To determine the efficacy of prisms when used for redirection of incoming images towards the preferred reinal loci (PRLs) for restitution of potential visual acuity (PVA) in low vision cases with age-related macular degeneration (AMD).
MethodsRetrospective comparative interventional case series review. Low vision rehabilitation (LVR) protocol used included best corrected visual acuity (BCVA), PVA, topographic PRL identification and use of prisms to produce image redirection to the presumed PRL. The primary outcome measure selected for analysis was BCVA for viewing distance targets after use of yoke prisms.
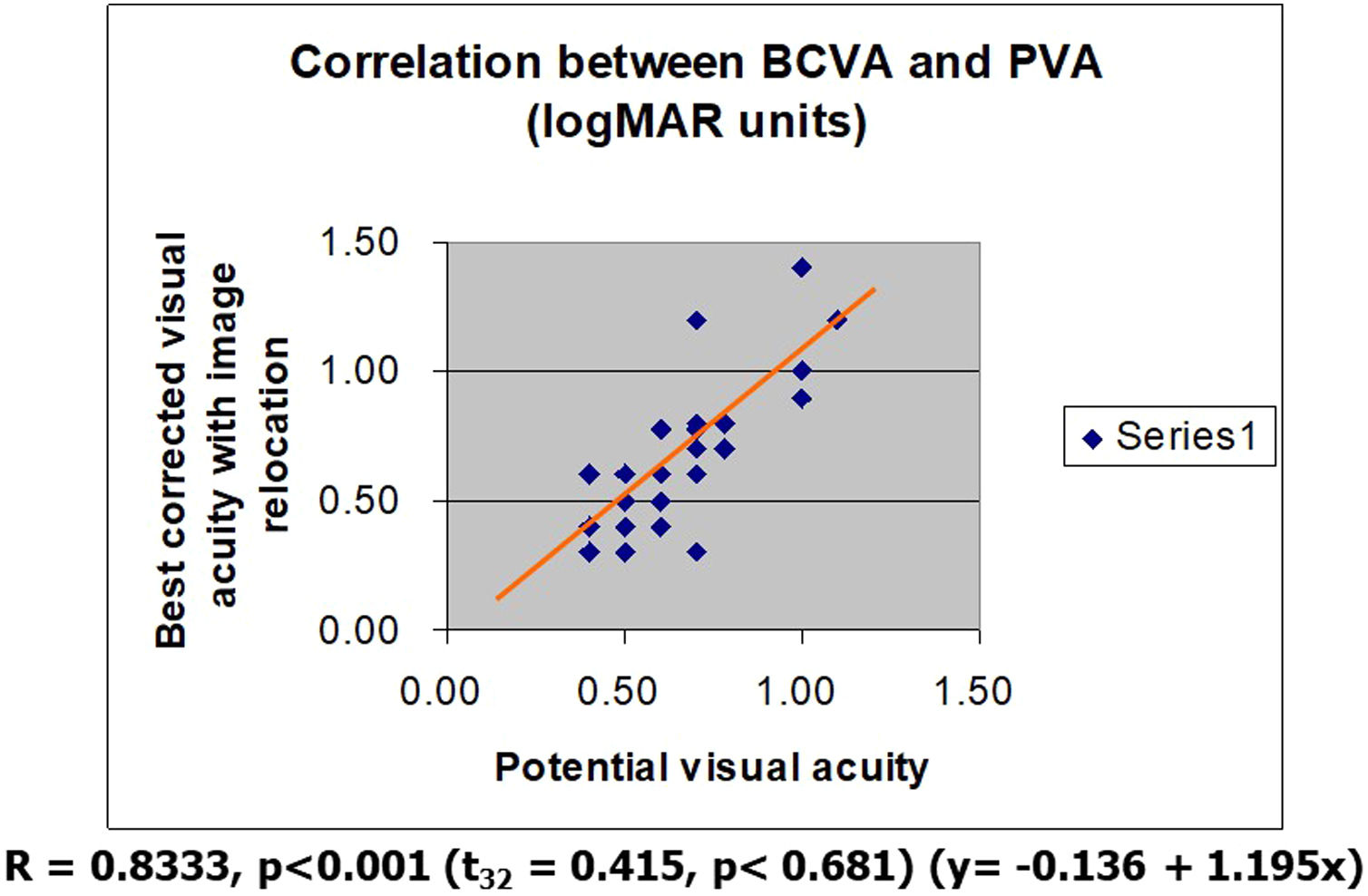
ResultsImage relocation with prisms in patients with AMD resulted in significantly better BCVA levels (t32 = 8.57, p < 0.0001) in the better eye. Distance BCVA levels achieved were almost identical to PVA levels (t32 = 0.415, p < 0.681) (y= -0.136 + 1.195x, r = 0.8333, p < 0.001).
ConclusionsUse of yoke prisms for image redirection towards a peripheral identifiable PRL may result in PVA restitution in most cases.
