation.3–5
A search of the scientific literature has shown scarce reports on the statistics regarding the contact lenses (CL) users (CLU) in the most recent years, in Spain. Santodomingo et al.,6 published that in 2017 the number of neophyte CLU was up to 700, whereas these data were about 19000 new CLU in other 29 countries. In 2019, a country report on the contact lenses and solutions in Spain https://www.euromonitor.com/contact-lenses-and-solutions-in-spain/report) concluded that an increased decision of CL instead of wearing glasses are occurring in the most active Spaniards, throughout the last years.
Among CL users (CLU), eye diseases affecting lubrication, usually involving tear production impairment, corneal surface disorders, conjunctival as well as palpebral gland dysfunction, are most frequent.7 Most CL-associated DEs manifestations have been managed by prescribing artificial tears during the day, and lubricant gels/creams at night, often with unsatisfactory results. But, according to Ablamowicz and Nichols,8 this is not the first-line recommendation, but changing to a CL with a more periodic replacement schedule. The main goal is to reach the biggest success for CLs continuation, by better understanding the causes of signs and symptoms of OS dysfunction.
The major etiopathogenic mechanism for CL-associated DEs is the lack of tear film stability, leading to lipid layer reduction, subsequent increase in aqueous layer evaporation and noticeable hyperosmolarity.7–9 To counteract these disorders, it has been shown that over-the-counter use of unpreserved artificial tears, eye gels and lubricants9,10 and phospholipid-liposome eye spray,11 as well as a combination of these, yields optimum results in the affected individuals.7–11 Furthermore, up to two-thirds of CLU make use of cleaning and disinfecting products that include chemicals (antimicrobial agents, preservatives, buffer systems, and surfactants) that can interfere with the integrity and function of the anterior eye and adnexa structures, resulting in a wide range of clinical manifestations. Recent reports have also confirmed the negative effects of these products (e.g., EDTA, polyhexanide, boric acid, hydrogen peroxide, poloxamer) on the tear film, corneal surface and conjunctiva.12
New strategies for preventing and treating DEs have therefore been proposed. The dietary precursor linolenic acid (ALA, C18:3) synthesizes eicosapentaenoic acid (EPA; C20:5 ω3) and docosahexaenoic acid (DHA; C22:6 ω3), the two major omega-3 (ω-3) long-chain polyunsaturated fatty acids (LC-PUFAs) essential for humans. These play fundamental roles in the regulation of OS morphology and function.13 A wide spectrum of reports have demonstrated that ω-3 LC-PUFAs either from food or oral food supplements induce positive responses on global health,14 as well as in several diseases, including DEs.5,15–18 Indeed, LC- ω-3 fatty acids are incorporated into the cell membrane phospholipids. Tridocosahexaenoine-AOX® (TDAH-AOX) is a highly concentrated DHA triglyceride, patented as a biological molecule that offers both cellular antioxidant protection and anti-inflammatory activity19–21 by (1) stimulating the intracytoplasmic synthesis of glutathione; (2) inhibiting E2 proinflammatory prostaglandins synthesis, but stimulating the anti-inflammatory E3 prostaglandins synthesis; and (3) favoring the resolution process of inflammation by inducing production of docosanoids as metabolic by-products called resolvins and protectins.
Nevertheless, it would be useful to prepare a preliminary personalized approach based on the risk-benefit assessment for CLU and NCLU. Indeed, effectiveness, side effects/safety, and route of administration are the usual considerations when addressing new treatments, especially for OS. We therefore performed a biocompatibility assessment and recommended indications of daily nocturnal administration of an eyelid gel containing TDHA-AOX for OS in CL, to give new practical advice in addressing DEs in CLU.
Material and methodsThe present work adheres to the Ethical Principles for Medical Research involving human subjects [Declaration of Helsinki (Edinburgh, 2000)], and the Ethics Committee standards of the study centers (no. 1017/2016-2017).
Study designA prospective, randomized, interventional, multicenter, masked clinical trial was conducted between 2017/2018.
The main goal of the present study was to evaluate the efficacy and safety of the nightly application on the right upper and inner eyelids, of a gel-cream containing TDHA-AOX, over 15 consecutive days. The left eye was used as the control. The evaluation criteria will lay over the skin features, as well as over the OS clinical parameters.
The secondary endpoint was to look at the effects of the above gel formulation on tear levels of pro-inflammatory mediators by multiplex analyses.
The sample size for this preliminary study was calculated by the eNe 3.0 (GlaxoSmithKline S.A.) statistical program, in order to reach an statistical power of 80 % that permit to detect differences in hypotheses contrast (Ho: p1 = p2) by means of the χ2 bilateral for 2 independent samples, with a significance level of 5%.
