Alzheimer's disease (AD) is a neurodegenerative dementia characterized by the deposition of extracellular β-amyloid (Aβ) plaques and the presence of neurofibrillary tangles. Until now, the techniques used to analyze these deposits have been difficult to access, invasive, and expensive. This leads us to consider new access routes to the central nervous system (CNS), allowing us to diagnose the disease before the first symptoms appear. Recent studies have shown that microglial and macroglial cell activation could play a role in the development of this disease. Glial cells in the CNS can respond to various damages, such as neurodegenerative pathologies, with morphological and functional changes. These changes are a common feature in neurodegenerative diseases, including AD. The retina is considered an extension of the CNS and has a population of glial cells similar to that of the CNS. When glial cells are activated, various molecules are released and changes in glial cell expression occur, which can be indicators of neuronal damage. The objective of this review is to compile the most relevant findings in the last 10 years relating to alterations in the eye in AD, and the role that glial cells play in the degenerative process in the retina in the context of neurodegeneration.
La enfermedad de Alzheimer (EA) es una demencia neurodegenerativa que se caracteriza por la deposición de placas beta-amiloides (βA) extracelulares, y la presencia de ovillos neurofibrilares. Hasta ahora, las técnicas utilizadas para analizar estos depósitos han sido poco accesibles, invasivas y costosas. Esto nos lleva a considerar nuevas vías de acceso al sistema nervioso central (SNC) que nos permitan diagnosticar la enfermedad antes de la aparición de sus primeros síntomas. Los estudios recientes han reflejado que la activación de las células microgliales y macrogliales podría desempeñar un papel en el desarrollo de esta enfermedad. Las células gliales del SNC pueden responder a diversos daños, tales como patologías neurodegenerativas, con cambios morfológicos y funcionales. Dichos cambios son una característica común en las enfermedades neurodegenerativas, incluyendo la EA. La retina se considera una extensión del SNC, y cuenta con una población de células gliales similar a la de dicho sistema. Cuando las células gliales se activan, se liberan diversas moléculas, y se producen diversos cambios en la expresión de las células gliales, que pueden ser indicadores de daño neuronal. El objetivo de esta revisión es recopilar los hallazgos más relevantes de los últimos diez años, con relación a las alteraciones oculares en la EA, y el papel que juegan las células gliales en el proceso degenerativo de la retina en el contexto de la neurodegeneración.
Alzheimer's disease (AD) is a disabling and progressive neurodegenerative disease with no cure that leads to cognitive impairment and memory loss, affecting millions of people around the world.1 Because the retina is considered an extension of the central nervous system (CNS), it seems clear that the use of new, non-invasive retinal analysis techniques could allow for the evaluation of retinal glial cell activation, and establish this as a new tool for AD diagnosis. This review gives a brief description of AD and its manifestations in the eye, as well as a description of glial cells and their changes in this disease.
Material and methodsA literature search was performed up to September 2017 using the MEDLINE database, PubMed, and Google Scholar search services with the following key words and word combinations: Alzheimer, glia, glia-retina, Alzheimer-glia, Alzheimer-astrocytes, Alzheimer-microglia, Alzheimer-eye, Alzheimer-retina, Alzheimer-glia-eye, Alzheimer-glia-retina, and Alzheimer-Müller cells. After filtering by author criteria (articles published in the last 10 years), English or Spanish language, and the condition that all address the relationship between AD and retinal glial cells as the main subject, 250 articles were considered.