Low vision rehabilitation (LVR) protocols must lead to conclusive assessments which are meaningful, productive and positive and which in turn will lead to interventions which will benefit the patient. Among principles in contemplated interventions to be followed is utilization of optimal residual visual functions as starting points and guidelines for rehabilitation. First and foremost among all, restitution of potential visual acuity (PVA) in low vision (LV) patients is the most coveted target of all for rehabilitation.
Many LV patients develop functional adaptations aimed at optimizing rehabilitation of residual functional vision.1–4 One such adaptive strategy attempts to reduce the impact from loss of macular vision by developing eccentric retinal areas empowered to assume macular function, areas commonly known as PRLs, areas presumably having the best residual vision.1,5
To that effect patients adopt compensatory viewing strategies aimed at facilitating on images entering the eye to reach such eccentric areas. As expected, such abilities in some may be excellent, in some totally absent, and in most may be present with various degrees of efficiency. Hence, utilization of PRLs is imperfect, mostly because of imperfect oculomotor control.2
In principle, two modalities are available for rehabilitation of oculomotor control: exercise the new oculomotor skills to do what they are intended to do6 or yoke prisms to assist the eye to redirect incoming images towards the PRL.7,8 Improvement in oculomotor control ultimately will result in improvement in visual acuity.7
Exercise of new oculomotor skills is the most prevalent method today in clinical practice aimed at rehabilitation of oculomotor control and subsequently helping recover best residual vision available at the PRL. The methodology is empiric and based on the obvious that the peripheral retina bordering the macular area has quite a large horizontal visual span and will host the new PRLs.
Variations of this methodology are many, however all reproduce a methodology commonly known in LVR as “clock face” display training.9
Other more specific methods aim at training the control of eye movements. In one study a remarkable improvement in reading speed was achieved by using exercises with a series of saccade tasks.6 Most recently biofeedback training with microperimetry instruments modules aim to do the same.10
Use of prisms known to bend light is one modality specifically available for assisting the eye to redirect incoming images towards existing peripheral PRLs.7,8 And since PVA is believed to reside at the PRL or close by, it is logical to postulate that prisms may assist in restitution of functional PVA. Hence the purpose for this study is to verify this assumption.
MethodsThe study was designed as a retrospective non-randomized observational case series. Consecutive LV cases were identified from archives. The study was performed in adherence to the guidelines of the Declaration of Helsinki. The study protocol was approved by the Research Ethics Committee of the University Health Network of Toronto and informed consent was obtained from each participant.
Included were cases with a binocular diagnosis of AMD of all age groups and with visual acuity levels in the better eye between 20/50−20/400. Included were only those with records of PRL identification and yoke prisms prescription for distance vision. Exclusion criteria included charts with incomplete data required by our study protocol. Excluded as well were cases with a history of cognitive impairment, other retinal pathology, previous retinal surgery and significant media opacity.
Demographic details on age and sex were collected as well as details of refractive error. Best corrected visual acuity (BCVA) was measured with Early Treatment Diabetic Retinopathy Study (ETDRS) charts. For the purpose of this study we recorded and analyzed the BCVA only from the better eye. PVA was tested with multiple Es tumbling charts as described by us before.11 PRL identification, fixation stability and fixation pattern estimates were assessed by using the Macular Grid Test as described by us previously.12 Contrast sensitivity was assessed with the Contrast Sensitivity Function Test (VCTS) chart.13 The VCTS chart allows testing of contrast at 5 different spatial frequencies from 1.5 to 18 cycles/degrees covering most of normal visual abilities. The chart provides contrast sensitivity estimates expressed in contrast log units for each spatial frequency tested.
Outcome measure selected for analysis were BCVA for distance pre and post intervention with prisms and PVA estimates.
Data analysis was based on descriptive statistics that include frequency distributions, a measure of central tendency (mean) and a measure of dispersion (standard deviation). Statistical comparison between populations was made by the t-test. The correlation between 2 variables (visual acuity and fixation stability) was assessed by calculating the correlation coefficient between the two. Differences were considered to be statistically significant at a p-value of less than 0.05.
ResultsWe collected data on 33 cases (eyes), 9 males and 24 females aged 47–91 years old (mean 80 ± 9.2). Distance mean ETDRS visual acuity assessed at baseline was 20/124 (logMar 0.79 ± 0.32) in the better eyes. Mean PVA assessed at baseline was 20/87 (logMar 0.64 ± 0.2) in the better eyes.
Contrast sensitivity estimates were obtained at 1.5, 3, 6, 12 and 18 cycles per degree and measured 1.15, 1.37, 1.28, 1.09, 0.45 contrast log units accordingly.
