To compare the efficacy and side effects of two combinations of tropicamide and phenylephrine as mydriatics for ophthalmoscopy.
MethodsIn this prospective randomized controlled trial, 51 Chinese outpatients were randomized to receive topical tropicamide 1.0% and phenylephrine 2.5% (Regime A), and 50 to receive a fixed combination of tropicamide 0.5% and phenylephrine 0.5% (Regime B). The change in horizontal pupillary diameter, subject discomfort upon instillation and the time elapsed between instillation and recovery from glare and near blur were studied.
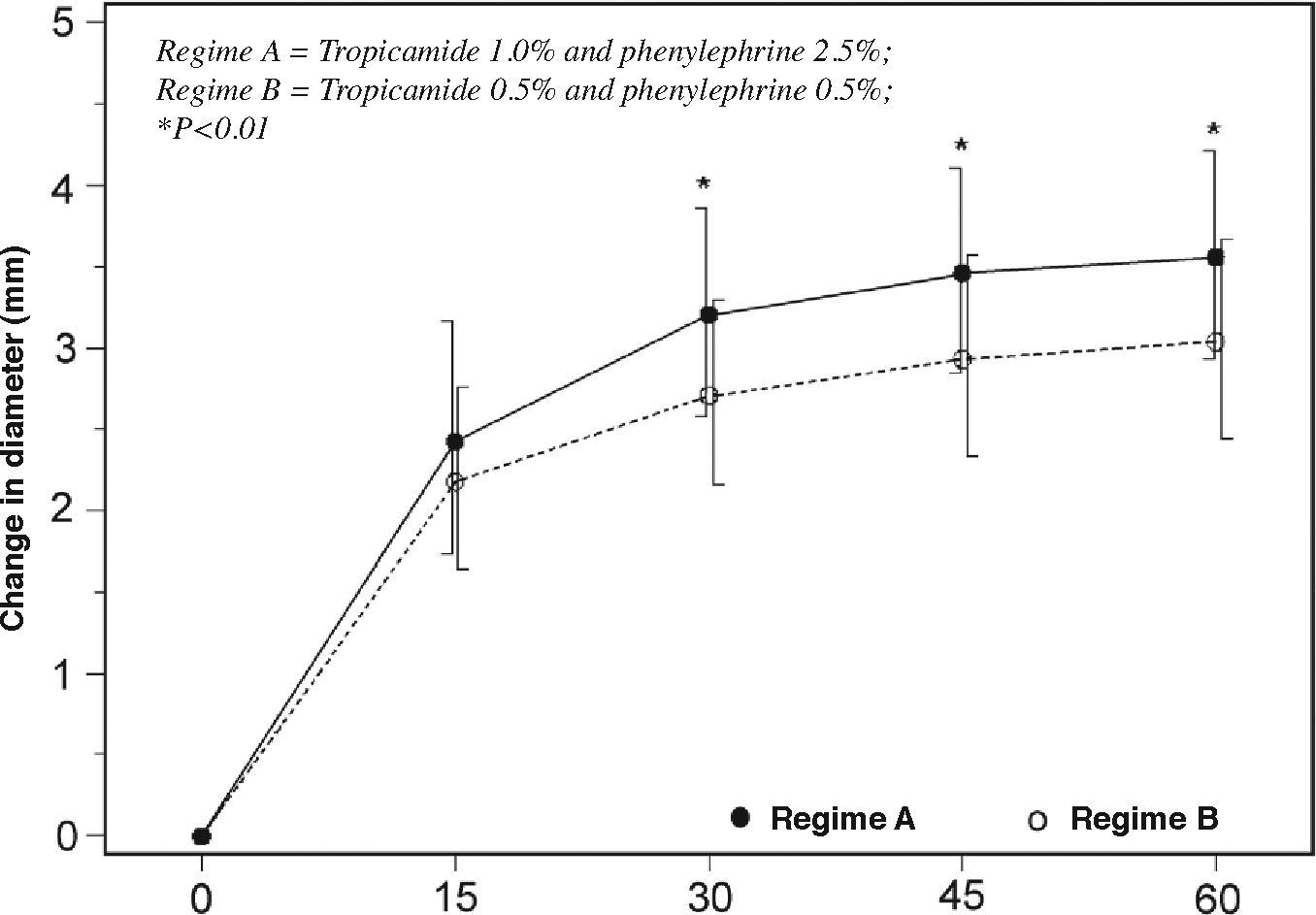
ResultsAfter 60 minutes, the mean increase in pupillary diameter was 3.56±0.65mm with Regime A, and 3.04±0.62mm with Regime B (P<0.01), but there was no difference in the proportion of subjects having a post-mydriatic pupillary diameter of 6mm or larger (P=0.54). No subjects required additional instillation. Regime B was better tolerated (P<0.001). The median times elapsed between instillation and recovery from glare or near blur was 7 hours, without a significant difference between the two regimes (P=0.5).
