Several clinical techniques have been described to evaluate visual performance and optical quality with intraocular lenses (IOL). However, subjective refraction remains one of the most important methods for assessing post-surgery results, taking decisions about retreatments, advanced spectacle prescription and the refinement of the constant for the formula used in the IOL power calculation. Beyond clinical refraction, defocus curve measurement has been described as a complementary tool for assessing visual performance and taking clinical decisions. However, to date, there are no clinical guidelines or evidence-based protocols published in the scientific literature recommended for pseudophakic patients implanted with either monofocal or multifocal IOLs. This narrative review highlights the importance of clinical refraction in pseudophakic eyes, its utility in the decision of different types of IOL implantation, and describes a clinical refraction protocol for eyes implanted with monofocal and multifocal IOLs.
Clinical refraction is an important step in any visual examination because it allows the detection of refractive errors, the most frequent reason for a decrease in visual acuity (VA). Anterior segment surgeons must be familiarized with the refraction process because of the extreme importance of detecting any error of the latter for optimizing the constant of any intraocular lens (IOL) power calculation formula. Thus, a postoperative refraction error in a patient implanted with a specific IOL may influence the residual refraction of future patients and may incline the clinician to use this information to adjust the formula constant or to use other formulas.1 Beyond clinical refraction, the defocus curve is an important and useful tool for measuring visual performance and making clinical decisions such as target refraction selection. Any refraction bias after IOL implantation and spectacle refraction required to achieve a given VA could be identified with its measurement. Several valid procedures for refraction evaluation have been reported in the scientific literature;2 however, to date, no evidence-based protocol has been published specifically for monofocal and multifocal IOLs, even though the need for this has been claimed years ago.3 Furthermore, refraction evaluation in pseudophakic eyes and its difference in comparison to phakic eyes is important in clinical practice, and it should be known by the clinician before proceeding through the measurement. The aim of this narrative review was to describe the importance of clinical refraction in pseudophakic eyes, its utility in different types of IOLs and to describe a clinical refraction protocol for eyes implanted with monofocal and multifocal IOLs.
Influence on intraocular lens power calculationTo achieve a better understanding of the role of postoperative refraction in the intraocular lens power calculation, it is important to know the concept of prediction error (PE). The PE is the variable that evaluates the formula accuracy and its value is the difference between the postoperative refraction and the refraction predicted by the formula for a particular IOL power.4
The postoperative refraction of an eye (i), represented in the Eq. (1) as Ri, corresponds to the sum of the refraction predicted by the formula R′i and an error attributed to the formula's imprecision (PEi). However, the PE may have a systematic error component (δ) and a random error (ε), which do not necessarily have to be caused by the formula (Eq. (2)).
At this point, it is important to understand the meaning of a systematic or random error, and how it can be originated. Systematic errors are generalized errors presented in the analyzed eyes, for example, the tendency to obtain myopic eyes to a greater or lesser extent. Formulas for IOL power calculations often eliminate systematic errors by adjusting their constants. For example, if a formula with a certain constant is adjusted or created for biometer A, when using the same formula for biometer B, and if biometer B systematically overestimates the corneal power in comparison to biometer A, the final PE obtained will be associated with a systematic error due to the change in the biometer and not to the formula. To solve this problem, the solution is to fit the constant of the formula used in biometer B. However, if biometer B is less repeatable or less exact than biometer A, the error obtained will be random and will affect the PE in its absolute value. In the latter case, the biometer should be changed or the protocols revised to obtain more accurate measurements. Although uncommon, some biometers can have systematic errors associated with the IOL power calculation owing to the axial length measurement or related to the prediction of the effective lens position.5,6 However, the systematic error from the latter will have less weight in the total error.7 Furthermore, due to the repeatability or lack of precision in the measurement of the included variables in the calculation formula (radius of curvature, axial length, etc.), the random error could exceed 0.5D in 5% of the eyes depending on to the biometer that has been used.7
The refraction process can also result in systematic and random errors. In the evaluation of PE, if we use objective refraction instead of subjective refraction, we will obtain PE with some myopic bias owing to the autorefractometers tendency to overestimate myopia or underestimate hyperopia.8 Nevertheless, the subjective refraction measured at a distance of 3 m instead of 6 m or infinite underestimates the myopia obtained because of the compensation exerted by the proximal vergence (−0.33D at 3 m). Both examples of systematic errors are generally compensated for with the formula constant adjustment. Moreover, there are random error sources attributed to the refraction process, such as depth of field,9 lens manufacturing,10 and patient pupil diameter.9 Additionally, the experience of the clinician in the refraction process11 or the patient VA12 would also be random error sources. All of these sources of systematic (δ) and random (ε) errors, which are part of the PE, will form the Eq. (3), where all errors will be added cumulatively, being δ1 and ε1 attributed exclusively to the formula.