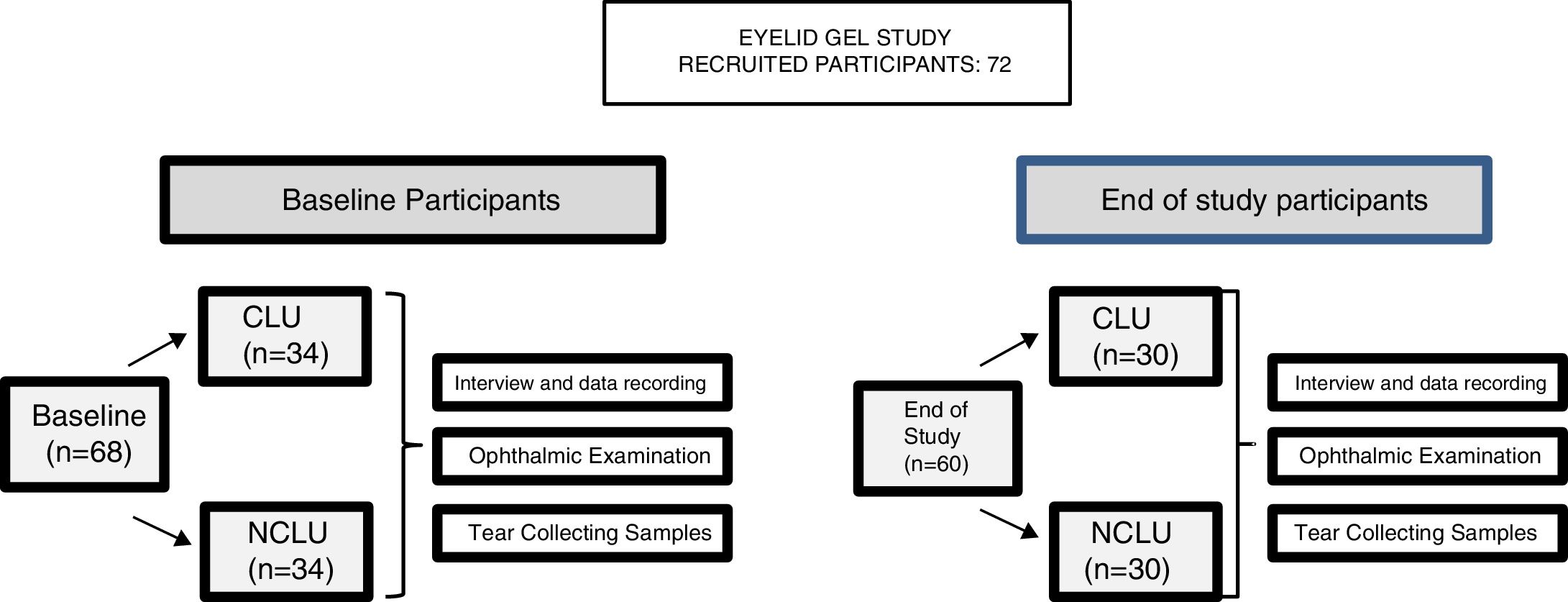
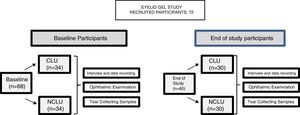
PatientsInitial eligibility interviews were conducted with 72 potential candidates of both sexes (144 eyes), aged 22–60 years, during the ordinary ophthalmologic appointments in the study centers, according to the study inclusion/exclusion criteria, as listed in the Table 1. 68 participants were initially included, extensively informed about the study characteristics and provided written informed consent. Subjects were classified into 2 groups: (1) contact lens users, CLU (n = 34) and (2) non-contact lens users NCLU (n = 34).
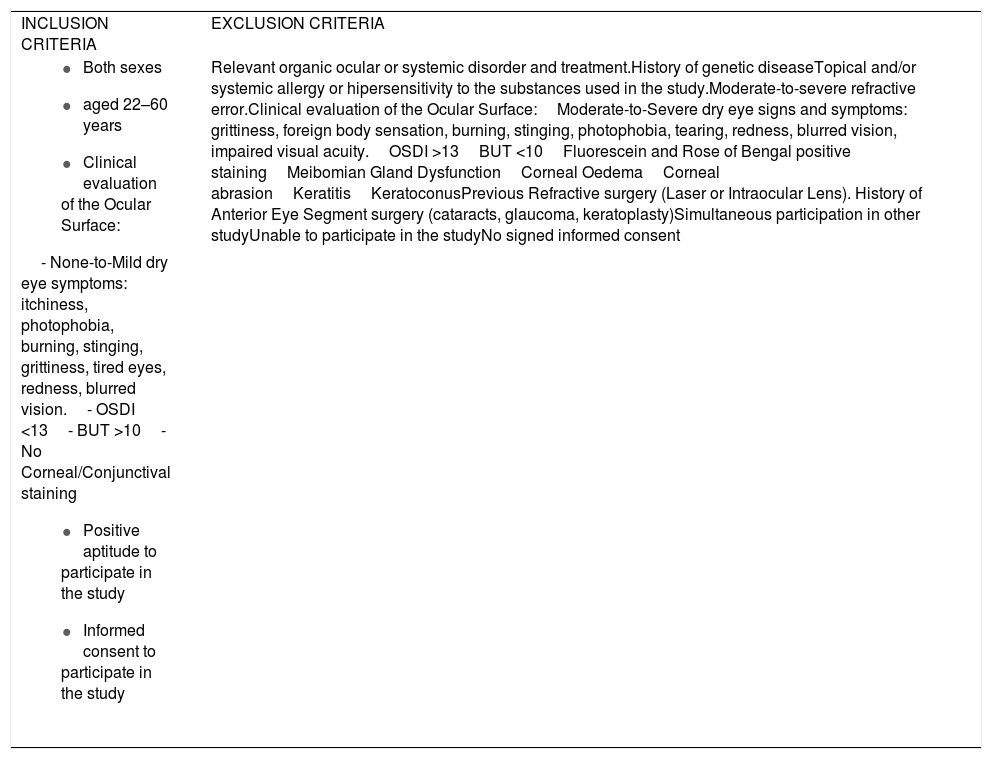
Inclusion and exclusion criteria for the study participants.