ConclusionsBoth regimes were effective and safe for ophthalmoscopy. However, Regime B was better tolerated. Subjects may be reassured that the side effects of glare and near blur are likely to disappear by the following day.
Comparar la eficacia y los efectos secundarios de dos combinaciones distintas de tropicamida y fenilefrina como midriático para oftalmoscopia.
MétodosEn este ensayo clínico prospectivo, controlado y aleatorizado, se determinó aleatoriamente que 51 pacientes ambulatorios chinos recibieran por vía tópica tropicamida 1.0% y fenilefrina 2.5% (Combinación A), y que otros 50 recibieran una combinación fija de tropicamida 0.5% + fenilefrina 0.5% (Combinación B). Como criterios de valoración se estudiaron: la variación del diámetro pupilar a lo largo del meridiano horizontal, las molestias sufridas por los sujetos cuando les instilan las gotas y el tiempo transcurrido desde la instilación del midriático hasta que desaparece el deslumbramiento y la visión cercana borrosa.
ResultadosA los 60 minutos, el aumento medio del diámetro pupilar fue 3,56±0,65mm con la Combinación A, y 3,04±0,62mm con la Combinación B (P<0,01), pero no se halló ninguna diferencia significativa entre los dos grupos en cuanto al porcentaje de sujetos que lograron con el midriático un diámetro pupilar igual o superior a 6mm (P=0,54). Ningún sujeto requirió la instilación de gotas adicionales para lograr la midriasis. Los sujetos toleraron mejor la Combinación B (P<0,001). En promedio, el tiempo necesario para que el deslumbramiento y la visión cercana borrosa desaparecieran fue de 7 horas, no observándose ninguna diferencia significativa entre los dos grupos (P=0,5).
ConclusionesAmbas combinaciones resultaron ser eficaces y seguras para ser utilizadas en oftalmoscopia. Sin embargo, los sujetos toleraron mejor la Combinación B. Los sujetos pueden estar tranquilos, pues es probable que los efectos secundarios de deslumbramiento y visión cercana borrosa hayan desaparecido ya la mañana siguiente al día de la instilación.
Detailed examination of the lens, vitreous and retina requires adequate pupillary dilatation. The ideal mydriatic should show a rapid onset of action, achieve adequate mydriasis, permit a quick recovery, and not cause discomfort or side effects. Tropicamide, an antimuscarinic agent, produces mydriasis within 20 minutes. Recovery of accommodation from its cycloplegic effects normally takes 6 hours. Phenylephrine is a sympathomimetic having predominantly alpha-adrenergic activity. Its side effects include local irritation and hypertension due to systemic absorption.1 Tropicamide 1% and phenylephrine 2.5% were supplied as separate preparations, and sequential instillation of one drop of each was required. Subjects reported different degrees of stinging sensation when these drops were instilled. Greater mydriatic effect could be achieved when these mydriatics were used in combinations.2,3 There were attempts to prepare fixed combinations from these two medications. However, solutions of tropicamide at a concentration of 1% or above may precipitate if this is mixed with phenylephrine. 4 A fixed combination of these two medications at a low concentration–tropicamide 0.5% and phenylephrine 0.5%–is chemically stable, with the advantage that only one drop of medication is required in each eye. This might be better tolerated by subjects and could save the staff time, especially at a busy outpatient clinic setting. Other possible benefits of low-concentration preparations include a lower risk of cardiovascular side effects,5,6 and a faster recovery from blurring of near vision and glare.3 A randomized controlled trial was designed to compare the efficacy and side effects of these two regimes.