Several studies have evaluated the random error produced by intra- and inter-examiner reproducibility of subjective refraction. The agreement between them show that in eyes with VA >0.7 decimal,12 the variability of the spherical equivalent can be >0.50D in 5% of the cases,12–14 and it could increase up to 0.75D with a VA of 0.5 decimal.12 However, a variability of >0.75D in 5% of the cases for pseudophakic patients with VA of 0.7 decimal has also been reported.15 For this reason, studies that compare IOL calculation formulas consider a minimum of 20/40 (0.5 decimal) VA or 20/30 (0.67 decimal) VA as an inclusion criteria.1,16
Important considerations before starting refractionThere are some factors that can improve the efficacy of the refraction measurement:
- -
Type of implanted IOL (monofocal, multifocal, extended depth of focus (EDOF)…).17 It is not even necessary to always know the exact model, sometimes we can identify the different technologies by slit lamp.
- -
Time elapsed since surgery. The clinical refraction won't be reliable until one month after surgery, given that it can be unstable until 3 months or even 6 months.8,18
- -
Target refraction expected by the surgeon, especially when the refractive residual error has been planned in order to program a monovision or improve the intermediate vision.19
- -
Corneal astigmatism in the implantation of non-toric IOLs, along with the objective refraction, will be the starting point of the astigmatism to be adjusted in the subjective refraction.
Objective refraction by retinoscopy, autorefractometry (AR), and aberrometry [Hartman-Shack (HS), ray-tracing (RT), or scanning-slit (SS)] can be used as the starting point of subjective refraction. The most advanced technology is aberrometry, although conventional autorefractometry has shown equivalent or less bias as a starting point for subjective refraction in patients implanted with monofocal and multifocal IOLs.15,20 Retinoscopy is a valid option; however, it relies on clinician experience and takes more time than autorefractometry.11
An agreement study between subjective refraction and objective refraction measured with AR (KR-8800, Topcon) and HS (Wasca, Zeiss) showed a mean bias of −0.3D in eyes with monofocal IOLs and with 5% of the measured eyes with a difference > 0.75D.15 This bias is consistent with that obtained using SS (OPD Scan III) for a central measurement area of 2.6 mm, however a higher myopic error has been reported for larger measurement areas, also in enhanced monofocal IOLs (Tecnis Eyhance).21,22 Less bias has been reported by other authors, such as around −0.14D for AR (KR-8800, Topcon) and RT (iTrace, Tracey), which increases until −0.39D for HS (LADARWave, Alcon).23 Despite the myopic bias in monofocal IOLs, there was no substantial difference between the biases observed in phakic eyes.24,25
Regarding multifocal IOLs, the bias may vary depending on the IOL design. It has been reported that there is a larger myopic bias in diffractive IOLs than in monofocal IOLs,23,26 however not all studies agree with it.20,27 For example, with the diffractive lens EDOF Symfony (J&J), a myopia overestimation from −0.45D to −0.85D can be obtained depending on the objective method used, increasing myopic refraction with the HS (RT > AR > HS).23,28 For PanOptix (Alcon), FineVision (Physiol) and Tecnis ZM900, (J&J) the myopic bias reported has been between −0.3D and −0.6D.20,26,27