Alzheimer's diseaseAD is a progressive neurodegenerative disease characterized by a decline in cognitive function and memory resulting from synapse and cell loss, accompanied by a strong neuroinflammatory response. It accounts for 60–80% of dementia cases, and is the leading cause of these in the population over 60 years old.2
AD may be familial or sporadic in origin. The incidence of familial cases is low (5–10%), and is related to mutations in three different genes: amyloid precursor protein (APP), presenilin 1 (PSEN-1) and presenilin 2 (PSEN-2). Sporadic AD accounts for 90–95% of all cases, and has a multifactorial pathogenesis consisting of a combination of genetic and environmental factors, in which age is the leading risk factor.3 The main gene associated with the sporadic manifestation of the disease is the apolipoprotein E gene (APOE). Of the three isoforms, APOE ɛ4 is found in 50% of patients affected by AD and carries a three-fold risk of developing the disease. In the CNS, APOE is expressed predominantly by astrocytes but also by microglia.4,5
Pathologically, AD is characterized by deposits of extracellular plaques of β-amyloid (Aβ) peptide or senile plaques and neurofibrillary tangles (NFTs) resulting from intracellular aggregates of hyperphosphorylated tau protein.6 Deposition of Aβ plaques, the first manifestation of the pathogenesis of AD, begins many years before symptoms appear. Although tau protein aggregation occurs after the appearance of the senile plaques, it is evident before the clinical onset of the disease. These characteristic changes are well-reflected in the cerebrospinal fluid (CSF), which demonstrates altered levels of tau protein and phosphorylated tau, as well as Aβ levels at the onset of the disease pathogenesis. The changes in these biomarkers can be seen even in the preclinical stages of the disease, and can be useful to determine which patients will develop AD over time.7 A clinical diagnosis of AD can only be definitively confirmed by a post-mortem examination of the brain. A diagnosis of probable AD is possible only when the disease has considerably progressed, and neurological damage has already occurred.8
Glial cellsGlia are a highly homogeneous population of non-excitable cells distributed throughout the CNS, and are essential for normal brain function.9 The main function of the glia is to maintain the homeostasis of the nervous tissue and to control, protect, and support neuronal function. They also respond to CNS injuries as a defense mechanism, and when dysfunctional, they can even be primary pathogenic agents.10 In the CNS, astrocytes and oligodendrocytes are neuroectodermic in origin, and make up the macroglia. Microglia originate from the mesoderm, and are considered the macrophagous element of the CNS.9
AstrocytesThe main population of glial cells consists of astrocytes. They account for about one in three cells of the brain mass. They are essential for correct homeostasis in the brain tissue and for neuronal function, and create a barrier around blood vessels that limits the passage of immune cells to the CNS. Astrocytes are resistant to cell death by apoptosis, and are therefore able to survive attacks from inflammatory processes.
The main functions of astrocytes include metabolic support of the neurons and their dendrites and synapses, glycogen storage and lactate export, neurotransmitter uptake, including glutamate, protection against reactive oxygen species (ROS), provision of neurotrophic factors to neurons and oligodendrocytes following injury such as nerve growth factor (NGF), ciliary neurotrophic factor (CNTF) and tumor necrosis factor (TNF-α) among others, homeostasis, blood-brain barrier (BBB) induction and maintenance, scar formation and tissue repair, and regulation of immune response in the CNS.11
The astrocyte processes stending radially out from the cell body give it a stellate appearance. The cytoplasm of these cells contains a large number of intermediate filaments composed of glial fibrillary acidic protein (GFAP), which is specific to astrocytes and reactive Müller cells and allows them to be selectively labeled via immunocytochemical techniques.12
Astroglia play a central role in responding to all forms of pathological conditions such as trauma, ischemia, and neurodegenerative diseases. It has been suggested that reactive astroglia (astrogliosis) either release neurotrophic factors to support cell survival or produce molecules that inhibit axonal repair and regeneration, triggering neurological cytotoxicity, which causes secondary damage in nearby neurons.13 A better understanding of glial reactions to lesions and their contribution to the development of the disease can assist in designing new treatment strategies.14
OligodendrocytesOligodendrocytes make up about 5–10% of the total glial cell population. They are essential for the rapid and efficient conduction of electrical impulses along the axons, as well as for preserving axonal integrity.15 Oligodendrocytes have a very limited capacity for regeneration; the injury of just a few can create an appreciable area of demyelination. They are the first nervous elements that degenerate in diseases of the CNS where myelin is affected.16 In the retinas of most vertebrates, the axons are unmyelinated. Oligodendrocytes, which are responsible for axonal myelination in the CNS, are therefore absent in the retina.17
MicrogliaMicroglia are the cells that make up the CNS immune system, and are found in all regions of the CNS, including the retina.18 They represent 5–10% of all adult human brain cells.4 Their functions include surveillance of the surrounding environment, elimination of invading microbes, clearance of cellular debris, and enforcement of programmed cell death via the removal of apoptotic cells. They also release brain derived neurotrophic factor (BDNF) and anti-inflammatory cytokines involved in neuronal survival. Therefore, in addition to their role as immune defense cells, they are vital for the endogenous repair of nerve pathways. Along with their anti-inflammatory and neuroprotective effects, they play a critical role in the normal development of angiogenesis.
Microglia are a dynamic cellular population and exist in various states of activation associated with distinct morphological transformations.19 In the absence of damage or pathologies, they present in their baseline or resting state.20 Each of these cells occupies one area and has no physical contact with the others.21 Even in the baseline state, they continuously monitor their surrounding environment.