Materials and MethodsThis was a prospective, single-blinded, randomized, controlled trial. The study was approved by the hospital's Clinical Research Ethics Committee and adhered to the tenets of the Declaration of Helsinki. Subjects were recruited among those patients who required comprehensive eye examination at the General Ophthalmology Clinic of the Hong Kong Eye Hospital. Chinese subjects, having dark brown irides, at least 18 years old, and a corrected near visual acuity of 20/400 or better were eligible to participate. Subjects were excluded if they had diabetes mellitus, uncontrolled systemic hypertension, or a history of anterior segment trauma, previous ocular surgery, use of topical ocular medications other than artificial tears or allergy to mydriatics. Other exclusion criteria were: intraocular pressure above 21mmHg, narrow anterior chamber angle, anterior segment abnormalities, abnormal pupillary light reaction, blepharoptosis, ocular motility defects, and instillation of topical anesthetics prior to pupillary dilatation during the study visit.
Written informed consent was obtained from those subjects who satisfied the recruitment criteria. Eligible subjects were randomized to receive either Regime A or Regime B. Regime A comprised the sequential instillation of one drop of tropicamide 1.0% (Mydriacyl; Alcon, Puurs, Belgium) and one drop of phenylephrine 2.5% (Mydfrin; Alcon, TX, USA) in each eye, whereas Regime B consisted of one drop of the fixed combination of tropicamide 0.5% with phenylephrine 0.5% (Mydrin-P; Santen, Osaka, Japan) instilled into each eye.
The sample size was set to detect with 80% power a 0.5mm difference between the two groups in the mean change in horizontal pupillary diameters of the two eyes. Considering possible multiple comparisons, the maximum false-positive error was taken as 1% for two-sided tests using the Bonferroni procedure. Around 50 subjects were recruited into each group.
One of the two mydriatic regimes, according to the randomization code, was instilled in the lower conjunctival fornices. The lacrimal sacs were compressed for one minute after eye drop instillation to minimize systemic absorption.6 Subjects were asked to grade the ocular discomfort they experienced at instillation time using a scale of 0 to 10; where 0 indicates no discomfort at all, and 10 indicates maximal discomfort.
The study was conducted in the consultation rooms with controlled ambient illumination at photopic condition. The horizontal pupillary diameter was measured to the nearest 0.5mm with a handheld infrared pupillometer (Colvard pupillometer; Oasis Medical, Glendora, CA, USA) immediately before, as well as 15, 30, 45 and 60 minutes after instillation of the assigned regime. During the measurement, the subject was instructed to look at the red fixation light of the pupillometer, while the fellow eye was left unobstructed. The investigator responsible for pupillary-diameter measurement was masked to the regime the subjects received. However, subjects were not masked as the number of instilled drops was different. Sixty minutes after eye-drop instillation, an ocular examination was conducted by means of a slitlamp biomicroscope and a binocular indirect ophthalmoscope (All Pupil; Keeler, Windsor, UK). Any pupillary constriction as a result of the light of the indirect ophthalmoscope was noted.
The duration of symptoms of glare and blurred near vision after mydriasis were investigated. Subjects were asked to read a standard near visual acuity chart (Chart “2”, Logarithmic Near Visual Acuity Chart “2000”; Precision Vision, La Salle, IL, USA) with reading glasses, if required both before and 60 minutes after the instillation of the eye drops. Those subjects who experienced post-mydriatic near blur were requested to read the near visual acuity chart again once every hour and record the result in a timetable given at the end of the eye examination, until they could read the same line achieved before mydriasis. Similarly, those subjects who experienced glare after pupillary dilatation were requested to check and record its progress once every hour, until no more glare was experienced.
Pulse rate, and systolic and diastolic blood pressure were measured using an automated sphygmomanometer (BP-8800C; Colin, Komaki City, Aichi, Japan) at baseline, and 30 and 60 minutes after instillation.