| INCLUSION CRITERIA | EXCLUSION CRITERIA |
| Relevant organic ocular or systemic disorder and treatment.History of genetic diseaseTopical and/or systemic allergy or hipersensitivity to the substances used in the study.Moderate-to-severe refractive error.Clinical evaluation of the Ocular Surface:Moderate-to-Severe dry eye signs and symptoms: grittiness, foreign body sensation, burning, stinging, photophobia, tearing, redness, blurred vision, impaired visual acuity.OSDI >13BUT <10Fluorescein and Rose of Bengal positive stainingMeibomian Gland DysfunctionCorneal OedemaCorneal abrasionKeratitisKeratoconusPrevious Refractive surgery (Laser or Intraocular Lens). History of Anterior Eye Segment surgery (cataracts, glaucoma, keratoplasty)Simultaneous participation in other studyUnable to participate in the studyNo signed informed consent |
The following baseline interview data were recorded: socio-demographic information, personal/familial background, lifestyle characteristics, and global ophthalmological and systemic aspects. At baseline, all participants underwent a precise ophthalmological examination in both eyes, including best-corrected visual acuity (BCVA); intraocular pressure (IOP); and biomicroscopy (BMC) of the anterior eye segment and adnexa. Among the latter, the major outcome variables were: eyelid status, Schirmer and fluorescein break up time (FBUT) tests, fluorescein corneal staining, Rose of Bengal staining, and presence of deposits on the CL surface. Other parameters—including OS response by means of the clinical evaluation (as above said), characteristics and status, user sensations, as well as adverse events—were fully evaluated at baseline, and also at the end of study. The ocular surface disorder index (OSDI [Allergan Inc., Irvine, California, USA]) questionnaire was carried out in all participants to differentiate those with normal, mild, moderate, or severe DEs. The OSDI includes 12 items and each answer is evaluated from 4 to 0 (higher to lower); the final OSDI score is the result of applying the following formula: Sum of scores from all questions answered × 25/Number of questions answered. The overall OSDI score delineates the OS from normal (0–12 points), mild disorder (13–22 points), moderate disorder (23–32 points), to severe stage of disease (33–100 points).21 The Contact Lens Dry Eye Questionnaire (CLDEQ) was set up to measure dryness symptoms among CLU.22 This tool was originally designed in parallel to the Dry Eye Questionnaire (DEQ) for NCLU to decipher the prevalence of discomfort, dryness, and blurred vision among CLU. However, the CLDEQ-8 (a short version of the CLDEQ), the one used in the present study, was developed to determine the opinion of CLU with respect to: new lenses, care products, and replacement schedules, as well as new eye therapies, as previously reported.23 Participants with a score higher than 12 are progressively considered vulnerable to developing DEs, while those with scores lower than 12 have apparently no risk of developing DEs. In addition, a questionnaire (personally designed to gather knowledge on the subjective sensations of the eyelid skin, product appreciation/adverse effects) was given to all individuals. Prior to the first study visit, participants were required to discontinue, for at least 1 month, the use of nutritional supplements, systemic antihistamines, and treatments for DEs (or meibomian gland disorder) such as eye cleaning wipes, antibiotics, non-steroidal anti-inflammatory drugs, and corticosteroids, as well as artificial tears. The study product was a gel protected from air by an airless container, containing 10 mL of gel, that provides 0.2 mL per pulse (approximately 50 doses per container), which has been previously tested for cosmetic use. The complete formula is reflected in Table 2, but essentially contains HA and a patented DHA triglyceride (European Patent EP 1 962 825 B1 held by Brudy Technology SL related to the use of DHA for treating a pathology associated with cellular oxidative damage. European patent granted, date April 2, 2014).15–20 The product was manufactured by the pharmaceutical company (Brudylab, Barcelona, Spain) and gently provided in unlabeled individual plastic containers (similar in size and shape to the eyedrop receptacles) to the investigators, and the doctors gave them to all participants without any charge. Neither the investigators nor the participants knew the specific composition of the product. The information provided by the manufacturers included that the study product did not contain harmful agents for the skin, eyes and vision in the formulation. All participants were instructed to apply the gel nightly, on the upper and inner right eyelids, to be maintained overnight. The procedure was as follows: the CLU previously removed the CL, no eyedrops were instilled, the eyelids have to be free of cleansing lotions, make-up, or beauty products, the participants have to apply a small amount of gel to the skin of the right closed eyelids, using the fingertip to give a brief, soft massage, and not on the left eyes, that will be considered as the controls. Application just before bedtime was especially recommended. Participants were specifically advised that this product was for external use on intact eyelid skin and should never be applied to the eye. At the starting point, 73 suitable participants were included in the study (according to the inclusion/exclusion criteria, precisely reflected in the Table 1). A summary of the visits and procedures is shown in Fig. 1.
