Artificial Intelligence, Data Science and E-health in Vision Research and Clinical Activity
More infoTo establish the baseline distribution of refractive errors and associated factors amongst a population that attended primary care optometry clinics.
DesignRetrospective cross sectional cohort study of electronic medical records (EMR).
MethodsElectronic medical record data was extracted from forty optometry clinics, representing a mix of urban and rural areas in Ireland. The analysis was confined to demographic and clinical data gathered over a sixty-month period between 2015 and 2019. Distribution rates were calculated using the absolute and relative frequencies of refractive error in the dataset, stratified for age and gender using the following definitions: high myopia ≤ -6.00 D, myopia ≤ -0.50 D, hyperopia ≥ +0.50 D, astigmatism ≤ -0.75 DC and anisometropia ≥ 1.00 D. Visual acuity data was used to explore vision impairment rates in the population. Further analysis was carried out on a gender and age-adjusted subset of the EMR data, to match the proportion of patients in each age grouping to the population distribution in the most recent (2016) Irish census.
Results153,598 clinic records were eligible for analysis. Refractive errors ranged from -26.00 to +18.50 D. Myopia was present in 32.7%, of which high myopia represented 2.4%, hyperopia in 40.1%, astigmatism in 38.3% and anisometropia in 13.4% of participants. The clinic distribution of hyperopia, astigmatism and anisometropia peaked in older age groups, whilst the myopia burden was highest amongst people in their twenties. A higher proportion of females were myopic, whilst a higher proportion of males were hyperopic and astigmatic. Vision impairment (LogMAR > 0.3) was present in 2.4% of participants. In the gender and age- adjusted distribution model, myopia was the most common refractive state, affecting 38.8% of patients.
ConclusionAlthough EMR data is not representative of the population as a whole, it is likely to provide a reasonable representation of the distribution of clinically significant (symptomatic) refractive errors. In the absence of any ongoing traditional epidemiological studies of refractive error in Ireland, this study establishes, for the first time, the distribution of refractive errors observed in clinical practice settings. This will serve as a baseline for future temporal trend analysis of the changing pattern of the distribution of refractive error in EMR data. This methodology could be deployed as a useful epidemiological resource in similar settings where primary eyecare coverage for the management of refractive error is well established.
Refractive errors are the most common cause of vision impairment (LogMAR VA > 0.3) worldwide.1 In many countries, this is due to the lack of access to refractive error correction.1 In countries with good access to eyecare, refractive errors still present a significant challenge as a leading cause of vision loss due to the various ocular diseases associated with refractive error.2,3 Myopia is of particular concern due to the continuing global increase in prevalence rates in recent years.4 Individuals with myopia are at increased risk of conditions such as myopic macular degeneration, retinal detachment, cataract and primary open angle glaucoma.2 Rising prevalence is also associated with a shift towards higher degrees of myopia across the population, which increases risk of disease and vision impairment.5 Other refractive errors can also impact ocular health. Hyperopia is a known risk factor for angle closure,6 age-related macular degeneration,3 amblyopia and strabismus.7 Astigmatism can lead to significant decreases in visual acuity, visual comfort, quality of life and independence if uncorrected,8,9 while anisometropia is associated with reduced binocular function and stereoacuity10 and can lead to amblyopia if uncorrected in children.11
The various epidemiological studies conducted to date have revealed that the prevalence of refractive error varies with age, race, gender and geographic region.12–19 The myopia burden is increasing globally, with reports of increasing prevalence in the USA,20 Europe21 and Asia.22 Increasing levels of vision impairment are directly related to these changes, with myopic macular degeneration related vision impairment projected to increase almost six-fold by 2050.23 Large prospective studies are expensive and time consuming to establish. They are also difficult to follow up due to costs and participant drop out.24 These problems have led to a relative lack of current data on refractive error epidemiology worldwide, which is particularly apparent in some countries. Ireland, for example, only has data available for two small pediatric cohorts.25 This absence of data has been identified as a limitation in predicting future trends in refractive error prevalence and the associated risk of vision impairment.4 There is also a need for current refractive error data to monitor population interventions such as in the emerging therapeutic area of comprehensive myopia management, and also to inform healthcare planning to meet the future healthcare needs of those affected by refractive error and associated disease.
Large databases from existing electronic medical records (EMR) have the capacity to be useful in epidemiology studies, especially where conventional studies are absent. This type of data has been used previously to assess distribution rates of refractive error.26,27 Selection bias is a common criticism of EMR research. In clinic population studies on refractive error, emmetropia and low hyperopia may be underrepresented in young people due to a lack of visual symptoms. In contrast, the omnipresence of presbyopia in the aging human eye, coupled with the exacting near visual demands of modern living means that older adults are highly likely to present to their primary eye care practitioner for near vision improvement. This should allow a reasonably representative analysis of refractive error prevalence in an older cohort of the population, as has been recently demonstrated.28 Symptomatic types of refractive error are likely to be very well represented in these datasets, particularly the higher levels of refractive error which are most strongly associated with ocular disease and vision loss.29 Indeed, a recent analysis of EMR data has demonstrated that this type of clinical data provides a very close approximation of the distribution of refractive error reported in conventional population studies such as the European Eye Epidemiology Consortium.28 The purpose of the current study was to use EMR data from primary care optometry clinics to assess the distribution of refractive errors, anisometropia, visual acuity, and vision impairment in a clinic-based population across the Republic of Ireland. This study will serve as a baseline upon which temporal changes in the distribution of these conditions can be evaluated in people attending for primary eye care assessment.