PRL location was recorded as present on all retinal quadrants (upper (73%), lower (12%), temporal (12%), nasal (3%)). Prescribed prisms used were directed to bend light towards the retinal periphery to match the PRL accordingly in the better eye. We used on the average 5.16 ± 1.44 prism diopters (mean ± sd) in each case. Matching yoke prisms were prescribed for the other eye. Yoke prisms were incorporated in glasses with a prescription for viewing distant targets. Distance ETDRS visual acuity assessed after image relocation with prisms measured 20/83 (logMar 0.62 ± 0.29) in the better eye. Image relocation with prisms in patients with AMD resulted in statistically significant better BCVA levels (t32 = 8.57, p < 0.0001). The BCVA levels achieved with prisms were almost identical to PVA levels in same eyes (t32 = 0.415, p < 0.681) (y = −0.136 + 1.195x, r = 0.8333, p < 0.001) (Fig. 1).
DiscussionModern LVR allows us today to use the latest scientific concepts available on visual impairments to design and implement most efficient clinical methods for vision rehabilitation.1,14,15
One of the most common tools used today in ophthalmology is the potential acuity meter (PAM) which provides estimates of PVA at the macula in the presence of pathology obstructing macular access to incoming light stimuli. The PAM estimates provide a measure of PVA after interventions to remove pathology like cataracts.16
The need to know PVA in cases with LV secondary to maculopathies is similar like in cases without maculopathies in order to plan and assess LVR interventions. A different sort of test is needed in low vision patients. The Markowitz-Gonzalez PVA Charts used by us (Precision Vision, Woodstock, IL. USA) is able to measure PVA on the residual retinal areas outside the macula and can provide estimates of best residual visual acuity in cases with low vision.11 The charts also provide an accurate measuring tool for assessing rehabilitation progress from vision rehabilitation or surgical interventions. Incidentally, the contrast sensitivity measured at the 6 cyc./deg spatial frequency (1.28 log units) which is the locus for the equivalent of visual acuity of 20/87, indeed confirms the availability of PVA estimated with the PVA charts.
Today we know that low vision patients also develop functional adaptations known as PRLs, areas where presumably the best potential for residual visual acuity (PVA) resides.1 Establishment of PRLs however, is directly linked to residual oculomotor abilities which are poor in AMD patients.7 Utilization of PRLs is imperfect, mostly because of imperfect oculomotor control.3 In principle, one modality available for assisting oculomotor control are prisms to assist the eye to redirect incoming images towards the PRL and hence to retrieve PVA.7
Modern vision therapy protocols currently in use aim to enhance awareness to PRLs either by teaching re-direction of incoming images towards the peripheral retina or by using yoke prisms for the same purpose. The few studies published on use of prisms for LVR after macular function loss showed a benefit from using this methodology.7 Most showed an improvement in levels of BCVA and one showed a significant positive effect size following use of yoke prisms.7
Participants in the current study were assessed accurately for PRL locations as well as for individual PVA estimates in each case. The assessment of cases in this study took place before the advent of microperimeters. We used the Macular Grid Test which provides estimates on PRL location indirectly from the analysis of scotoma displacement on the perimetry record.17 The LVR protocol used in each case assured exact matching between the individual PRL location and their polar orientation to match the amount of prisms needed.
As hoped and expected the use of prisms assisted in redirecting incoming images into the eye towards the retinal periphery and the PRL. This effect of prisms on moving images on the retina was known for a long time however was demonstrated unequivocally just recently.14 As seen also from this study images were displaced on the retina towards the existing PRL. The prisms amount used was minimal representing on the average about 3 spatial angle degrees on the retina, yet sufficient to reach the PRL and facilitate restitution of better distance visual acuity very close to PVA values. We investigated this intervention only on the better seeing eye. Perfunctory analysis of data on the poorer seeing eye did not allow to obtain meaningful results.
Among outcomes in LVR, improvement in visual acuity for distance vision using spectacle glasses is the most coveted one. Usually correcting for refracting errors in low vision patients does not achieve satisfactory rehabilitation for distance vision. Restitution of PVA using yoke prisms incorporated in spectacle glasses as described in this paper is usually satisfactory and achieved easily and much appreciated by patients.
A practice template for LVR practice is probably the most useful way to practice LVR. It usually allows assessment of residual visual functions such as visual acuity as it allows assessment of residual functional vision such as reading. Interventions are aimed at improving vision for near, intermediate and far distances with options detailing other interventions like vision therapy training.18 Traditional LVR uses today plus lenses in glasses and hand held magnifiers, telescopic devices, monoculars and binoculars, hand held and head borne. We also use prisms and selective transmission lenses. However today we have also at our disposal modern tools for LVR.
Ideally today, any protocol for LVR practice should include in addition to traditional methods also elements of modern LVR such as assessment of PRLs, PVA and restitution of PVA as described in this paper. Restitution of PVA should be considered as a first step in any LVR attempt and as evidence suggests it will not only improve distance vision but it will also benefit all subsequent steps of the LVR process.10
ConclusionsModern LVR teaches us that residual visual functions are much better than thought before the advent of the newer techniques for assessment and intervention. A core concept in LVR is restitution of BCVA to the maximum possible. Today concepts like PRL and PVA make this core concept possible to achieve. This paper present how one can use a simple and affordable device like yoke prisms to enhance significantly distance visual acuity in low vision patients.
Conflicts of interestThe authors have no conflicts of interest to declare.