Complete formula of the gel-cream tested.
| List of ingredients |
|---|
| DHA concentrated triglyceride 0,7% |
| Sodium hyaluronate 0,2% |
| Aloe Vera 3,0% |
| Propylene glycol |
| Glycerine |
| Avocado oil |
| Caprylic/Capric Triglyceride |
| Olive oil |
| Xanthan gum |
| Sesame oil |
| Polysorbate 80 |
| Soybean lecithin |
| Grapefruit oil |
| Sodium citrate |
| Sorbitan monolaurate |
| Tris(hydroxymethyl)aminomethane |
| Tocopheryl acetate |
| Grape seed oil |
| Hydroxypropyl methyl cellulose |
| Citric acid monohydrate |
| Tea tree oil |
| Geranium oil |
| Phenethyl alcohol |
| Caprylyl glycol |
| Water |
A systematized eye examination was performed and all data recorded in a database specifically designed using the Microsoft Excel program: best corrected visual acuity in each eye (BCVA), biomicroscopy of the anterior eye segment and adnexa (BMC) by the slit lamp (Hagg-Streit AG, Koeniz, Switzerland), to explore the OS structures with/without fluorescein and rose of Bengal staining; Schirmer’s test with anesthesia, fluorescein break up time (FBUT), and IOP by the aplanation tonometer (Haag-Streit AT-900, Koeniz, Switzerland) after instillation of a single drop of Fluotest ® (sodic fluorescein plus oxibuprocaine. Alcon Cusi, Barcelona, Spain). Briefly, a standard Schirmer test with topical anesthesia (Anestesico Doble ® 0.5% oxibuprocaine hydrochloride plus tetracaine hydrochloride eye drops. Alcon Cusi, Barcelona, Spain) was performed. A sterilized filter paper strip (5 mm × 35 mm) was placed in the external canthus of the inferior eyelid and left in place for 5 min. Wetting of the paper after 5 min was recorded in mm according to the scale provided. The wetting strips <10 mm per 5 min were diagnosed positive, while ≤5 mm per 5 min were diagnosed as strongly positive. The OS of both eyes was then stained with 2 μL of Fluorescein ® (2% fluorescein eye drops, Alcon Cusi, Barcelona, Spain) that were instilled into the inferior fornix of the conjunctiva. The ophthalmologist instructed participants to blink actively for 3−5 sec to examine the cornea, conjunctiva, eyelids (free lid margin and outer/inner eyelid border), and eyelashes. The FBUT was determined as the time period between the last blink and the apparition of a random corneal black spot on the fluorescein stained tear film. For each participant the FBUT was determined three times and the value was expressed as the mean ± standard deviation. FBUT was considered positive if the final value was less than 10 sec. The rose of Bengal test (1% sodium rose of Bengal sterile ophthalmic strips), was performed when applying one single strip on the inferior tarsal conjunctiva, and the OS was examined by BMC. The positive staining marked the damaged epithelia. At the end of the 2-week period of daily application of the eyelid gel, the participants underwent the same examination protocol, as performed in the baseline visit.
Sample collection and processingThe tear sampling technique used by our research team has been previously described elsewhere.5,15–18 During the baseline study visit, participants attended the ophthalmological clinic at 9 a.m. to undergo the above ophthalmological examination. The following day, reflex tear samples were extracted with a capillary Pasteur micropipette from the inferior tear meniscus of the right and left eyes separately, using a gentle rubbing softly technique (Fig. 2). Tear samples were then placed into microeppendorfs (100 μL) and these were placed into cryotubes that were conveniently labeled and stored in boxes at −80 °C until processing. In cases of decreased tear production, the sampling procedure was repeated until an acceptable volume was obtained (up to 20–30 µL). Great care was taken during tear sample conservation and manipulation. Tear sampling was scheduled at baseline and at the 2-week follow-up for biochemical assays. All experiments were performed in duplicate and the protocols are described below.
Basal and final OSDI index scoring of the total group of participating volunteers (n = 60), and of the separate groups of CLU (n = 30) and NCLU (n = 30) evaluated after one single night gel-cream application on the right eyelid skin over two weeks.
OSDI: Ocular Surface Disease Index; CLU: contact lens users; NCLU: non-contact lens users.