MethodsThis study was designed as a retrospective analysis of an existing EMR database on refractive error and visual acuity. Forty individual practice owners gave consent for their anonymised patient demographic, refractive and visual acuity data to be extracted remotely by the optometric EMR provider (Ocuco Ltd., Dublin, Ireland). The analyses were confined to data captured during eye examinations conducted between January 2015 and December 2019. The EMR provider removed all personal identifiable information in such a way that the researchers could not reverse the anonymisation. The study was approved by the Research Ethics and Integrity Committee of Technological University Dublin.
The spherical equivalent refraction (SER) (defined as sphere plus half cylinder) was used for myopia and hyperopia analyses. Myopia was defined in line with the recommendations of the International Myopia Institute as a SER of ≤ −0.50 dioptres (D), high myopia as a SER of ≤ −6.00 D.30 Hyperopia was defined as a SER of ≥ +0.50 D, while participants with less than 0.50 D of ametropia were classed as emmetropic (−0.49 to +0.49 D). Astigmatism was defined as ≤ −0.75 cylindrical dioptres (DC). With-the-rule (WTR) astigmatism was defined as cylinder axes from 1°−15° and from 165°−180°, against-the-rule (ATR) astigmatism as axes 75°−105°, and oblique (OBL) astigmatism as axes 16°−74° and 106°−164°.31 Traditional sphero-cylinder correction was transformed into J0 (primary) and J45 (oblique) astigmatic components using the following formula:
Anisometropia was defined as a difference between right and left SERs of ≥ 1.00 D. The values for SER were calculated from the final refraction result (for spectacle dispensing) recorded in the EMR (refraction method unknown). To enable statistical analysis, the best corrected visual acuity (BCVA) was converted to the logarithm of the minimum angle of resolution (LogMAR). Vision impairment (VI) was defined according to previous literature as corrected LogMAR VA > 0.3 (equivalent to Snellen 6/12) in the better seeing eye,32 and was further stratified into the following categories – mild VI (LogMAR > 0.3, ≤ 0.48), moderate VI (LogMAR > 0.48, ≤ 1.00), severe VI (LogMAR > 1.00, ≤ 1.30) and blindness (LogMAR > 1.30).32 Children under ten years old were omitted from visual acuity and vision impairment analyses as higher VA scores are normal dependent on the child's age.
The data was validated and cleaned prior to analysis to remove biologically implausible data entry errors. The inclusion criteria were age 0 – 110 years, sphere between −25 and +25 D, cylinder between −15 and +15 DC, axis between 1 and 180°, addition between 0 and +4 D and gender recorded. 2.76% of records were removed due to the age criterion, mostly due to age not being recorded (2.4%) and the remainder due to likely typographic errors of biologically implausible age values. A little over 1% were eliminated due to the refraction criteria (sphere 1.0%, cylinder 0.003%, axis 0%, addition 0.1%). The omission of gender data accounted for 6.35% of data removed. Calculations were based on each person's most recent visit during the study timeline (2015 – 2019). The age groups were stratified into intervals of 10 years, except for the oldest age group (80 years and over) where there were fewer patient numbers, which were grouped together. A descriptive analysis was performed using mean, median, standard deviation (SD) and interquartile range (IQR). Distribution rates were calculated using the absolute and relative frequencies of refractive error in the dataset, stratified for age and gender. As there was no statistical difference between the right and left SER (r = 0.94, p < 0.001), the right eye was chosen for further analysis. The Pearson correlation coefficient was used for simple correlation analysis. Welch's independent samples t-test was used to assess differences between groups. Chi-square testing was performed to assess the relationship between gender and age on different refractive error types. Confidence intervals were calculated for all relative frequencies of refractive errors in the population. Refractive error distribution rates were also calculated using a gender and age-adjusted subset, created using random sampling without replacement to match the proportion of patients in each age grouping to the equivalent gender and age distribution in the most recent (2016) Irish census. This subset omitted the youngest grouping (0 – 9-year-olds) as the EMR data contained relatively few patients in this age category (children in this age group are usually screened by public health nurses and referred to ophthalmology if any abnormality is detected). Persons with a history of eye disease, cataract or other refractive surgery could not be excluded due to limitations with the database received. The statistical programming language R (R Core Team, (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/) was used to perform statistical calculations.
ResultsThere were 153,598 unique, individual patient records with refractive information after data cleaning, of which 57.8% were females. The mean age of participants was 47.94 ± 22.26 (SD) years (range 0 – 106 years). The median age was 50 years old (IQR 31 – 65). The mean age of females and males was 47.88 ± 22.08 (SD) and 48.01 ± 22.49 (SD) years respectively (t = 1.13, p = 0.26). Descriptive data pertaining to the frequency distribution of the study population according to refractive error type and patient demographics are provided in Table 1, while central tendency, variability, skew and kurtosis of the refractive error data are illustrated in Table 2.
Number of patients with each refractive error type, mean age, refraction with gender and vision impairment statistics.
| Vision Impaired | Vision Impaired | Vision Impaired | ||||
|---|---|---|---|---|---|---|
| Age (Years) | SER (D) | (incl. Blindness) | Age (Years) | SER (D) | ||
| Refractive Error Type | n (%) | Mean ± SD | Mean ± SD | n (%)* | Mean ± SD | Mean ± SD |
| Myopes | 50,297 (32.75) | 41.63 ± 20.12 | −2.45 ± 2.16 | 933 (2.39) | 61.25 ± 22.53 | −3.09 ± 3.54 |
| Emmetropes | 41,666 (27.13) | 45.65 ± 21.69 | 0.02 ± 0.23 | 523 (1.66) | 67.42 ± 21.50 | 0.03 ± 0.23 |
| Hyperopes | 61,635 (40.13) | 54.64 ± 22.48 | 1.82 ± 1.44 | 1326 (2.91) | 71.91 ± 18.22 | 2.07 ± 1.61 |
| Gender | ||||||
| Female | 88,807 (57.82) | 47.88 ± 22.08 | −0.14 ± 2.44 | 1710 (2.52) | 68.46 ± 20.53 | −0.03 ± 3.20 |
| Male | 64,791 (42.18) | 48.01 ± 22.49 | 0.04 ± 2.29 | 1072 (2.22) | 65.94 ± 21.43 | −0.06 ± 3.39 |
Descriptive statistics of refractive error and visual acuity in the EMR database.