Data were analyzed using a computer statistical package (SAS version 9.1.3; Cary, NC, USA). Pupillary diameter data were checked for univariate and multivariate normality. For the purpose of the drug-efficacy comparison, the average increase in horizontal pupillary diameter observed in the two eyes of each subject quarter-hourly after instillation were analyzed using multivariate analysis of variance. Mydriatic response of the two eyes from the same subject are highly correlated, readings from the right and left eyes of one subject cannot be regarded as two independent samples for the analysis of variance. Taking the average increase of pupillary diameter of the same subject for computation does not affect the values of the means. The values of standard deviations are likely to be smaller and confidence intervals narrower. Multivariate analysis of variance was also used to compare the changes in pulse rate and blood pressure across the two groups (i.e., regimes). Discomfort scores were compared using the Wilcoxon rank-sum test. The times elapsed between instillation and recovery from post-mydriatic near blur and glare were compared using the logrank test on their Kaplain-Meier survival functions. When a subject went to bed before recovery from glare or near blur, but had recovered from these effects on awakening, the last recorded time before going to bed was regarded as a censored observation.
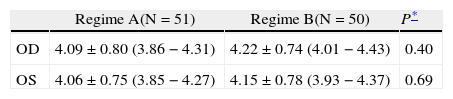
ResultsOf the 101 subjects recruited, 51 were randomized to Group A and 50 to Group B. Both groups were comparable in terms of age, sex, prevalence of hypertension and heart disease (Table 1). Pupillary diameters at baseline were similar in both groups (Table 2). Twenty-five subjects had unequal pupil size at baseline (24.8%). Their baseline blood pressure readings and pulse rates were comparable (Table 3).
Demographic data
| Regime A (N=51) | Regime B (N=50) | P | |
| Age (years) mean ± SD (range) | 60.04±16.88 (18 to 86) | 61.36±15.34 (18 to 86) | 0.84* |
| Sex, M:F | 16:35 | 15:35 | 1.0§ |
| Hypertension n (%) | 16 (31.4%) | 19 (38.0%) | 0.54§ |
| Heart Disease n (%) | 3 (5.9%) | 5 (10.0%) | 0.49§ |
Regime A = Tropicamide 1.0% and phenylephrine 2.5%; Regime B = Tropicamide 0.5% and phenylephrine 0.5%
Baseline photopic horizontal pupillary diameter including mean ±SD and 95% confidence intervals (lower-upper limits). Values are in mm
| Regime A(N = 51) | Regime B(N = 50) | P* | |
| OD | 4.09 ± 0.80 (3.86 − 4.31) | 4.22 ± 0.74 (4.01 − 4.43) | 0.40 |
| OS | 4.06 ± 0.75 (3.85 − 4.27) | 4.15 ± 0.78 (3.93 − 4.37) | 0.69 |
Regime A = Tropicamide 1.0% and phenylephrine 2.5%; Regime B = Tropicamide 0.5% and phenylephrine 0.5%
Pulse rate and blood pressure at baseline including mean ±SD
| Regime A(N = 51) | Regime B(N = 50) | P | |
| Systolic blood pressure (mmHg) | 137.90±25.87 | 141.72±28.36 | 0.56 |
| Diastolic blood pressure (mmHg) | 70.98±11.54 | 72.50±12.15 | 0.43 |
| Pulse Rate, min−1 | 74.43±13.98 | 69.42±7.59 | 0.09 |
Regime A = Tropicamide 1.0% and phenylephrine 2.5%
Regime B = Tropicamide 0.5% and phenylephrine 0.5%
*Mann-Whitney U test, §Fisher's exact test.
When the pupillary data were checked for normality, one outlier in group B was identified and removed, leaving a total of 49 subjects in this group. From 30 minutes after instillation, the mean increase in horizontal pupillary diameter of both eyes became significantly different, reaching 3.56±0.65mm with Regime A, and 3.04±0.62mm with Regime B (P<0.01, Figure 1) at 60 minutes. Subjects who received Regime A had a larger post-mydriatic pupillary diameter (7.61±0.86mm) than those receiving Regime B (7.22±0.80mm) (P=0.015). Most subjects attained a pupillary diameter of 6mm or above during indirect ophthalmoscopy (98.0% with Regime A and 96.0% with Regime B; P=0.55). No subject required a second instillation or noticed any pupillary constriction during binocular indirect ophthalmoscopy. Subjects on Regime A reported higher discomfort scores than those receiving Regime B (P<0.001).