Synaptic formation is regulated by the microglia, and seems to depend on the production and release of molecular mediators such as BDNF or interleukin-10 (IL-10), among others.22 The ability of microglia to enhance the release of BDNF in the context of aggression accelerates healing and constitutes a damage reduction mechanism. It seems that cytokines considered classically injurious, such as IL-1β and TNF-α, facilitate the phagocytosis of cellular debris and trigger many other events necessary for neural repair.19
Since adenosine is an important neuromodulator of the immune-inflammatory system, microglia express all subtypes of adenosine receptors A1, A2A, A2B and A3. In the case of A2AR, this protects neurons from primal proinflammatory neurodegeneration, however other brain injuries that trigger neuroinflammation cause upregulation of this chemical in the microglial cells, where it appears to play an important role in the pathophysiology of degeneration.22
Microglia express both class I and class II major histocompatibility complex molecules (MHC). In the presence of certain cytokines these increase, allowing the microglia to act as antigen-presenting cells and to become an integral part of the immune network.19
Glial responseIn situations such as neural damage, disease, or even aging, the glia can respond by undergoing morphological and functional changes accompanied by the production of proinflammatory cytokines.23 This general glial response to aggression or inflammation in the adult brain is called reactive gliosis.24 Reactive gliosis has a neuroprotective effect, however chronic gliosis exacerbates disease progression by increasing vascular permeability, infiltration of toxic compounds, and even through neovascularization.25 Oligodendrocytes do not usually demonstrate reactive changes; astrocytes and microglia are the main populations of reactive glia.23
Microglia exist in a symbiotic relationship with neurons, where microglia sustain the neurons and neurons maintain the microglia in a noninflammatory phenotype.19 Because of their ability to activate rapidly upon aggression,24 the microglia normally initiate and direct the coordinated neuroinflammatory response.
Activated microglia change from a ramified form to a non-phagocytic hyper-ramified or hypertrophic phenotype, and finally to an amoeboid morphology (especially if there is degeneration of terminals and/or neurons), retracting their extensions. This allows migration through the parenchyma to the site of the injury.21 There are two activated polarization states.19 Under stress conditions, microglia release anti-inflammatory cytokines and growth factors, transition to the M2 phenotype induced by IL-4 and IL-13 and express mannose receptor CD206 and arginase 1, which downregulate inflammation and promote tissue remodeling/repair and angiogenesis.4 When overactivated, they transition to the M1 phenotype, characterized by the production of nitric oxide (NO), superoxide radicals, proinflammatory cytokines such as IL-1β, TNF-α, and chemokines, in order to amplify the inflammatory response and eliminate the agent causing the aggression,21 thereby playing a central role in the defense against pathogens and tumor cells.19 The M1 phenotype alters the permeability of the BBB, allowing the entry of immune mediators, natural killer cells (NK) and lymphocytes. This immune response promotes both tissue repair and neuronal damage.21 The attack on native neuronal cells can have serious consequences if the process deregulates19 and becomes chronic, where the M1 phenotype becomes phagocytic and harmful, as occurs in neurological diseases, including AD.4 Additionally, deregulated, active microglia may contribute to pathological neovascularization in the brain and retina. The precise mechanisms of this process are not yet fully understood.19
Reactive astrogliosis is an astrocyte response to polyetiological insults to the CNS, characterized by a jump in astrocyte numbers (hyperplasia/proliferation), increases in the number and length of astroglial processes, larger cell body size (hypertrophy), migration, and upregulation of cytoskeletal components such as GFAP, vimentin, and nestin.25 Thus, an astrocyte phenotype change results in the expression of non-detectable molecules in resting astroglia,21 such as the enzyme heme oxygenase 1 (HO-1),26 and a reduction in the synthesis of glutamine synthetase (GS). The cerebral astrocytes of AD patients have demonstrated increased GFAP expression.26 Reactive astrocytes produce NO as an autocrine apoptosis mediator21 and can secrete inflammatory cytokines such as IL-1β, IL-6, IL-8, inflammatory chemokines, and other chemokines involved in recruiting microglia, monocytes/macrophages, T lymphocytes and dendritic cells.25 Because of this, astrocytes are considered immunocompetent cells. Astrogliosis may favor either neuronal survival or the formation of a glial scar that prevents the regenerative process.21 Reactive astroglia have been associated with the stimulation of higher metabolic activity, upregulation of cytoprotective factors and antioxidant defenses that protect neurons from free radicals, restoration of the neurotransmitter balance, and regulation of ion and water concentrations. When the CNS is injured, reactive astrocytes migrate to the damaged site, isolate the injured area, and remove pathogens, dead cells, and cellular debris, and then remodel the nerve tissue. Chronic astrogliosis, however, is typically detrimental, directly or indirectly damaging neurons and the vasculature while also inhibiting tissue repair. Through the production of vascular endothelial growth factor (VEGF), astrogliosis exacerbates disease progression by increasing vascular permeability, affecting the properties of the BBB, facilitating the infiltration of peripheral immune cells, and even by facilitating neovascularization. Astroglia also produce molecules that inhibit axonal repair and regeneration, triggering neurotoxicity and secondary damage in nearby neurons and glial cells. When dysfunctional, astroglia can also constitute the primary pathogenic element.25