J0 = Primary axis, J45 = Oblique axis, SD = Standard Deviation, Min = Minimum, Max = Maximum, Q1 = 1st quartile, Q3 = 3rd quartile.
Values expressed in dioptres (except visual acuity).
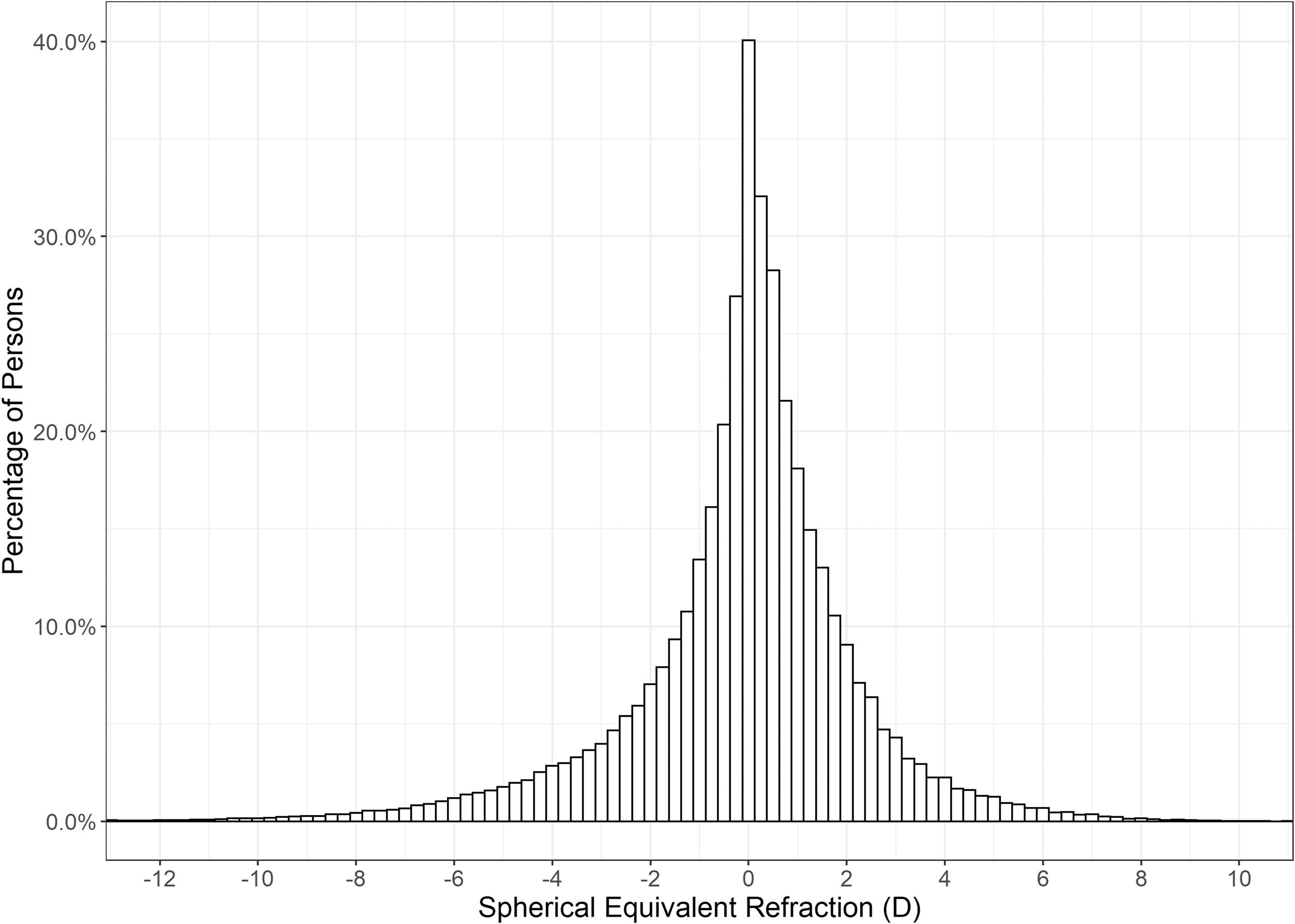
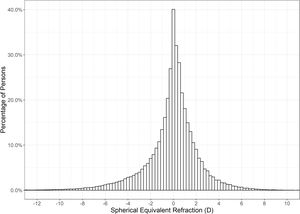
Hyperopia was the most common refractive condition, with 40.13% of individuals (95% CI 39.88 – 40.37%) falling into this classification, ahead of myopia, which accounted for 32.75% (95% CI 32.51 – 32.98%), and emmetropia at 27.13% (95% CI 26.90 – 27.35%). High myopia was identified in 2.38% (95% CI 2.31 – 2.46%) of individuals. The mean SER of the right eye was −0.06 ± 2.38 (SD) D. The distribution was leptokurtotic with a median SER of +0.125 D (range −26.00 to +18.50 D). Fig. 1 shows the distribution of SER was asymmetric with a greater occurrence of persons with higher myopic corrections.
Within this clinic population, females were found to have a more myopic mean SER than males (−0.14 ± 2.44 D compared to +0.04 ± 2.29 D; t = 14.253, p < 0.001). Amongst those classified as myopic, mean SER was −2.52 ± 2.22 D in females compared with −2.34 ± 2.06 D in males (t = 9.097, p < 0.001), whilst amongst those classed as hyperopes, mean SER was +1.83 ± 1.42 D in females and +1.81 ± 1.46 D in males (t = 2.043, p = 0.04). The proportion of myopia and high myopia was statistically significantly higher in females compared to males (χ2 = 318.17, p < 0.001 and χ2 = 116.51, p < 0.001 respectively), whilst the proportion of hyperopia was higher in males than females (χ2 = 76.55, p < 0.001) (Table 3).