Times for recovery from post-mydriatic glare and blurring of near vision were analyzed using their Kaplain-Meier survival functions. The latter did not follow any simple distribution model and, therefore, these data were compared using the non-parametric logrank test. On a first inspection the durations of these side effects appeared to be more prolonged with Regime A than with Regime B; however, the differences were not statistically significant neither for glare (P=0.53, Figure 2) nor for near blur (P= 0.48, Figure 3). The median duration was around 7 hours for both side effects. Glare could last up to 13 hours with either regime. It could take as long as 15 hours to recover from near blur after instillation of Regime A, and 13 hours with Regime B.
Kaplan-Meier survival function on the recovery from glare after mydriasis.
The y-axis indicates the probability of suffering from post-dilatation glare and the x-axis indicates the time for recovery. With either regime, the median time for recovery was 7 hours, the range being 0 to 13.3 hours with Regime A and 0 to 13.2 hours with Regime B (P=0.53).
Kaplan-Meier survival function on the recovery from blurred near vision.
The y-axis indicates the probability of suffering from post-dilatation blurred near vision and the x-axis indicates the time for recovery. With either regime, the median time for recovery was 7 hours, ranging from 0 to 13.6 hours with Regime A and 0 to 15.2 hours with Regime B (P=0.48).
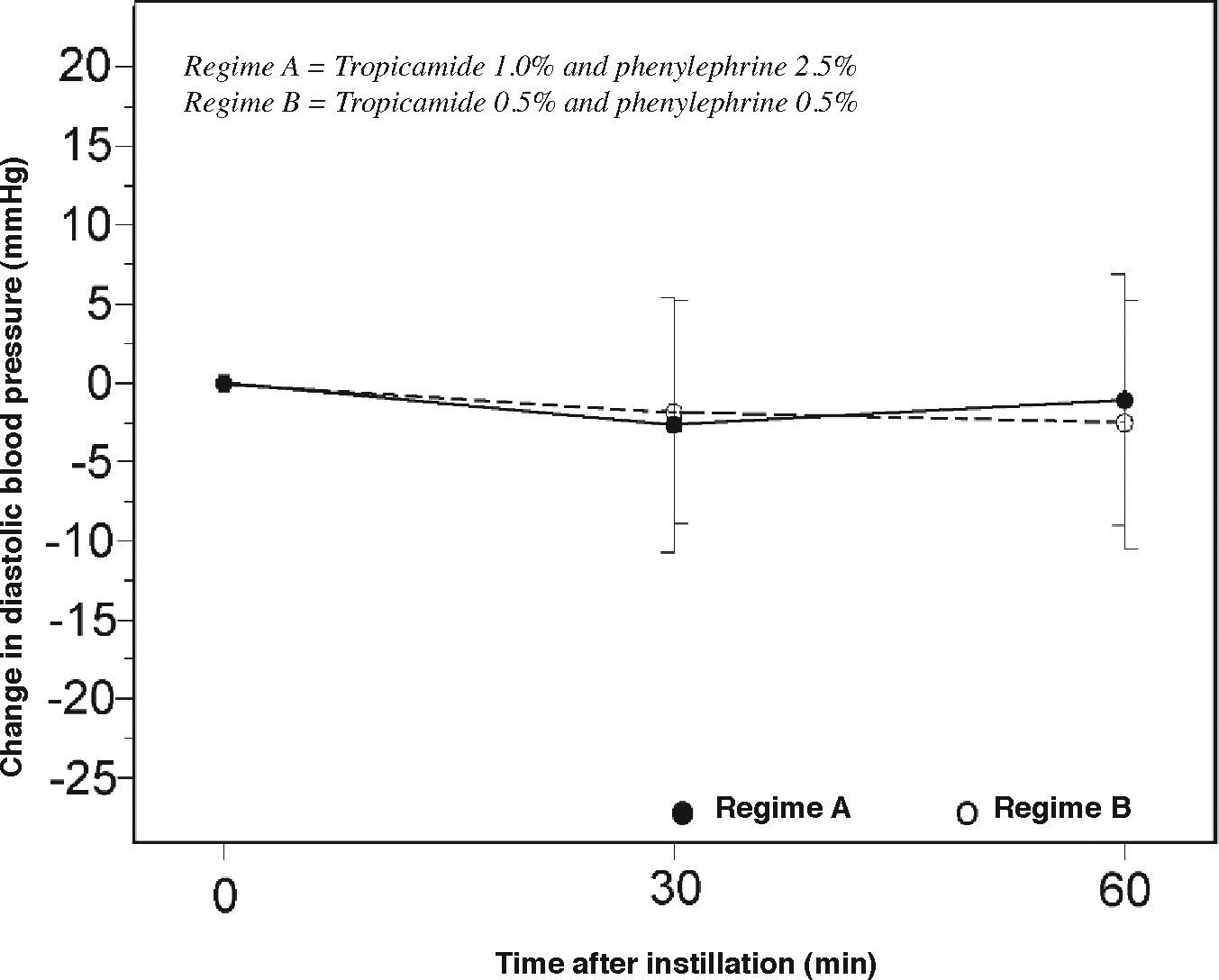
Subjects in both groups had minimal changes in pulse rate, systolic pressure and diastolic pressure after mydriasis (Figures 4, 5 and 6), and no adverse reactions were noted during the study.