In zonal or sectorial refractive lenses, the myopic bias is much higher than that of diffractive IOLs. A myopic overestimation of −0.84D (sphere) and −1.0D (spherical equivalent) with the AR (Topcon KR-8000) in zonal refractive lens Rezoom has been reported.29 Nevertheless, clinically relevant mean differences among the Tecnis ZM900 and ReStor diffractive lenses were not found.30 The higher bias reported in refractive IOLs generally coincides with half of the addition in the spectacle plane, which has been reported with sectorial lenses such as Lentis (Teleon) and Precizon (Ophtec).31,32 In EDOF refractive lenses (Mini WELL) some studies have shown a bias <|0.3|D, such as those found in monofocal or diffractive lenses.33 Ucar and Cetinkaya included in their study several diffractive and refractive IOL technologies measured with the Tonoref II autorefractometer (Nidek, Aichi, Japan) and reporting bias for RayOne (−2.18D), Lucidis (−0.85D), PanOptix (−0.02D), Lentis Mplus (−1.11D), Tecnis Symfony (−1.13D) and Acriva Trinova (−0.88D).34
There are no clinically significant differences reported in the range of astigmatism between the autorefractometry and subjective refraction in diffractive and refractive lenses,29,30 although an overestimation around 0.5D has been described for Precizon Presbyopic.32
In conclusion, objective refraction overestimates myopia more markedly in refractive multifocal IOLs than in diffractive and monofocal IOLs. There is enough clinical evidence to consider AR as the standard objective refraction method because of its lower bias compared with aberrometry in multifocal IOLs.
Trial frame, phoropter and refraction unitsCurrently, there are several alternatives for subjective refraction, such as manual and digital phoropters, and new units that combine subjective and objective refraction.35,36 In recent literature, no differences have been found between these new refraction technologies, conventional trial frames, and phoropters. However, an improvement in the operating comfort and time has been reported during the process of subjective refraction.35,37 Nevertheless, more positive refractions (< 0.25) are obtained with trial frames.37 This could be explained due to larger pupil diameters obtained when the refraction process carried out with instruments which provide less light to the eye,37 or with monocular refraction instead of binocular refraction methods.38 The other two main considerations, in addition to the different refraction evaluation instruments, are pupillary distance adjustment and head tilting.
Subjective refraction protocolThere is not much difference between the subjective refraction procedure in patients with monofocal or multifocal IOLs and the refraction procedures for phakic patients.2 Subjective refraction procedure consists in two stages, the monocular refraction and the binocular adjustment to refine the refraction in binocular conditions.
The proposed optotype was an ETDRS located at 4 m with a background luminance of 85 cd/m2. The environmental light in a clinical setting must be approximately 100 lx (photopic conditions). The infinite refraction will be obtained by adding −0.25D to the sphere component once the subjective refraction is finished. When the distance is different from 4 m, vergence correction adding −1/d(m) to the refraction obtained instead of −0.25 D should be considered (d is the distance of the optotype from the patient's corneal vertex during refraction).
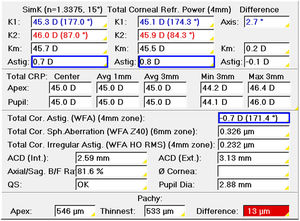
The first step before monocular refraction is the selection of the sphere and cylinder provided by an autorefractometer, aberrometer, or retinoscope. Corneal topography or tomography can be used to select the cylinder only in patients with non-toric IOLs when astigmatism in the objective refraction is zero. Otherwise, the objective refraction is the starting point. Refracting in negative notation, the cylinder to correct corneal astigmatism would be the power difference between the meridians of minimum (flatter) and maximum power (steeper) with the axis to the meridian of minimum power (Fig. 1). Total corneal astigmatism calculated from the measured anterior and posterior cornea by corneal tomography is probably a better predictor of refractive astigmatism than keratometric astigmatism, which only considers the anterior surface.39 Any time the astigmatism is increased −0.50D, the sphere must be increased by +0.25D in objective refraction.