Distribution of High Myopia, Total Myopia, Emmetropia, Hyperopia, Astigmatism and Anisometropia across different age groups and genders (Bold indicates highest proportion).
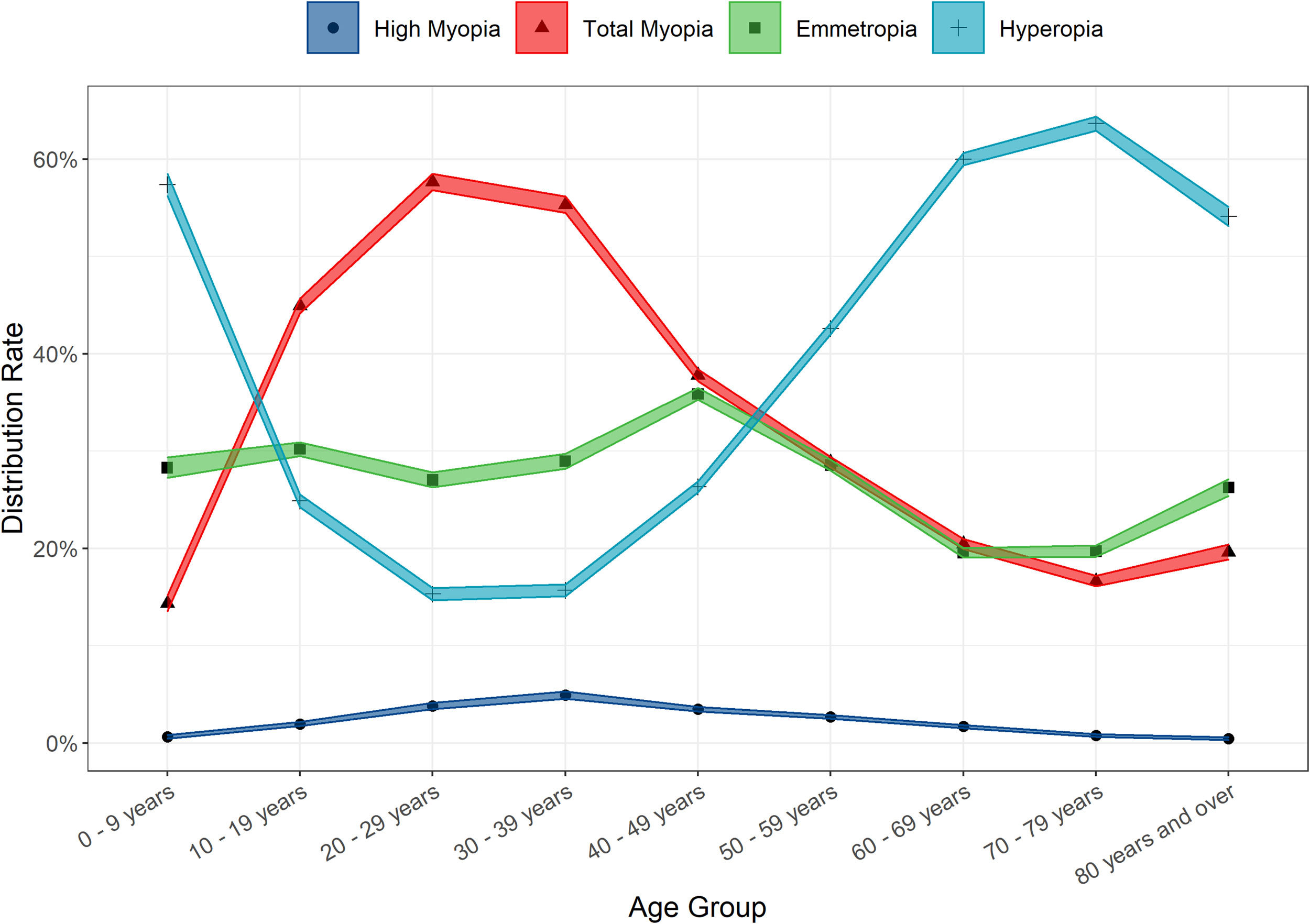
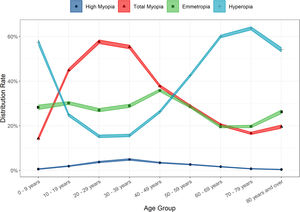
Large variances in the distribution of refractive error amongst the different age groups were observed (Table 3 and Fig. 2). Young children were most likely to be hyperopic, with 57.38% of the 0 – 9-year-old group displaying this refractive error type. A greater proportion of teens, young adults and pre-presbyopes presented with myopia relative to hyperopia or emmetropia. The most myopic age group were 20 – 29-year-olds (total myopia 57.67%, including 3.78% with high myopia), whilst the proportion of individuals with high myopia was highest in 30 – 39-year-olds (4.91%). Hyperopia was the most prevalent refractive state for those aged over 50, and peaked at the 70 – 79 age group (63.65%). (Table 3 and Fig. 2) Age was found to be positively associated with SER (r = 0.18, t = 69.69, p < 0.001).