Neuroinflammation essentially depends on the activation of microglia and astrocytes, which respond to various injuries and stimulations through the expression and release of molecules that subsequently cause oxidative stress. Cytotoxic activation and inflammatory factors stimulate glial cells to release proinflammatory signals, creating a vicious cycle of neuroinflammation and oxidative stress, resulting in an autoinflammatory condition. In this situation, proinflammatory cytokines secreted by active microglia inhibit astrocytic gap junction communication, and the astrocytic neuronal support function is affected. This has been observed in AD and could explain the cell death, although it may also play a defensive role, preventing the diffusion of toxic molecules. The microglia also release the neurotransmitter glutamate, which triggers excitotoxic neurodegeneration and cell death in astrocytes and oligodendrocytes.4 Astrocytic glutamate uptake leads to neuronal loss. Elevated levels of TNF-α have been shown to stimulate BDNF secretion, supporting neuronal survival.25 Pathogens themselves are able to recruit proinflammatory microglia and perpetuate the pathology.19 The accumulation of active microglia and reactive astrocytes is a common feature in neurodegenerative diseases, including AD.21
Neurodegeneration, glial response and Alzheimer'sIt has been shown that both microglia and astrocytes, not neurons, are the main source of N-terminally truncated Aβ peptides that can be found in amyloid deposits.2 Aβ plaques stimulate the factors and proteins that induce the production of inflammatory cytokines and chemokines. Deregulated proinflammatory cytokines and chemokines have been found in AD. Certain forms of inflammation and microglial activation are beneficial in pathologies such as AD.4 It is not known whether glial activation is the cause or a consequence of AD, but it certainly contributes to its development.
Aβ oligomers induce a potent inflammatory response and distort the phagocytic microglial response, leading to the excessive and sustained production of inflammatory mediators that could explain neurotoxicity and neuronal loss. Astrogliosis is also involved in the neural degeneration associated with AD.2 Dysfunction in the astrocytic regulation of microglial phagocytosis has been observed in AD.4
One way in which Aβ accumulation may lead to neurodegeneration is through oxidative stress whereby molecules, such as deoxyribonucleic acid (DNA) and proteins, are damaged due to excessive oxidation resulting from an imbalance between oxidant and antioxidant activity. This imbalance may be due to the oxidizing properties of the Aβ peptide, which is thought to produce hydrogen peroxide and induces ROS accumulation. It appears that astrocytes are able to release NO, which is an ROS, in an attempt to counteract the reduced cerebral blood flow in AD. Such a release could be counterproductive if NO levels become toxic. In degenerative disorders, oxidative stress initiates apoptosis in healthy cells. In response to this apoptosis, the complement system can be activated to remove debris derived from cell death which, under normal circumstances, is deactivated upon completion of the process. However, it has been observed that in AD, this deactivation process can be abandoned when the complement system response is exacerbated, which also leads to cell death. When microglia detect that the complement system has been activated, they become active as well, and have been associated with the pathogenesis of AD, age-related macular degeneration (AMD), and glaucoma.27In vivo and in vitro studies suggest that microglia recruited to Aβ plaques sites are able to surround and phagocytize Aβ peptides. The Aβ deposits are responsible for microglial activation in a way that is strictly dependent on the amyloid load.4 In turn, microglia can produce complementary factors and thus reinforce the activation of the complement system response by creating a vicious cycle.27
Microglial activation and the production of proinflammatory mediators by neurons positive for phosphorylated tau can contribute to neuronal death and disease progression in neurodegenerative tauopathies such as AD. Although plaques and tangles remain the major hallmarks of AD, studies show that activated microglia are one of the major players in neuroinflammation, along with plaque deposition and astrocyte stimulation.4
AD and the eyeThe increasing prevalence of AD, combined with the need to treat the disease in its early stages before irreversible neurological damage occurs, prompts us to search for accessible in vivo brain structures that allow for early diagnosis of the disease.8
The retina and optic nerve share an embryologic origin with the brain, and have similar patterns of vascularization, self-regulating blood flow, and blood–tissue barrier functions. As part of the CNS, the retina has a specific immune privilege provided by the BBB. The blood–retinal barrier (BRB) is similar in structure to the components and mechanisms of the BBB.2
Because of these similarities, the retina can be inspected to detect AD markers such as Aβ deposition. The retina has been shown to be an important tool for the in vivo visualization of changes in AD, their pathogenesis, and progression and response to AD therapies (Table 1).