The first stage is spherical equivalent determination. As the objective refraction is the starting point, a positive lens of +1.00D will be added.2 In phakic patients, the objective is to relax the accommodation, whereas in pseudophakic patients, there is no accommodation, so this positive lens is added to compensate for the autorefractometer myopic bias described above in Section 4. Clinician should be conscious that even though a +1.00 D can be enough for major part of IOLs, a higher positive lens could be required for some multifocal IOL designs.34 In the case of refractive multifocal IOLs, the recommendation is to start with half of the addition in the lens plane instead of +1.00D, as we previously described, the higher bias reported in refractive IOLs generally coincides with half of the addition in the spectacle plane.31,32 In diffractive IOLs depending on the technology, the bias has been reported up to −2.18 D, therefore we recommend to always review the bias to select the best starting point for a particular IOL and autorefractometer.34 In phakic patients, after positioning a +1.00D lens, VA is expected to decrease by at least three lines.2 However, in pseudophakic patients, this could be different, especially in EDOF lenses, as described below.
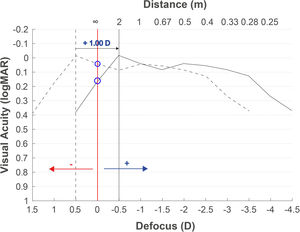
Fig. 2 shows the defocus curve of the eye implanted with a multifocal IOL. The objective refraction obtained with the autorefractometer overestimates the myopia (−0.50D in this case), and the defocus curve (dotted curve) is therefore displaced with respect to the optimal infinity subjective refraction (red vertical line) if the objective refraction is used. After adding +1.00D to the objective refraction (this myopization power can vary depending on the implanted IOL as described in the objective refraction as the starting point section), the curve is shifted to the position of −0.50D (solid curve). In a patient with this multifocal IOL, the VA would have decreased by only one line (the difference between the two blue circles over the red line), while in EDOF lenses, it may be even lower because the defocus curves present flatter patterns.
Expected defocus curve based on objective refraction to obtain the subjective refraction. A defocus curve hyperopic shift will be obtained when the refraction provided by the autorefractometer is used, due to the myopia overestimation. Adding a positive lens of +1.00D or higher depending on the multifocal IOL, the best focus will be myopically shifted.
After patient myopization with positive lenses, negative lenses will be added in steps of −0.25D to achieve maximum positive maximum visual acuity (MPMVA). Adding −0.25D in a patient with a multifocal IOL from the example (Fig. 2) should improve at least three letters (half line of VA). If the patient does not improve the three letters, no more negative lenses must be added, and the spherical lens obtained will be spherical equivalent. This is the reason why knowing the mean normal defocus curve of the particular implanted IOL could help us to understand the number of letters commonly read for each −0.25 D added. Specifically, in EDOF or enhanced monofocal lenses, the rule should be to increase −0.25 D only if three letters in a five-letter row are completely read and avoid advancing in negative lenses despite the patient seeing one or two letters more at a distance or reporting clearer vision. This is because a small increase in distance visual quality after adding −0.25 D could lead to a large decrease in near vision due to the higher slope decrease of visual acuity in the near range for these lenses.
Several authors have used the red-green duochrome27,31,33 before or after the cylindrical adjustment,40 which could be valid in phakic patients but does not necessarily have to be valid in monofocal or multifocal IOLs. There is some evidence observed in multifocal IOLs technologies, which are based on the energy distribution with dominance in the red color for far vision.41 Therefore, in the spherical adjustment of multifocal IOLs, we do not recommend the use of the duochrome test because of the lack of evidence regarding its reliability, especially for these particular designs.