Measurement of pupillary diameter using conventional methods, such as a millimeter ruler or a Rosenbaum pocketcard pupil gauge is difficult and often inaccurate.7 With our local Chinese population, who have dark brown irides, pupillary measurement could be difficult since the color contrast between iris and pupil is low. Diffuse illumination using a hand torch, a direct ophthalmoscope or a slit-lamp biomicroscope often induces variable amounts of pupillary constriction due to the direct light reflex, reducing the reproducibility of the measurement. The use of an infrared pupillometer avoids the use of illumination, and the red fixation light of the pupillometer allows subjects to fixate steadily, without stimulating accommodation. Based on its precision and reliability, the Colvard pupillometer was chosen for this clinical trial.8 During pupillary-diameter measurement, the patient was instructed to look at the fixation light while the fellow eye could see across the consultation room unobstructed. This avoided miosis due to near reflex, but allowed the measured pupil to respond to ambient illumination via the consensual reflex.
The differences in change in mean pupillary diameter observed from 30minutes onwards were significant. In a study on preoperative mydriasis for cataract surgery among Chinese patients comparing the same mydriatic combinations but instilled four times, Lam et al reported that the mydriatic effect (i.e., increase in pupil diameter) with tropicamide 1.0% plus phenylephrine 2.5% (3.70±0.97mm) was greater than that observed for the fixed combination of tropicamide 0.5% and phenylephrine 0.5% (3.32±1.00mm).9 Weiss and co-workers also noted a 3.56±1.26mm increase in pupillary diameter with tropicamide 1% plus phenylephrine 2.5% in diabetic patients.10 However, there was a large variability regarding the time required to achieve maximum mydriasis (from 10 to 135 minutes). Forman did not find a clinically significant difference in mydriasis resulting from the use of a mixture of tropicamide 0.75% and phenylephrine 2.5% (mean 7.4mm) or of separate tropicamide 1.0% and phenylephrine 10% (mean 7.6mm) in Caucasian eyes.11 Sinclair et al. reported a greater mydriatic effect with tropicamide 1.0% and phenylephrine 5.0%, compared with tropicamide 1.0% and phenylephrine 2.5%.4 However, in this study the use of a topical anesthetic may have enhanced the effect of the dilating drops. Apt and Henrick demonstrated that the instillation of topical proparacaine before mydriatics could bring about an additional mydriasis of 1mm,12 while Ghose et al showed that the time to attain a 6mm pupillary diameter was shorter if an additional drop of topical lidocaine was instilled.13 In this study, subjects who had received a topical anesthetic prior to pupillary dilatation, e.g. for gonioscopic evaluation or Goldmann applanation tonometry, were excluded. Our results could be applicable to the clients attending general optometry clinics where topical anesthetic was not available.