In the gender and age-adjusted analysis (n = 59,938; mean age 43.15 ± 20.43; female gender 51%), the distribution of myopia increased to 38.83% (95% CI 38.44 – 39.22%), while high myopia increased slightly to 2.83% (95% CI 2.70 – 2.96%). This was matched by the relative decrease in the proportion of individuals classified as hyperopic to 33.50% (95% CI 33.12 – 33.88%), whilst the proportion of emmetropia was marginally higher than in the unadjusted data at 27.67% (95% CI 27.31 – 28.03%). Within this gender and age-adjusted analysis, females were also found to have a more myopic SER than males (−0.42 D vs −0.30 D, t = 6.514, p < 0.001).
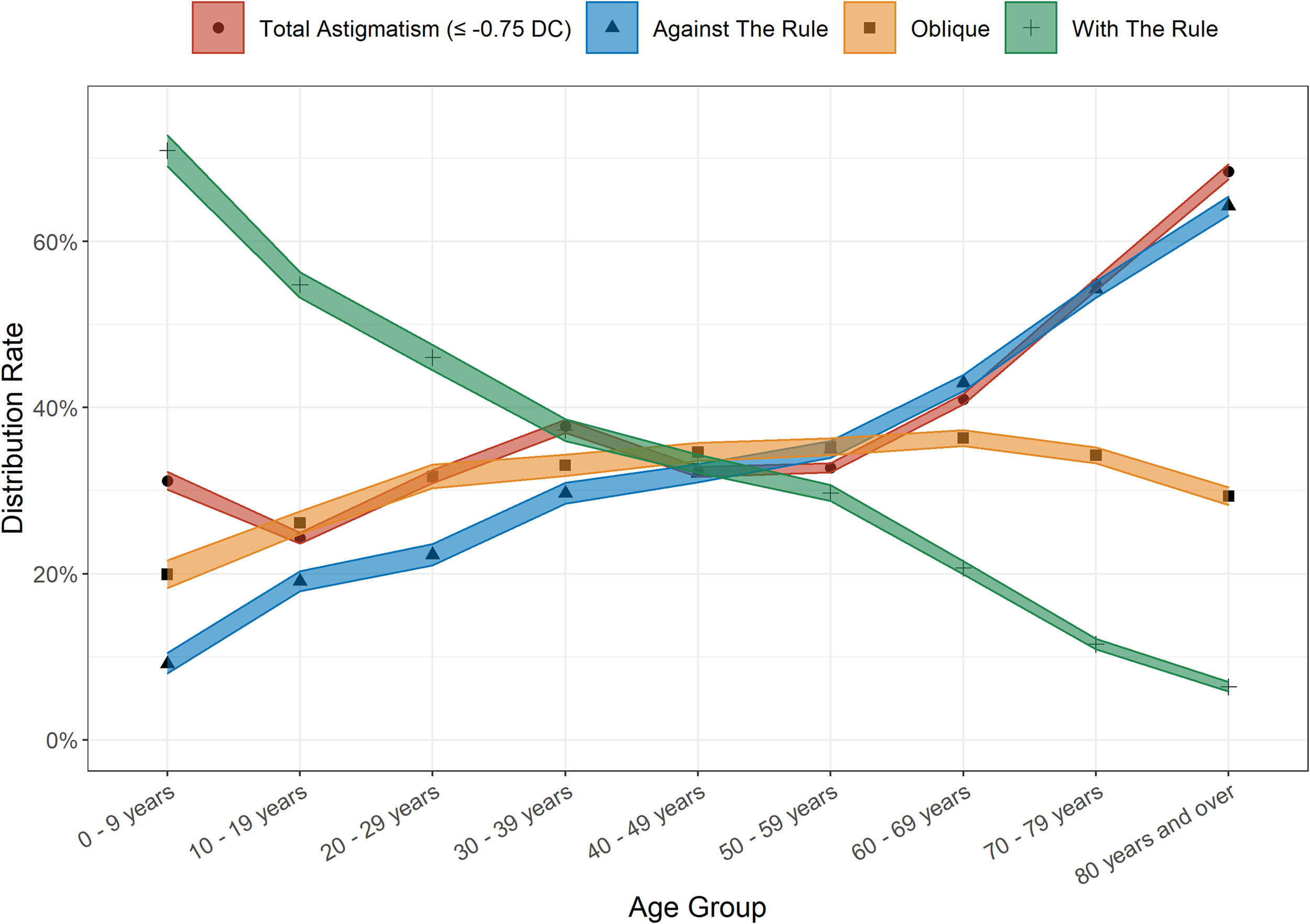
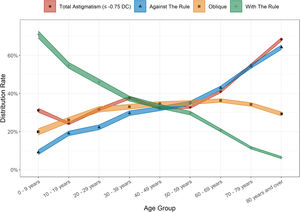
AstigmatismAstigmatism was present in all age groups of this clinic population. Overall, 38.30% (95% CI 38.06 – 38.55%) of persons exhibited astigmatism ≤ −0.75 DC, and was slightly more common amongst males than females (39.07% vs 37.47%; χ2 = 61.78, p < 0.001). The mean level of astigmatism was also marginally higher amongst males (1.43 ± 0.92 DC in males, 1.39 ± 0.87 DC in females; t = 5.94, p < 0.001). The proportion of people who presented with astigmatism increased with age – 24.29% of the 10 - 19-year-old group were affected, compared to 68.41% of the 80 and older group (Fig. 3). Cylinder magnitude was found to be associated with the magnitude of SER, both for myopes (r = 0.17, t = −39.53, p < 0.001) and hyperopes (r = −0.22, t = 56.97, p < 0.001). Against the rule (ATR) astigmatism was most common in the overall sample with 39.22% of persons affected, followed by oblique astigmatism (32.82%) and with the rule (WTR) astigmatism (27.96%). WTR astigmatism was most common in the 0 - 9-year-old group (70.92%) with a steady decline in proportions with this subtype in each subsequent older age group to a minimum in those aged 80 years and over (6.37%). Conversely, ATR astigmatism was at its lowest in the youngest age cohort (9.16%) and peaked in those aged 80 years and over (64.28%). (Fig. 3). Males were more likely to be classified as ATR astigmatism (41.35% vs 37.58%; χ2 = 86.19, p < 0.001), whilst females were more likely to have oblique astigmatism (34.65% vs 30.44%; χ2 = 115.86, p < 0.001). There was no significant gender difference in the rates of WTR astigmatism (male 28.2%, female 27.8%; χ2 = 1.35, p = 0.25). The gender and age-adjusted astigmatism rate was slightly lower at 36.75% (95% CI 36.37 – 37.14%).