The level of expression of pro-inflammatory mediators was done by determining several cytokines/chemokines in the tear samples by using the Luminex R-100 multiplex system (Luminex, Austin, TX, USA).24 The system is able to perform immunodetection with a minimum amount of approximately 20 µL. The flow-based Bio-Plex suspension array system (Bio-Rad Laboratories, Hercules, CA, USA) was used to identify and quantify each particular antigen-antibody reaction. Identification of the assayed molecules was based on bead color and fluorescence, using fluorescent-labeled reporter molecules associated with each target protein. Unknown cytokine and chemokine concentrations were calculated automatically by the Bio-Plex Manager software using a standard curve derived from a recombinant cytokine standard. Cytokine and chemokine concentrations were corrected for the initial total protein concentration of each human tear sample during analysis. The inflammation and immune response molecules analyzed herein were: interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, and IL-12; vascular endothelial growth factor (VEGF); tumor necrosis factor α (TNF-α); granulocyte-macrophage colony-stimulating factor (GM-CSF); and interferon γ. The experiments were done in duplicate and the results are shown as the mean (SD) in pg/mL.
Statistical analysisThe Microsoft Excel program and IBM Statistical Package for the Social Sciences SPSS V.24.0 program (SPSS Inc., Chicago, IL, USA) were used. For continuous variables, nonparametric (Mann–Whitney U) and parametric (t test) statistics were used, and the results are expressed as mean ± standard deviation (SD). Categorical variables are expressed as percentages. A p-value less than 0.05 was considered statistically significant, adjusted by Bonferroni correction when pairwise comparison in multiple groups was conducted.
ResultsA total of 60 participants (30 assigned to the CLU [33% male/67% female] and 30 assigned to the NCLU [30% male/70% female]) completed the 2-week follow-up; 120 eyes were analyzed both in separate, and also as a whole (classified as pertaining to each of the two study groups). Rate of withdrawal was 9%. Lack of motivation and tear samples failing to show any data constituted the most common causes for withdrawal. Mean age of total participants was 36 ± 12 years (32 ± 12 years in CLU and 38 ± 11 years in NCLU). Distribution by sex and age was similar in both groups.
The CL status was investigated in the corresponding group of the study participants (CLU). Regarding the biomaterials of the CL utilized by our participants, 100% corresponded to soft lens of silicone hydrogel (SiHy), and the frequency distribution according to their distinguishing properties was: 62% were reusable spherical SiHy CLs [from which: 31% were Ultra mensual moisture seal -samfilcon A-, 46% water content (WC); and 69% were Biofinity mensual -comfilcon A-, 48% WC], 28% were reusable spherical SiHY CLs (replacement frequency two weeks: Acuvue Oasys -senofilcon A-, 36% WC), and, finally 20% of the CLs were torical SiHy CLs (Airoptix for astigmatism -Iotrafilcon B-, 24% WC). Other physical CLs-related factors, such as thickness/bulk and/or edge configuration did not were considered for the present work. All study participants utilized the CLs during the daytime. Mean usage full time was: 8,2 ± 3,1 h.
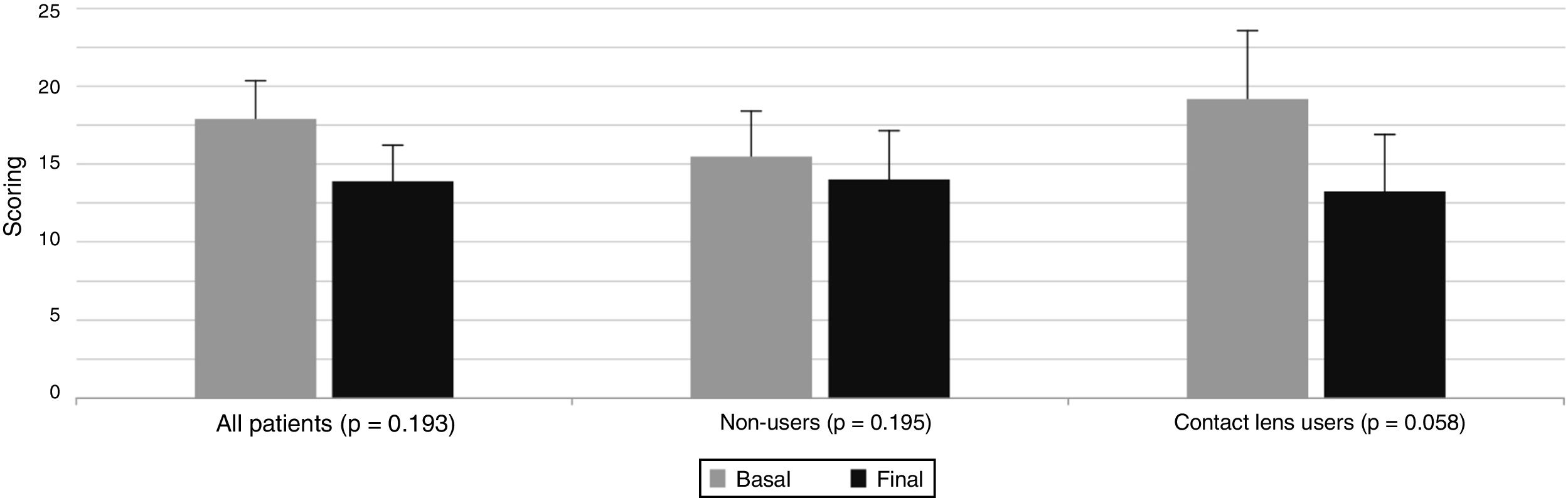
The OSDI questionnaire revealed that the CLU group had higher OS dysfunction than the NCLU group, with a significant improvement on the overall scores among CLU individuals for the two study points (Fig. 3).