Retinal changes in Alzheimer's disease.
| Reduction in the number of retinal ganglion cells (RGC) | 6,28,32,41–43 |
| Decreased thickness in the retinal nerve fiber layer (RNFL) | 6,28,31,34–36,38,42–44 |
| Decreased choroidal thickness in the foveal area | 6,30,45–47 |
| Vascular disorders | 39,48–50 |
| Visual field reduction (VF) | 6,40,51,52 |
| Accumulation of tau and Aβ | 29,37,53,54 |
Post-mortem analyses of the retinas of AD patients have revealed significant axonal degeneration in the optic nerve head and a reduced number of retinal ganglion cells (RGCs) associated with reduced thickness in the retinal nerve fiber layer (RNFL).28
Using immunohistochemical techniques, the presence of both tau and Aβ has been demonstrated in the human retina. Accumulation of APP has been shown in RGCs and the RNFL. Retinal deposits of phosphorylated tau have also been reported in the retinas of AD patients.29
The use of several different non-invasive techniques makes it possible to analyze in vivo the retinal changes that occur with AD.
OCT allows us to determine RNFL thickness and RGC loss. Patients with AD present peripapillary decreases in RNFL thickness, with the most pronounced changes occurring in the superior quadrant of the retina,31 as well as a decreased number of RGCs in the temporal foveal region,32 which is most pronounced in the foveal and parafoveal region.33 Using OCT, several patterns of RNFL thinning are evident in Alzheimer's patients, including retinal nerve fiber loss in the superior retina,34 superior and nasal retina,35 and the superior and inferior retina.36 It has been postulated that RNFL deficiencies may be the earliest sign of AD, even before damage to the hippocampus region occurs, resulting in memory changes.30 A statistically significant generalized loss of foveal choroid thickness in patients with mild and moderate AD has been found using optical coherence tomography (OCT).30
Recent studies suggest that fundus auto-fluorescence (FAF), a method for detecting highly fluorescent structures, may be used in conjunction with OCT to analyze structures of interest, leading to a possible visualization of perimacular and perivascular Aβ deposits, primarily in the outer plexiform layer, the ganglion cell layer (GCL) and the RNFL of AD patients.37
Confocal scanning laser ophthalmoscopy has revealed changes at the optic nerve head in AD patients in comparison to age-matched controls, demonstrating a significant decrease in RNFL thickness in AD patients compared with controls, as well as larger cup-to-disc ratios.38
Using retinography, vascular alterations have been identified in patients with AD. The retinal vessels of AD patients show vascular attenuation, arterial and venous caliber decreases, and decreased venous flow.39
The visual field (VF) reduction shown in AD patients, occurring predominantly in the lower field, is directly related to RNFL thinning found in the superior quadrant.40
Glia and the eyeThe retina, although developmentally part of the brain, is a unique CNS structure in many aspects. The glial cells of the retina are one example of this. In the retina, the principal glial cells are the Müller cells,17 which together with the astrocytes constitute the macroglia of the human retina. In addition to macroglial cells, the retina contains microglial cells, the resident immune cells of the retina.55 The microglia are blood-derived phagocytic cells.56
The retinal microglia are mainly located in the plexiform layers, the GCL, and the RNFL, and possess highly mobile extensions that interact with the surrounding environment.57 Microglial cells are essential for normal retinal growth, neurogenesis,58 and proper formation of retinal blood vessels.59 They interact with Müller cells to form a network that serves as a control system for trophic factor in retinal degeneration.2
Microglial communication with neurons, the vascular system, and the macroglia is mediated by a diverse range of factors. These include chemokines, cytokines, nucleotides, surface proteins and adhesion molecules. The microglia may be activated by a perturbation of chemokines and/or receptor expression.56 Activated microglia can proliferate and migrate to regions of retinal damage, where their morphological changes are often accompanied by changes in signaling and gene expression.60 They are involved in phagocytosis, secretion of trophic factors, and active remodeling of neurons and synapses.56
Retinal microglial cells perform various tasks that are essential for the normal physiological development of the retina, and are constantly in a close relationship with neurons.25 The morphology of astrocytes in the human retina changes from the star shape in the GCL to the elongated shape in the RNFL. The somas of human astrocytes can mainly be found in the layers mentioned above, but extensions of these can be found even in the inner nuclear layer.61 Mouse astrocytes, however, are star-shaped and can only be found in the GCL and in the RNFL.62