AnisometropiaThe overall proportion of anisometropic patients was 13.42% (95% CI: 13.25 – 13.59%) in this clinic sample but varied as a function of age. Table 3 illustrates that the proportion affected was higher in the youngest group of children relative to older childhood and early adulthood groups, with minimal difference observed across older age groups, thereafter, up to age 59. The anisometropia rate became steadily higher, however, in older age groups and peaked at 25.52% in the oldest group of participants aged 80 years and over. There was no statistical difference in the occurrence of anisometropia between females and males (13.55% and 13.25% respectively, χ2 = 2.84, p = 0.09). In addition, there was no difference in mean anisometropia (persons with ≥ 1.00 D) between the female and male cohort (female 1.83 D ± 1.21 D, male 1.82 D ± 1.13 D, t = 0.60, p = 0.55). The proportion of anisometropia was substantially greater amongst highly myopic persons (40.11%, 95% CI: 38.52 – 41.73%) compared to other refractive error types (Total myopia 17.84%, hyperopia 13.98% and emmetropia 7.26%). The gender and age-adjusted distribution of anisometropia was marginally lower at 12.49% (95% CI 12.23 – 12.76%), (Table 4).
Distribution of refractive error types in clinic and census-adjusted population.
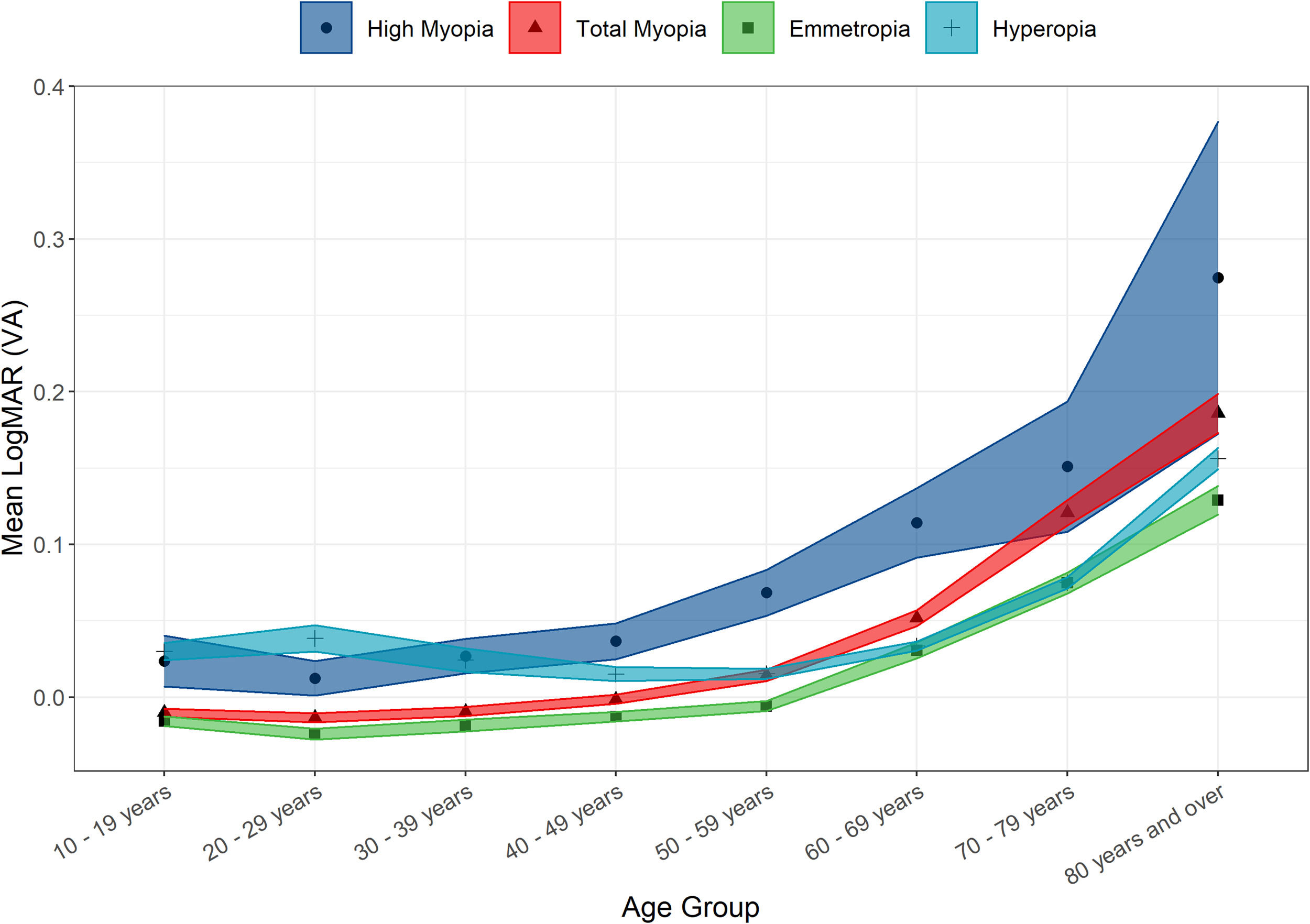
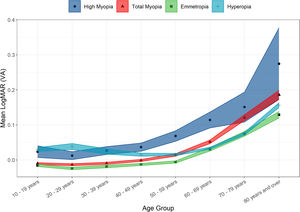
There was a non-clinically significant difference in mean LogMAR BCVA between genders. Amongst those attending optometry clinics, females’ BCVA was slightly lower than in males (0.028 ± 0.158 compared to 0.025 ± 0.168; t = 2.83, p = 0.005). It was also noted that highly myopic and hyperopic persons had statistically significantly worse mean LogMAR VA (0.053 ± 0.168 and 0.048 ± 0.180 respectively) than emmetropes or myopes (0.009 ± 0.147 and 0.017 ± 0.150 respectively; χ2 = 2256.6, p < 0.001). (Fig. 4) As expected, mean LogMAR BCVA was statistically significantly worse in older age groups (r = 0.23, t = 80.532 p < 0.001).