Basal and final OSDI index scoring of the total group of participating volunteers (n = 60), and of the separate groups of CLU (n = 30) and NCLU (n = 30) evaluated after one single night gel-cream application on the right eyelid skin during two weeks.
OSDI: Ocular Surface Disease Index; CLU: contact lens users; NCLU: non-contact lens users.
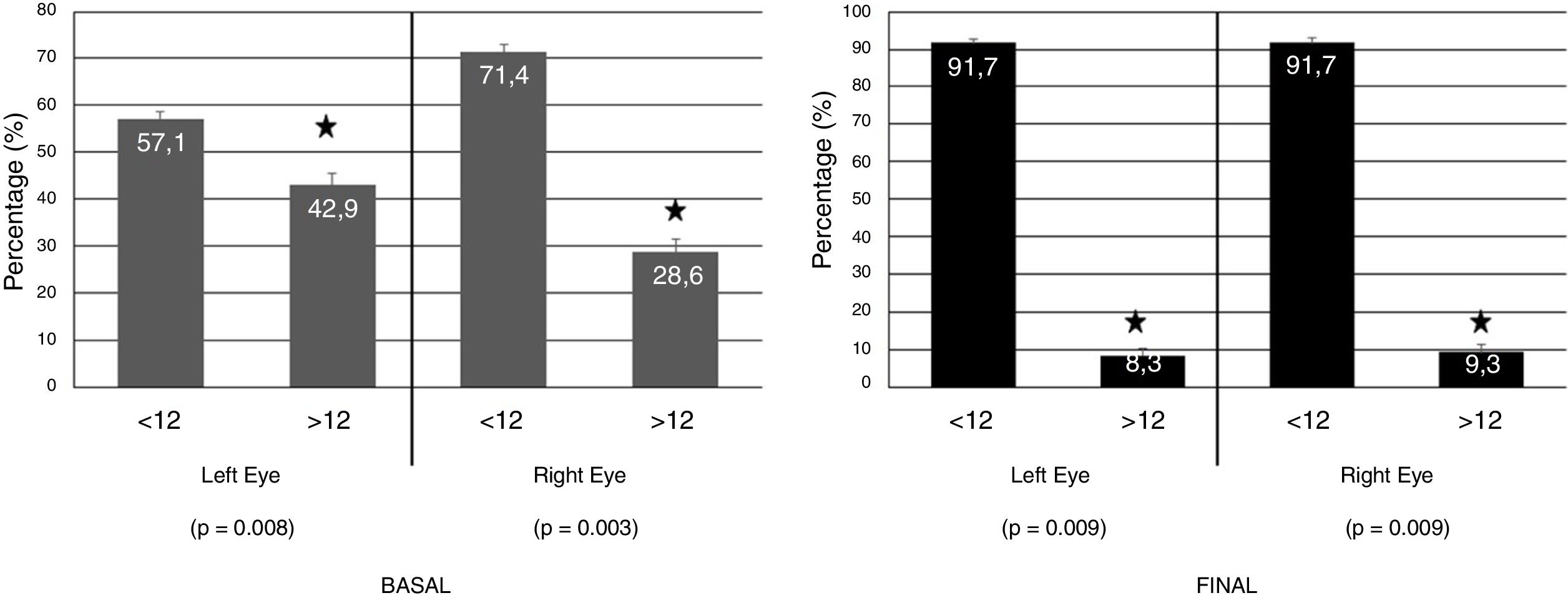
Regarding CLDEQ-8 scores obtained by the CLU, there were differences between the start and end of study relating to the application of the eyelid gel (regarding alteration of the OS). This means that the participants improved their initial risk of developing DEs by applying the eyelid gel (Fig. 4).
Basal and Final CLDEQ-8 scoring evaluating the risk of developing dry eye (Scoring >12) in the right and left eyes of the CLU (n = 30) after one single night gel-cream application on the right eyelid skin during two weeks.
CLDEQ-8: Contact Lens Dry Eye Questionnaire-8; CLU: contact lens users.
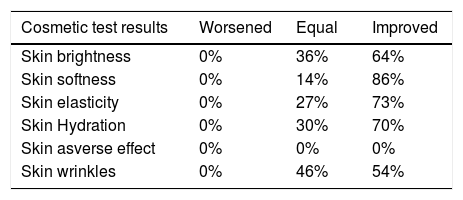
The questionnaire, designed by researchers to gather opinions on the effect of the nightly application of the eyelid-gel over 2 consecutive weeks showed interesting data on the positive participant appreciation for this intervention, as shown in the Table 3. Significantly higher percentages of patients responded that the palpebral skin of the RE had greater brightness/smoothness and more elasticity/hydration, as well as amelioration of wrinkles than the LE at the end of the study. No adverse effects were suffered or declared by any participant.