At this point, the circle of least confusion was positioned on the subject's retina. It is extremely important to be in the circle of least confusion before the cylindrical adjustment by the Jackson's Cross-Cylinder (JCC), especially in multifocal IOLs.42 In clinical practice, the JCC test is considered the standard to the cylindrical adjustment, while other subjective methods, such as the clock dial test, are not used due to their subjective limitations.15,43–45 A single letter with a size of two lines more than the VA obtained in the last step will be showed in the screen to adjust the axis and power of the cylinder. The patient must answer which of the two positions the letter is clearer in.46 The process of adjustment will be the same than in a phakic patient. Regarding the JCC power, some authors have used ±0.50 D,45 although in a VA higher than 20/20, it is recommended to use ±0.25 D.46 As an alternative to the objective cylinder estimated in the corneal topography, if the cylinder provided by the objective refraction is zero, we could conduct a power sweep in 90°/180° with a cylinder of −0.50D and the cross-cylinder, always adding +0.25D to the sphere before the sweep. The sweep must be done according to the refractive astigmatism prevalence in the study population 180° - 90° - 45° - 135°.47 For the last step, to obtain the sphere, a positive lens of +0.50D is added, and the positive lenses are decreased until monocular MPMVA is achieved. Once monocular subjective refraction is completed in one eye, the process will be the same for the fellow eye.
Binocular adjustmentIn phakic eyes, the subjective refraction process finishes with binocular balance to equal the accommodative stimulus in both eyes.2 Binocular balance is not required in pseudophakic eyes because of the absence of accommodation. However, binocular adjustment of the sphere can be conducted to obtain subjective refraction in smaller pupil diameter conditions,37 even though still evidence is required that justifies this step in pseudophakic patients. For this purpose, we could add a positive lens of +0.75D in both eyes and decrease the sphere in steps of −0.25D simultaneously, so that in each step, the patient should improve one line of VA in monofocal IOLs or phakic eyes, and half of the line with multifocal IOLs, approximately, depending on the defocus curve showed by each lens.
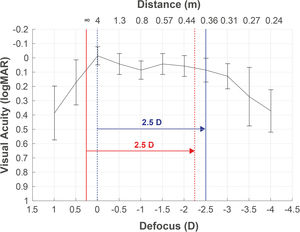
Defocus curve as a complementary tool for taking decisionsDefocus curve purpose is to measure visual performance at multiple distances, maintaining the visual test at a far distance and using spherical lenses (defocus), which provide similar vergence light changes as moving the test distance. The corresponding defocus lens (L) is calculated using the formula L=1/d0 - 1/d1, where d0 is the static distance where the visual test is placed during the defocus curve measurement, and d1 is the distance that simulates the light vergence change produced by the lens. For example, in a defocus curve measured at 4 m, the defocus lens that simulates 40 cm would be L=1/4 - 1/0.4= −2.25D. This is due to the infinite refraction is underestimated in −0.25D, resulting in an overestimation of the DCNVA (at 40 cm) in comparison to the reported at −2.50D in the defocus curve (Fig. 3). This partly explains the monocular overestimation of monocular DCNVA in comparison to the −2.5D location reported in the defocus curve for some clinical studies.48
Defocus curve obtained from a patient with the best correction at 4 m (correction to infinity not applied). A refracted sample at 4 m (blue dotted line), without the adjustment to infinity (red solid line), will obtain poorer infinity vision and better vision at 40 cm (red dotted line) than the measured by the defocus curve at −2.5D (blue solid line).
The maximum vision peak should be centered at 0D. Thus, the visual refraction obtained at 4 m needs to be adjusted to infinity by adding an additional negative lens of −0.25D, as described by the international standard ISO-11,979–7:2018 of clinical investigations with intraocular lenses.49 This means that, the refraction (−0.25)(−0.75)x90° obtained at 4 m, will correspond to an infinity refraction of (−0.50)(−0.75)x90°.49 The infinity visual refraction should be used to measure intermediate and near distance corrected visual acuity (DCIVA and DCNVA).