Müller cells are an anatomical link between the retinal neurons and structures such as the retinal blood vessels, the vitreous body, and the subretinal space, which need to exchange various molecules.63 Astrocytes and Müller cells maintain the ion and water homeostasis of the retinal tissue, including the pH,64 as well as the homeostasis of neurotransmitters such as glutamate and gamma-aminobutyric acid (GABA).65
Macroglial cells are also involved in retinal glucose metabolism, providing retinal neurons with the necessary nutrients for oxidative metabolism, and in removing metabolic waste products. Müller cells and astrocytes have also been demonstrated to be more resistant to oxidative damage than neurons, due to their high concentrations of antioxidants that protect them against such damage.25 These antioxidants, such as glutathione, which are provided to the neurons, eliminate free radicals and reactive oxygen compounds.63 Another neuroprotective mechanism is the uptake and/or detoxification of potentially harmful substances, which involves the phagocytosis of debris from dead neurons or retinal pigment epithelial cells.66 Astrocytes and Müller cells are involved in regulating local blood flow in response to changes in neuronal activity. Substances such as prostaglandins (PG), NO, and arachidonic acid (AA), which regulate blood flow in the CNS, are produced by astrocytes.67 Macroglial cells exercise their barrier properties in the retinal capillaries, called the BRB.25
As mentioned above, astroglial cells defend the CNS, and therefore the retina, from damage through a process called reactive gliosis. This gliosis is triggered in response to trauma, ischemic damage, neurodegeneration, or neuroinflammation.68 Müller cells, due to their radial distribution, are usually one of the first types of glial cells to detect retinal damage, providing rapid response to any change in the retinal microenvironment.69 Müller cell gliosis is characterized by both non-specific and therapeutic responses, upregulation of GFAP, and the activation of extracellular signal-regulated kinases (ERK).63 GFAP upregulation is therefore used as a common marker for reactive Müller cells and as an indicator of retinal stress, retinal injury, and Müller cell activation. Practically all retinal diseases are associated with Müller cell gliosis.70
After retinal detachment, Müller cells migrate to the affected area and react by establishing a glial scar, replacing degenerated neurons and photoreceptors.71 Glial scars involve the expression of inhibitory molecules on the surface of reactive glial cells, which inhibit both regular tissue repair and neurodegeneration, harming the function and structure of retinal neurons.69
Müller cell gliosis has both cytoprotective and cytotoxic effects on retinal neurons.72 In the context of a threat to the retina, the so-called conservative or non-proliferative gliosis is neuroprotective, releasing neurotrophic factors and antioxidants which favor neuronal survival and limit the extent of the tissue damage.73 The most severe injuries provoke another response described as massive or proliferative, in which gliosis becomes detrimental to the retinal tissue and increases neuronal death.63
A possible trigger for the transition from conservative to massive gliosis is a breakdown of the BRB, augmenting both the retinal and vitreous content of growth factors, cytokines and inflammatory factors, and allowing the infiltration of blood-derived immune cells.74 The excessive and prolonged expression of VEGF after massive gliosis can lead to retinal inflammation, neovascularization and vascular lesions.75 High concentrations of NO after Müller cell activation can damage neurons,76 while lower levels may have a protective effect, as in the protection of neurons against glutamate excitotoxicity and decreased retinal ischemia via its vasodilator effect.77
Another important feature of gliotic Müller cells is their potent cross-communication with cells from the immune system. Molecules from inflammatory cells may activate Müller cells, and these cells may express a wide variety of inflammation related factors, such as TNF-α.69
Retinal glia in ADRetinal glial cells can establish significant communication amongst themselves, which have been suggested to act as mediators of neuronal-glial interactions, acting as sensors for neurotransmission signals, and contributing to the maintenance of neuronal activity and homeostasis in the healthy CNS.2
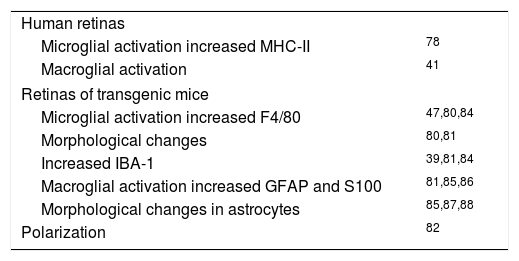
The glia, as a population of immune cells residing in the retina and optic nerve, are able to activate and respond rapidly to any type of damage.25 Changes in glial cells have been found in the retinas of AD patients (Table 2). Upregulation of MHC-II expression (a microglial reactivity marker) has been observed in the donor retinas of AD patients in comparison to those donated by normal patients. This increase occurs in the absence of lymphocytic infiltrate, suggesting that the pathogenesis of the retina in AD may be different from that observed in certain areas of the brain.78
Glial activation in Alzheimer's disease.