Vision impairmentBased solely on BCVA in the better seeing eye, 2.40% (95% CI 2.31 – 2.49) of the clinic population were classed as vision impaired. Of these, 1.99% had mild VI, 0.39% had moderate VI, 0.01% had severe VI and 0.01% were blind. The overall rate of VI was relatively similar (∼1%) in all younger age groups until age 40 to 49, but a gradual increase in the proportion affected was observed for those in their 50 s and 60 s, with a significantly higher proportion affected in the two oldest age groups, reaching 12.75% among those aged 80 and older. Compared with emmetropes (1.66%), the hyperopic group had the highest proportion of persons with VI (2.91%, χ2 = 123.81, p < 0.001), followed by the myopic group (2.39%, χ2 = 46.03, p < 0.001). The high myopia subgroup exhibited the highest proportion of VI (4.26%; χ2 = 96.06, p < 0.001). However, the myopes with VI were significantly younger, which is an important finding as presented in Table 1 (χ2 = 133.01, p < 0.001). In this clinic population, highly myopic VI persons were even younger (54.09 ± 18.20 years). Females were found to have a higher rate of VI compared to males (2.52%, 2.22%; χ2 = 11.14, p < 0.001). Higher proportions of VI were also observed among those with (compared to without) astigmatism (3.77%, 1.52%; χ2 = 601.18, p < 0.001) and among those with anisometropia compared to isometropes (4.93%, 2.01%; χ2 = 482.20, p < 0.001).
DiscussionThis study evaluated the refractive status of a large clinic going population of people living in the Republic of Ireland. To our knowledge, this study is the first analysis of refractive error and vision impairment in both adults and children carried out in Ireland. Our findings show that the most common refractive error in the overall clinical population was hyperopia, followed by astigmatism and myopia. The relative distributions, however, were highly age dependent and the census-based gender and age-adjustment revealed a higher proportion of myopia overall. High myopes were most likely to be vision impaired, followed by hyperopes, whilst the mean age of those affected by vision impairment was ten years younger in myopes compared to hyperopes.
The hyperopia rates observed herein are higher compared to those reported in the two largest assessments of refractive error in European adults.18,19 However, both studies used a higher threshold of ≥ +1.00 DS SER as their definition, which resulted in 25.2% and 27.6% of persons being labelled hyperopic in the E318 and Biobank19 studies respectively. Using this threshold, we observed similar rates of hyperopia of 27.7% (unadjusted) and 22.1% (age-adjusted) in our study population. Those most likely to be hyperopic were males and older persons, which is consistent with findings in the relatively limited number of previous studies that have explored risk factors for hyperopia.18,19
The clinic distribution of myopia was highly age dependent, with a clear trend for higher proportions in younger adults, and a marked fall off in older age groups. The over 80s age group proved to be the exception where an increase in the proportion affected by myopia was observed – most likely due to cataract.15–17 Similarly, high myopia was most frequently found in younger adults. The overall observed rates of myopia and high myopia were broadly in line with that found in prevalence studies conducted in similar European populations15,18 although lower than findings in East Asia.33 Females were found to have slightly higher proportions of myopia as have been observed elsewhere,19 although several studies have also found no differences.34 A recent population-based analysis of risk factors for myopia in Irish school children (ages 6–7 and 12–13) found no relationship between gender and myopia,35 however, the difference in age ranges means these results are not directly comparable. Higher risk of myopia in females may be associated with increased level of education, a significant risk factor for the development of myopia,21 with the latest Irish census reporting a much higher proportion of young women with third level (university) education than men.36
Astigmatism was the second most common refractive error in the unadjusted and adjusted analyses, and was observed to increase with age, a finding that has been observed previously.12–14,16,17 Astigmatism type shifted from WTR dominance in younger age groups to ATR dominance in older groups, consistent with previous reports.12,14 Astigmatism prevalence around the world appears to be highly variable, with lower prevalence rates observed in some similarly aged populations13,15 and higher prevalence found in others.12,14,17 The reasons for differing rates of astigmatism across populations remain elusive,38 although it has been suggested that higher levels of refractive error are associated with higher levels of astigmatism, 38 which is supported by our findings herein.
The proportion of anisometropia in this clinic-based study was broadly in line with observations in prospective studies.12,14,15 Anisometropia prevalence was observed to increase with age, a common finding in other epidemiological studies12,14,15 and likely as a result of age-related changes in the crystalline lens. The anisometropic burden amongst hyperopes aged 9 and under was remarkably high, affecting one in five children in that cohort. This number is overstated relative to true population levels due to the relative lack of asymptomatic children in the optometry clinic population. Nevertheless, this is concerning given the implications for amblyogenesis in this age group. Anisometropia of 1 D has been shown to cause reduced visual acuity in the weaker eye amongst hyperopic children,11 and was reported by Harrington et al.39 as the leading cause of amblyopia in the Ireland Eye Study (IES), a population study of refractive error in a pediatric cohort (ages 6–7 and 12–13).