Any refraction bias may be identified by measuring the defocus curve as well as post-surgery refraction changes required to reach an objective VA at a given distance. Therefore, if subjective refraction is the procedure that provides the maximum vision peak to infinity, the defocus curve will be the method by which the real position of the maximum vision peak can be identified. The peak should be at infinity, or it can be slightly myopic and targeted to reach a larger depth of field.50
Nowadays, a clinical conflict between the constant adjustment in formulas for IOL calculation and defocus curve testing exists. The constant is usually adjusted for refraction at 6 m, adding a negative lens (1/6–1/d1).50 In clinical practice, this means that patients will be slightly myopic (−0.16D), being perfectly graduated to obtain the best vision at 6 m. Fig. 3 shows the consequences when the refraction measured at 4 m is not adjusted to infinity. The maximum peak is located at −0.25D (4 m) rather than at 0D (infinity). Consequently, the constant adjustment for a refraction at 4 m (blue dotted line) could lead to a worse vision at further distances than 4 m (red solid line). Vision at 40 cm (red dotted line) will be slightly better if the correction to infinity is not applied previously to the constant adjustment (solid blue line).
We recommend the constant adjustment (infinity or 6 m) when the refraction room is ≤ 5 m. The difference between 6 m or infinity would be −0.17D, which could be below the perception of blurriness caused by the diopter change depending on the IOL.9 Thus, the constant adjustment in monofocal IOLs might be justified with the objective of reaching better near vision.50
The assessment of the visual performance and focusing profile of any multifocal or EDOF IOL will be inaccurate if the adequate infinity distance is not adjusted for far focus. The distance between focal planes will be mismeasured with an underestimation of the IOL “near addition” as the far and near focus gets closer (i.e., by 0.25 D if the refraction is measured in a 4 m lane).
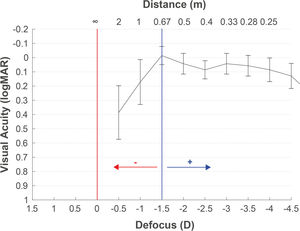
The defocus curve measurement may also help determine the target refraction. The concept of the target can be defined as the final refraction for which the patient reaches a binocular balance at one or more distances. With the defocus curve results of any IOL, we can select the power that generates the expected residual to reach the patient's desired vision to any distance. It is common to find in the scientific literature defocus curve results with the maximum peak of vision centered at 0D, although the refraction has not been adjusted to infinity. This can lead to interpretation errors in decisions based on the defocus curves. Hence, as previously described, we recommend the refraction adjustment to infinity with the objective of avoiding interpretation mistakes in the target selection based on the defocus curve. Moreover, in the postoperative period, the defocus curve helps us to decide the retreatment needed or the refractive prescription to improve the vision at any particular distance. For example, Fig. 4 illustrates an example recommending +1.25D for moving the maximum peak of vision to 67 cm if the intermediate vision expectative without spectacles is not achieved by the patient. The patient also had improved visual acuity at a near distance of 33 cm. However, wearing this compensation for viewing a distance of more than 67 cm would result in poor vision. Table 1 presents some key recommendations for measuring defocus curves.
Key recommendations for measuring defocus curves.
This narrative review describes the importance of the role of clinical refraction in PE evaluation, which is relevant for fitting the constant of the formula for IOL power calculation. Subjective refraction should be used to achieve this purpose, as objective refraction is just the starting point, owing to myopic overestimation in some IOLs, especially in refractive multifocal IOLs. Moreover, sufficient clinical evidence has been reported to consider AR as the standard objective refraction method of measurement with either monofocal or multifocal IOLs. Measurement of the defocus curve should be used as a subjective refraction complement for making clinical decisions. Knowing the monocular average defocus curve of a multifocal IOL provides information on VA changes during the subjective refraction process. Finally, it is extremely important to differentiate subjective refraction from the objective and subjective refraction (target) expected from the surgeon, which is determined by the patient's needs at multiple distances. The subjective refraction adjusted with the MPMVA will be used for PE evaluation, whereas the refractive target will be adjusted to achieve satisfactory vision at a far distance, maximizing the vision at intermediate and near distances.
None.