| Human retinas | |
| Microglial activation increased MHC-II | 78 |
| Macroglial activation | 41 |
| Retinas of transgenic mice | |
| Microglial activation increased F4/80 | 47,80,84 |
| Morphological changes | 80,81 |
| Increased IBA-1 | 39,81,84 |
| Macroglial activation increased GFAP and S100 | 81,85,86 |
| Morphological changes in astrocytes | 85,87,88 |
| Polarization | 82 |
Blanks et al.79 observed an increase in the proportion of astrocytes and their immunoreactivity, especially in RGCs, as well as in the radial processes of Müller cells in the retinas of AD patients.
Different models of transgenic mice have been used to study the retinal glia in AD. Ning et al.47 used two strains of mouse models of AD with gene mutations for PSEN-1 and APP to study the immunoreactivity of F4/80 glycoprotein (mouse retinal microglial marker), monocyte chemotactic protein 1 (MCP-1), APP and Aβ, as well as to perform a TUNEL test (Terminal deoxy-nucleotidyl-Uridin-triphosphate, dUPT, Nick-End Labeling assay) which determines cell death by apoptosis. Sample comparisons were made between different age groups (7.8, 10.5, and 27 months) and between transgenic mice and the wild-type control group (WT). The results showed that there was an age-associated increase in the inflammatory cytokine MCP-1 and the microglial marker F4/80 in the APPswe/PSM146L transgenic mice. This suggests that RGCs increase the production of MCP-1 in response to Aβ, and that the microglia proliferate to the stimulation of MCP-1. This occurs in parallel with the increased apoptosis in the GCL.
In a study by Pérez et al.80, the retinas of APPswe/PS1ΔE9 transgenic mice and non-transgenic mice between 12 and 19 months of age were compared, finding greater microglial activation in the mutant mice than in the control group at all ages evaluated. This was evidenced by an increase in F4/80 immunoreactivity as well as in morphological changes, including increased thickness in the microglial processes, which made them appear more dendritic in the transgenic group than in the control. However, no changes were observed in cell body size. The same study evaluated patterns of GFAP immunoreactivity, revealing similar patterns in both mutated and non-transgenic mice regardless of age and sex. This immunoreactivity was restricted to the distal portion of Müller cells and astrocytes.
The retinas of Tg2576 transgenic mice studied by Liu et al.81 showed Aβ plaques with increased microvascular deposition of Aβ and inflammation. Immunization with Aβ and amylin hormone, or islet amyloid polypeptide (IAPP) reduced retinal Aβ deposits, but increased microvascular deposition of retinal Aβ and increased neuroinflammation. The neuroinflammation was observed to be partly due to microglial infiltration, as evidenced by increased immunoreactivity in microglial marker IBA-1 (ionized calcium binding adaptor molecule 1), which in some cases was associated with a loss of retinal architecture from the GCL to the outer nuclear layer, and partly due to astrogliosis (labeled with anti-GFAP), which was observed in transgenic mice not only in the NFL and in the GCL, as in the control group, but also in the astrocyte extensions that penetrated the GCL to reach deeper layers. Müller cell process staining was also increased. These observations support the idea that Aβ immunotherapy increases the neuroinflammatory response in the retina. Microglial activation and astrogliosis may play an important role in the clearance of Aβ from the CNS.81
Macroglial cells have been found to be polarized, meaning that their membranes have different domains with differentiated molecular functions and organization. Some studies indicate that this polarization is lost in some diseases, including AD, and that this loss is characterized by a lack of some molecules in certain membrane domains normally rich in these, which entails a loss of normal function. The membrane domain of the perivascular endfeet of astrocytes and Müller cells contains a large amount of aquaporin-4 protein (AQP4) and other integral membrane molecules that differentiate it from the presynaptic membrane. This high concentration of AQP4 is the result of several anchoring mechanisms, including interaction with DAPC, a dystrophin associated protein complex such as α-syntrophin. Enger et al.82 performed a mouse study to analyze the effect of a lack of dystrophin and α-syntrophin on the macroglia polarization in three brain regions and in Müller cells using AQP4 as a marker. They concluded that both proteins played an important role in the organization of the plasma membrane molecules in the endfeet of these macroglial cells, but that they were not solely responsible for polarization. Other studies demonstrated how β1-integrin deletion affects the polarized expression of AQP4 and dystrophin. In addition, the increase of utrophin following a lack of dystrophin suggests that utrophin may also play a role in structuring the membranes of the terminal feet.82