The rate of vision impairment found in this population was much lower than that estimated by the Vision Loss Expert Group for Western Europe (2.4% vs 7.5%).1,40 A possible explanation for this difference is that the data from this study does not include vision impairment due to undercorrected refractive error as it was not possible to determine this from the EMR dataset. It is unlikely however that this represents a significant source of vision impairment in Ireland as there is particularly good access to eyecare due to two widely used Irish government subsidised schemes providing free eye exams and subsidised or free spectacles to the majority of the population.41,42 Criteria for registering blind with the National Council for Blind in Ireland (NCBI) include BCVA of 6/60 or less and reduced field of vision (not greater than 20° at its widest). Previous studies have estimated this to be around 0.2% of the Irish population 16 years and over.43 The equivalent value within this EMR dataset was 0.02%. The lower proportion affected herein may be partly explained by the lack of visual field information in the EMR data and the likelihood that persons who meet NCBI criteria for blindness no longer attend primary care optometry clinics and are seen within specialist low vision clinics and hospital ophthalmology clinics for more comprehensive care.
The main limitation of this study is that it was clinic- rather than population-based. This means that some groups are underrepresented, most notably younger emmetropes and low hyperopes who do not yet require refractive correction. Indeed, our EMR data overestimated myopia rates when compared to the IES for very young children (10.1% vs 3.3% in 6 – 7-year-olds).25 There was also a gender imbalance in the attendance of the optometry practices, with higher numbers of females attending. This is a common finding in other healthcare settings.44 Given the gender and age-related variations in the distribution of refractive error observed herein, the EMR data was sub-sampled to mirror the population distributions recorded in the most recent Irish census. This census-based gender and age-adjusted analysis showed a higher rate of myopia and a lower rate of hyperopia than the original EMR data. This is not surprising as the original EMR data contains an older age distribution (mean age 47.9 years) which is more likely to be hyperopic compared to the younger national population (mean age 37.4 years). Despite this, the variance in the adjusted and unadjusted refractive error rates is small and the census-matched rates were still similar to other European population studies.15,18 This selection bias is likely to be less significant in older age ranges due to the universal need for near vision correction due to presbyopia. This is particularly the case in countries like Ireland where the sale of prescription eyewear and contact lenses are regulated, whilst access to free eye examinations and eyewear removes one of the most significant barriers to eyecare for the majority of the public. This is evidenced by survey results from the UK, where eyecare is also subsidised, which demonstrated almost 100% of adults over the age of 50 used corrective eyewear.45 In addition, medical records-based estimation of clinically significant refractive error distribution rates has been shown to be a valid methodology,28 particularly where conventional studies are absent. This implies the results for the older age groups herein (> 50 years) might be generalisable to the population of older adults where the majority of people will have symptomatic refractive error, including emmetropes who develop presbyopia. For the younger groups (< 50 years) there is less certainty that these results are generalisable due to selection bias. The gender and age-adjusted subset helps to deal with this issue.
Other limitations include that it was not possible to assess the effect of urbanicity on refractive status. The EMR data, however, had good geographic coverage, with all 26 counties in the Republic of Ireland represented, including 55.6% in the east (most populated region), 21.7% in the west, 20.8% in the south and much lower coverage (1.8%) in the north. Ethnicity was also not recorded in the EMR data, but the Irish population is predominantly white (91.7%), so ethnic influences on the distribution in Ireland is likely to be small. The method by which refraction was carried out was also unknown, but likely to be largely based on non-cycloplegic subjective refraction. Cycloplegic refractions are considered the gold standard for refractive error epidemiology, particularly in younger people.46 The use of cycloplegia is not widespread in adult studies of refractive error epidemiology47 and hence this study should compare well in older age groups given the low variability in refractive error between cycloplegic and non-cycloplegic eye exams.48 Nevertheless, this limitation could potentially lead to some misclassification, particularly among younger individuals close to the hyperopia and myopia classification thresholds. One other limitation is the use of the spectacle prescription for the analyses, which the prescribing optometrist may alter for a variety of reasons. However, if these modifications are made, they are often small and can occur in both positive and negative directions and hence, should have a minimal effect on the results herein.49 It should also be noted that persons with diseases and history of ocular surgery were not removed from the data. The EMR data may, therefore, include some individuals that have had cataract or refractive surgery which may affect their reported refractive status. The literature indicates, however, that although the rates of surgery have increased, they still represent less than 1% of all individuals.50 Given the large number of participants, it is unlikely that this would have a significant impact on the reported distributions.
Although the findings should be interpreted with caution, this study has several strengths. Most importantly, the clinic sample size was relatively large relative to the population size (equivalent to 3.2% of the Irish population as per the 2016 census), which affords small confidence intervals. As the data is relatively current, future extractions of EMR data can facilitate longitudinal comparisons relatively easily, quickly and inexpensively.
ConclusionThere are currently no ongoing epidemiological studies on refractive error in Ireland, and gathering fully representative data would take many years and significant funding to design and implement. Although the IES25 explored refractive error in a small representative cohort of school going children aged 6 – 7 and 12 – 13 years, this EMR study provides the first data on refractive error distribution across the full age profile of the Irish population. EMR data is a useful resource and may be reasonably representative in older age groups in which ocular disease and vision impairment associated with refractive error is most likely to become manifest. Critically, the most important contribution of this study is to provide a comprehensive baseline assessment of the distribution of refractive error and associated factors in the clinic-going population. This will be particularly useful from a health planning perspective when combined with future EMR data extractions to facilitate contemporaneous evaluation of the changing distribution patterns in this clinical population.
Conflicts of interestNone of the authors have any conflicts of interest related to this submission.
Financial SupportNone.
This paper has not been published or simultaneously being considered for publication in any other journal.