In prior studies on pharmacological mydriasis with healthy volunteers, the two eyes of the same subject received different regimes,13-15 or a different regime is given to both eyes again after a wash-over period, e.g. cross-over study.12 These methods effectively control intra-subject variability, reducing the sample size. In the clinical setting, however, subjects usually receive the same mydriatic regimes in both eyes and they seldom need to return for ophthalmoscopy within a short period. In this trial, we compared the efficacy of the two regimes using the average increase in horizontal pupillary diameter observed in both eyes. Practically, this has avoided some possible bias arising from the selection of the readings from one eye for analysis, when the baseline pupillary diameters of both eyes were different. Lam, Thompson and Corbett showed that 19% of subjects in their study had physiological anisocoria,16 and the prevalence in the present study was even higher (24.8%). Bias could also arise from choosing the eye that was the first or the second to receive the regime. Lowenstein cautioned against the dilution effect provoked by reflex tearing caused by instillation of mydriatic to the first eye.17 Excess tears in the conjunctival fornix could diminish the amount of mydriatic reaching the second eye.
In the literature, various endpoints were used to define adequate pupillary dilatation when evaluating mydriatic regimes.3,12,13,18,19 Using the Schepens’ small pupil indirect ophthalmoscope,20 together with modern condensing lenses, the observation of the retinal periphery should not pose any difficulty with a 6mm or larger pupil. In this study, 97% of subjects attained a pupillary size of 6mm or above at 60 minutes and no subject from either group required an additional instillation.
Blurring of near vision after mydriasis may affect the subjects’ working and reading ability, while glare may affect walking stability and safety, especially in the elderly.21 Previous studies have also reported a drop in visual function after mydriasis. Eyeson-Annan et al. evaluated glare and the drop in reading ability after pupillary dilatation using topical phenylephrine 10% alone or in combination with tropicamide 1.0%.3 They showed that the effects were greater with the combined regime. However, the duration for recovery from these effects was not specified. Reversal of mydriasis with thymoxamine could have sped up the recovery from mydriasis. Paggiarino et al. also found that 30% of eyes still experienced a reduction in their amplitude of accommodation 7 hours after instillation of tropicamide 0.5% and phenylephrine 2.5%.2 Subjects with brown eyes required longer recovery times than those with blue eyes. Reduced accommodative amplitude,21 reduced visual acuity and increased glare sensitivity have been reported after the use of tropicamide 1.0%.22 These studies involved young volunteers (under 35 years) and, therefore, their results could not be generalized to our subjects, whose mean age was above 60.
The effects on visual function were evaluated in this study using two subjective measures – blurred near vision, based on the duration of reduced reading ability, and glare, which was based on subjective sensation. Both regimes induced post-mydriatic near blur and glare, with a median duration of around 7 hours. However, there were large intersubject variations, ranging from no visual disturbances to side effects lasting up to 15 hours. The duration of visual disturbances was, in any case, above the 4-to-5-hours stated in Regime B's product insert.23 It would be therefore prudent to warn subjects that their vision may be affected for the rest of the day and that most likely it will be back to normal by the next morning, regardless of which mydriatic regime was being used.
Subjects receiving Regime A reported higher discomfort scores. This could be due to a higher concentration of drugs in this regime (phenylephrine in particular),1 to the mere instillation of four drops in this regime, or to a combination of these two factors. There were minimal changes in pulse rate and blood pressure after mydriasis in both groups. Neither regime caused any significant cardiovascular side effects. Lam et al. showed that these regimes were safe in a cohort of cataract patients whose mean age was 73 years.9
In conclusion, Regime A had a greater mydriatic effect than Regime B. Using a threshold of 6mm or more as criteria for adequate pupillary mydriasis, we did not find a significant difference between the two regimes in terms of the proportion of subjects who achieved this threshold pupillary size. The use of one single drop of the fixed combination of tropicamide 0.5% and phenylephrine 0.5% appeared to be as effective (in clinical terms) but much better tolerated than the use of one drop of tropicamide 1% plus one drop of phenylephrine 2.5% in subjects with dark irides for ophthalmoscopy. Subjects should be warned that the side effects of glare and near blur may last for the rest of the day but are likely to have disappeared by the next day.
Financial disclosure: Supported in part by the Action For Vision Eye Foundation, Hong Kong. The authors have no financial and proprietary interest in the materials and equipment in this study.