In the study by Edwards et al.26 with 3xTG-AD mice and a control group found signs of glial activation in Müller cells and astrocytes in the retinas of the transgenic group. Müller cells activation was evidenced via antibodies against GFAP and S100 proteins. These proteins are not normally expressed by Müller cells, however, GFAP is expressed when they become reactive. Astrocyte activation was evidenced through the observation of a large number of these cells demonstrating a reactive hypertrophic morphology in the 3xTgAD retinas in comparison to the control group. It should be noted that these signs of retinal glial activation preceded the signs of glial activation in the brain. The S100B protein increases the production of Aβ, which may potentially contribute to the formation of plaques. In the study by Edwards et al. there also appeared to be an increase in the secretion of the S100 protein in the retinas of the transgenic mice, although it remains to be investigated whether this protein is secreted by the astrocytes and Müller cells, and what consequences this increase has in the retina. A study of changes in the expression of the GS enzyme in the retinas of these mice was also conducted, finding no significant changes. This was contrary to expectations, since reduced levels of protoplasmic astrocyte expression of this enzyme had been observed in the brain. However, it was noted that changes in GS expression were observed mostly in astrocytes associated with amyloid plaques, and no such plaques were observed in the sections of the retinas of the 3xTG-AD mice used for this study.
Studies of the retina using this 3xTgAD mouse model that focused on the microglia also detected microglial activation, but without conclusive results with respect to the control group.26
A study of 19-month-old TgF344-AD transgenic mice by Tsai et al.45 showed glial activity through GFAP expression revealed by Müller cell staining. Although the control group also showed this type of activity, it was much less significant than in the transgenic mice.45
There is still a long road ahead in investigating retinal glia to understand glial activation. Attention must be paid to the size and number of processes in the microglia, their protein markers of activation, and the increased number of these cells observed in retinal diseases.26 The loss of macroglial polarization has become a feature to be studied in AD and its models. Future research should address studies with mouse lines lacking utrophin and dystrophin to provide an additional insight into the polarization mechanisms of macroglial cells like Müller cells.82 Bosco et al.83 observed early and progressive microglial activation in the retinas of mouse models of glaucoma. Several authors describe similarities between the retinal manifestations of glaucoma and AD, so it is fair to consider that the techniques used in the study of glia in glaucoma can be used to evaluate these cells in the retina with AD.2
Given the functional importance of mitochondria in microglial activation, and that mitochondrial alterations are recognized in the brain in AD, but have not yet been evaluated in the retina (specifically in the retinal microglia), it seems to be a good hypothesis that the early stages of microglial activation can be detected using neuroimaging of specific mitochondrial markers.8 Along these lines, mitochondrial translocator protein (TSPO; 18kDa), which is expressed by reactive glia and is a biomarker of gliosis, seems a promising candidate as a molecular marker for the visualization of retinal inflammatory cells.2
ConclusionAs part of the CNS, and because of its similarities to brain tissue, the retina can be analyzed to detect markers of AD, and is therefore an important tool for visualization of the disease. Analyses of the retinal tissue of AD patients in combination with analyses of populations of transgenic mice models reveal the changes that retinal glial cells undergo as Alzheimer's disease develops. The study of morphological changes, numbers, microglial and macroglial protein markers, microglial mitochondrial markers, and macroglial polarization status are new opportunities for research in AD.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was supported by the Ophthalmological Network OFTARED (RD16/0008/0005) of the Institute of Health of Carlos III of the Spanish Ministry of Economy; by the PN I+D+i 2008–2011, by the ISCIII-Subdirección General de Redes y Centros de Investigación Cooperativa, and by the European programme FEDER. Grants to Elena Salobrar-Garcia are currently supported by a Predoctoral Fellowship (FPU13/01910) from the Spanish Ministry of Education, Culture and Sport.