Results of the Cosmetic Survey including the opinion of all the participating volunteers (n = 60) relating to the cosmetic properties and possible detected skin adverse effects, evaluated after one single night gel-cream application on the right eyelid skin during two weeks.
| Cosmetic test results | Worsened | Equal | Improved |
|---|---|---|---|
| Skin brightness | 0% | 36% | 64% |
| Skin softness | 0% | 14% | 86% |
| Skin elasticity | 0% | 27% | 73% |
| Skin Hydration | 0% | 30% | 70% |
| Skin asverse effect | 0% | 0% | 0% |
| Skin wrinkles | 0% | 46% | 54% |
No changes in the BCVA and IOP values between groups were observed between the two study points. The clinical probes to address the OS status, as described before (Material and Methods section) were performed to all participants at baseline and at the end of study. In fact, descriptive statistics for 5 min wetted lengths (mm) of Schirmer strips reflected that up to 18% of both examined eyes from the CLU participants and 9% of the NCLU eyes showed test reading <5 mm. Average Schirmer test value was of 8.8 ± 2.6 mm in the CLU eyes, versus 13.6 ± 2.3 mm in the NCLU eyes; p < 0.05). Average TBUT scores displayed no statistically significant differences between groups (4.8 ± 1.6 sec in the CLU eyes versus 6.8 ± 1.5 sec in the NCLU eyes). However at the end of follow-up, a significant improvement respect to baseline values was noticed in the probes from the RE of the CLU participants (Schirmer test: 13.2 ± 2.2 mm, p < 0.05; TBUT: 7.8 ± 1.2 sec, p < 0.001) that were similar to the baseline values of the RE from the NCLU. The biomicroscopy examination showed no significant fluorescein corneal staining and no significant CL additional deposits in any case at the end of study (applicable to the eyelid gel administration).
Data from the biochemical analyses were quite surprising. Up to 86% of the molecules of the human cytokine/chemokine panel were detected in tears of the study participants in the picomolar range. Moreover, noticeable higher expression of IL1β, IL6, VEGF and TNFa were detected in the CLU as compared to the NCLU, at baseline. However, it was not fully demonstrated changes in the expression pattern of cytokines/chemokines in tears from the two study groups at the end of study (regarding the CL wearing). It was not detected statistically significant changes in cytokines/chemokines tear expression between the RE and LE of the participants (regarding the eyelid-gel application in the RE but not in the LE). Only the VEGF expression was noticeably lower in tears from the CLU group from baseline (RE: 1200 pg/mL; LE: 550 pg/mL) and at the end of study (RE: 800 pg/mL; LE: 460 pg/mL).
Overall, our data demonstrated a final risk reduction (2 weeks from baseline) among CLU after nightly eyelid gel applications. Improvement was detected when comparing final vs. initial symptom scores and clinical signs among study participants. Final scores were noticeably lower than the initial ones, pointing to a decrease in sensations and signs of DEs in relation to OS changes induced by the application of the eyelid gel. The present work with the palpebral cream received high scores for comfort and personal positive sensations at day 15. The parameters revealed noticeable differences between the RE and LE.
DiscussionWe evaluated the effects of nightly application in the RE of an eyelid gel containing TDHA-AOX and HA on the OS in CLU and NCLU, taking into consideration the contralateral eye as the control. At 2-week follow-up, we found a significant increase in the Schirmer and FBUT tests in RE assigned to the cream application compared to the contralateral eyes that were considered as the controls. Moreover, noticeably lower cytokine/chemokine expression was seen in the tears of the NCLU group than in the CLU, but the results showed a lack of statistical significance. In addition, it was evident that the gel-treated eyes displayed a trend to diminish the cytokine/chemokine values as compared to the non-treated eyes, at the end of follow-up, without statistical significance. Our data also indicate that overnight external exposure to this formula noticeably improved the palpebral skin with an important repercussion on the OS integrity in CLU individuals, based on the presence of DHA in triglyceride form (DHA-TG).
Chalmers et al., reported a prevalence of DE signs and symptoms of about 50% in CLU, twice the percentages reported by NCLU of similar age.23 It has been widely stated that wearing CL is one of the most frequent external causes of DEs.1–6,8,25,26· In our study, the CL type, biomaterial properties, wearing/replacement schedule and eye care was quite similar between the participants. In our opinion, when the summed CLDEQ-8 scores were obtained from the study participants, an excellent response in terms of overall opinion of the CLU was seen, which reinforces the participant-reported satisfaction with the eyelid gel treatment. This questionnaire can therefore be strongly recommended for identifying new products that may reduce and manage DE symptoms in CLU.
The eyelid skin possesses specific biochemical constituents (collagen and elastin) that provide structural/functional characteristics to defend the ocular globe they cover, as well as to regularly spread tears on the OS to keep it appropriately moistened. Because of this, any improvement of the penetration efficiency combined with the release of active products in the skin can facilitate the treatment of eyelid diseases, as well as favor the integrity of the structures of the OS. A wide variety of in vivo techniques have been used to evaluate the penetration of pharmacological products into the skin, including electron paramagnetic resonance spectroscopy, confocal laser scanning microscopy, and confocal Raman microscopy. Moisturizers topically applied affect skin barrier homeostasis. Lodén has recently reported that some substances may also penetrate deeper into the skin and induce changes in the lipid production.27 In this context, we speculate that the eyelid gel can induce changes in the palpebral skin as well as in other components of the OS.
It is widely accepted that external/internal agents can adversely affect tear film stability and osmolality resulting in a variety of OS disorders. Taking into consideration that DEs have an important, sometimes subclinical, inflammatory component, the clinical probes are far from complete for DE diagnosis. Normally, tear secretion by the main lacrimal gland is approximately 4.5 μL. It has been described that a small amount of tears (2 to 4 μL is needed on the OS for the appropriate integrity of the anterior eye structures. TFOS. Quantitative probes demonstrated that CLU eyes had significanly lower tear volume than the NCLU participants (Schirmer: 8.8 ± 2.6 mm in the CLU, versus 13.6 ± 2.3 mm in the NCLU). The nightly eyelid gel application in the RE induced a significant increment of the Schirmer and TBUT levels in the CLU participants. Average TBUT scores displayed no statistically significant differences between groups (4.8 ± 1.6 sec in the CLU group versus 6.8 ± 1.5 sec in the NCLU participants). However at the end of follow-up, a significant improvement respect to baseline values was noticed in the probes from the RD from the CLU participants (Schirmer test: 13.2 ± 2.2 mm, p < 0.05; TBUT: 7.8 ± 1.2 sec, p < 0.001).
Scientific research has accumulated much immunological evidence establishing that the DEs highly involve a immune response and the release of cytokines/chemokines and its downstream effectors.28 The biochemical analyses carried out in the present work showed changes in cytokine/chemokine tear levels between the two groups (CLU, NCLU) as well as the two study points, with a trend to decrease in the RE exposed to the eyelid gel compared to the LE. However our data did not show statistical differences between groups for the assayed panel of human cytokine/chemokine.
The composition of the eyelid gel with LC-PUFAs -more specifically T-DHA- must also be considered. Up to 70 DHA metabolites (biologically active) have yet to be identified. Among the pleiotropic effects of DHA metabolites, the stimulation of lipid catabolism, the regulation of the immune response by helping to resolve inflammation, and the activation of wound healing/tissue regeneration have to be considered. The anti-inflammatory properties of ω3 LC-PUFAs have been extensively investigated. Human microarray data strongly demonstrate that dietary intervention with ω-3 fatty acids, flavonoids, and resveratrol enriches immune response and disease pathways, including their beneficial effects on DEs.5,15–18 Because of this, we hypothesize that DHA exerts an inhibitory effect on the activation of nuclear-κβ factor, hindering the synthesis of inflammatory cytokines (IL-6, TNF-α, IL-1β) as well as VEGF. Regarding the latter, the decreased VEGF levels in tears from CLU individuals at the end of study may reflect the amelioration of the subclinical inflammation processes occurring in the OS as a consequence of using the CL after eyelid gel application. These results need further long-lasting studies with larger sample sizes to assess the pathogenic role of VEGF in DEs.
Globally, the results of the present study can be explained as follows. (1)Our participants were healthy CLU and NCLU individuals, without any previous diagnosis of DEs or ocular pathology. It is plausible that this was the reason why the cytokine/chemokine expression did not significantly change between the two study points. (2) The results are positive in tolerability and appreciation of the eyelid-gel, because the application did not induce any signs or symptoms in the OS or inflammatory response in tears from the participants after 2 weeks of nightly application. (3) The time of intervention was perhaps too short to detect changes in the expression patterns of cytokine/chemokine in tears. (4) The subjective data on the cosmetic effects of the eyelid gel application were satisfactory for the majority of participants.
This is the first study demonstrating that the external application of an eyelid gel containing T-DHA and HA has a positive and safe impact on the OS. An extensive search of the scientific literature did not reveal studies similar to ours.
Among the study limitations, as has already been mention, the relatively small sample size must be considered. Large cohorts should be analyzed over a longer duration to better elucidate the effects of eyelid gel administration on the OS in CLU and NCLU individuals. Our participants were also recruited on the basis of not suffering DEs. Finally, the 2-week follow-up may be too short a time to assess changes in tear cytokine/chemokine levels. It may also have been difficult for the participants to accurately discern between the RE and LE subjective sensations.
To summarize, the results of the present study reveal that the application of the eyelid gel overnight is efficient and safe for further use in clinical practice. These data also suggest that a reduction of the inflammatory background present in CLU can be counteracted by the tested eyelid gel, registered as a medical device formulation, containing DHA-TG and HA to better manage OS disorders and eyelid skin dryness.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank to Brudylab (Barcelona, Spain) for kindly given the eyelid gel tubes used in this study, without any cost. Vicente Zanón-Moreno was the recipient of a research grant from Brudylab, SL (Barcelona, Spain). Maria D. Pinazo-Durán and Jorge Raga-Cervera share first authorship of this work.
This work was supported by the Thematic Network of Cooperative Research in Ophthalmology (OFTARED, RD16/0008/0005 and RD16/0008/0026)‚ Carlos III Health Institute, Spanish Ministry of Science, Innovation and Universities.