The purpose of this study was to characterize the central epithelial thickness (CET) of penetrating keratoplasty corneal specimens obtained from patients with keratoconus (KC) and correlate the histological patterns with their clinical history.
MethodsEx vivo histological imaging was performed to measure CET and total corneal thickness (TCT) in 56 patients with KC. Microscopic slides from penetrating keratoplasty corneal specimens, stained with hematoxylin and eosin were evaluated using bright field microscopy. CET and TCT were measured, and morphological features were studied. Clinical history regarding duration of KC prior to surgery and length of and tolerance to contact lens wear were compared and analyzed.
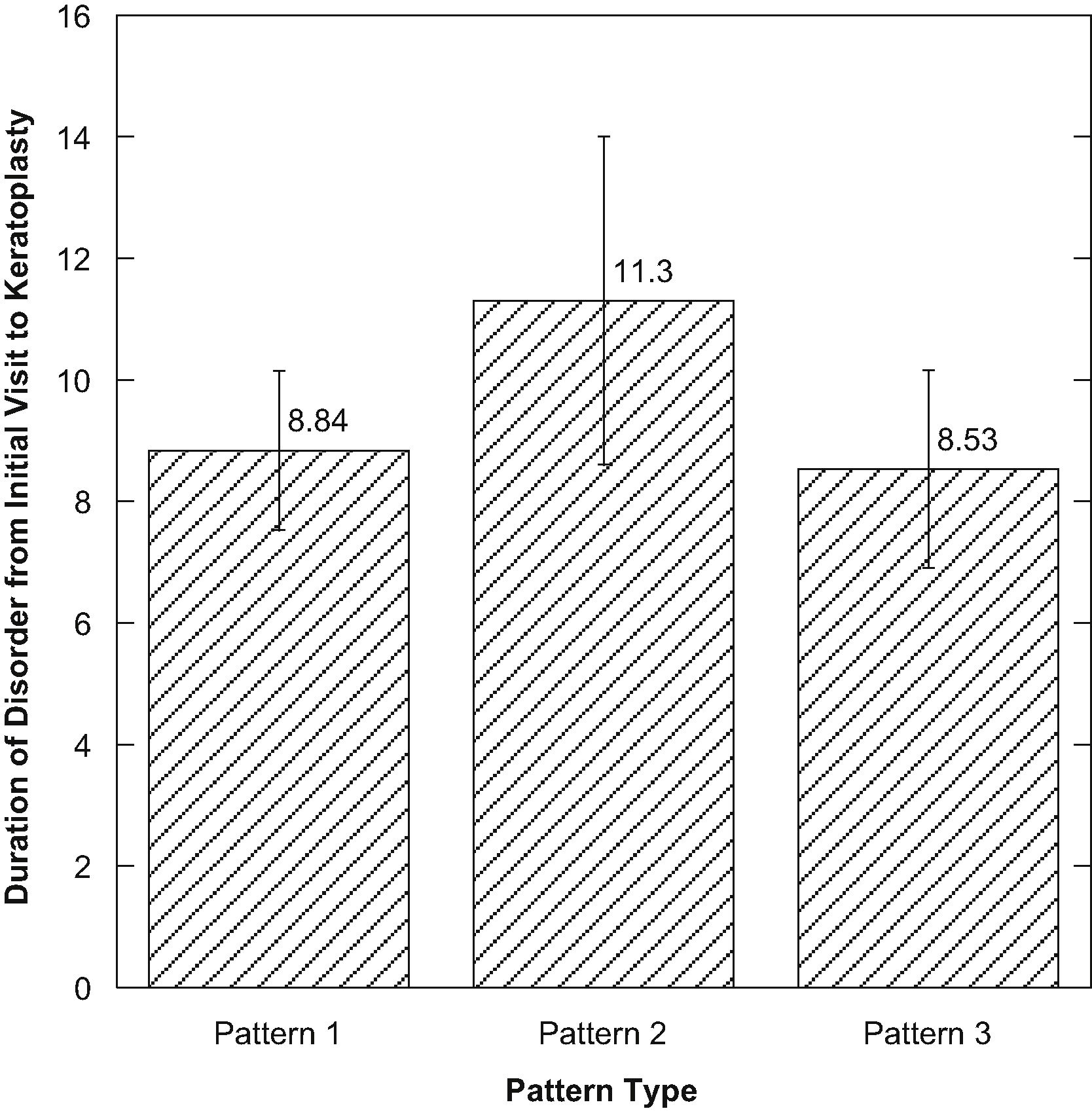
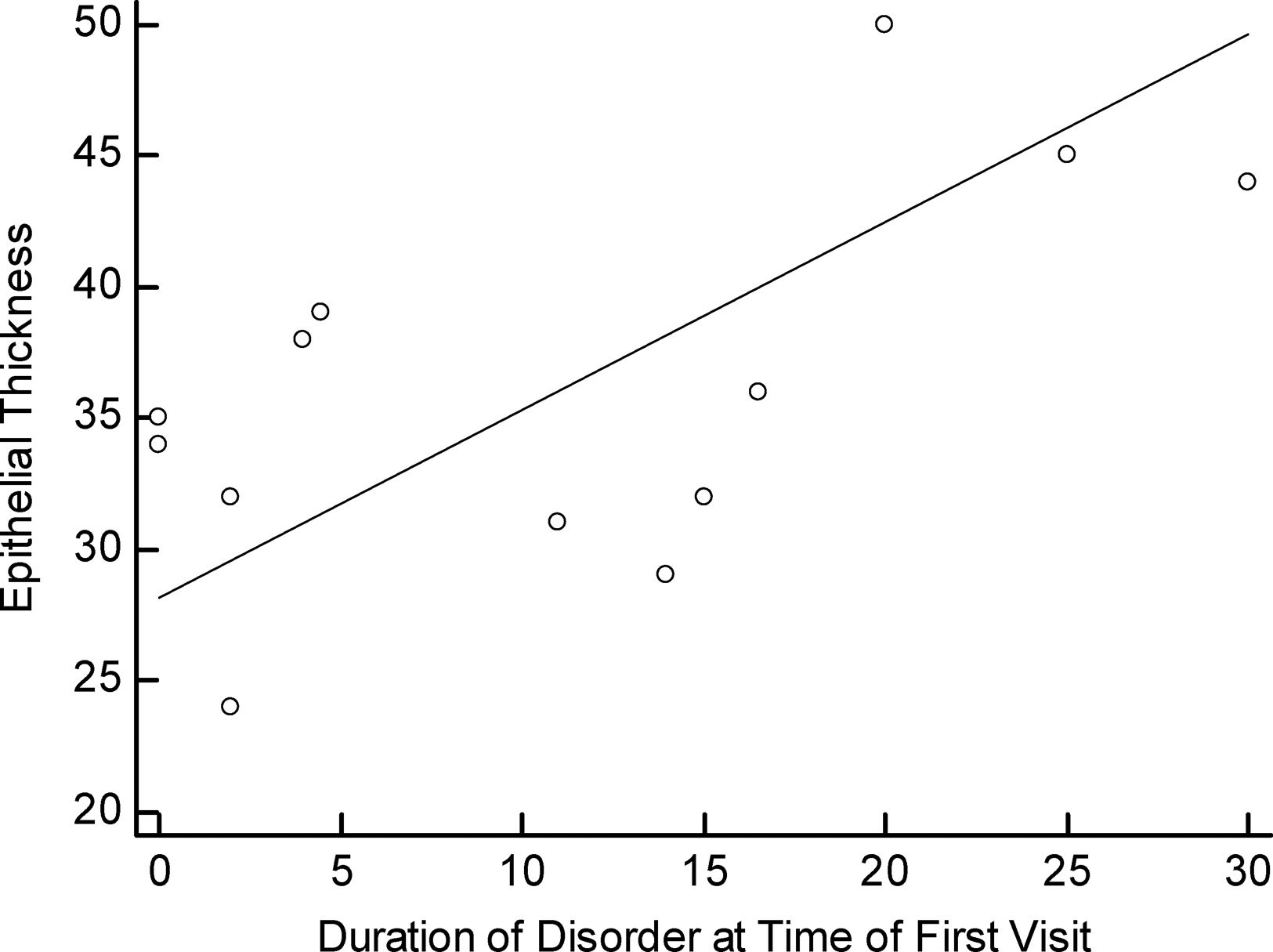
ResultsThe microscopic slides of all patients available for follow up (n=48) were analyzed and CET and TCT were measured. The histological evaluation revealed 3 distinctive epithelial patterns. Pattern 1 with central hypertrophic and hydropic changes (n=19) measured 70.89±25.88μm in CET and 308.63±100.74μm in TCT; Pattern 2 (n=14) had not changed, similar to normal epithelium CET and TCT measuring 36.5±7.02μm and 260.14±87.93μm respectively. Pattern 3 (n=15) demonstrated thinner central epithelium characterized by atrophy and focal hydropic changes measuring 19.93±4.60μm and 268.00±79.39μm in CET and TCT respectively (all p<0.0001). The presence of Pattern 2 characterized by similar to normal CET was correlated with the duration of the condition (R=0.600, p=0.030). There was a significant difference in the length of CL wear comparing those with patterns 1 and 2 versus 3 (least no. of CL years) (p=0.05 and p=0.33 respectivelly).
ConclusionsPatients with advanced disease have various central corneal epithelial changes detected with histology. Although each central epithelial pattern type was distinctive comparing the 3 patterns, there was no correlation with years of CL wear but only with the duration of the condition.
Keratoconus (KC) is a bilateral degenerative disease characterized by a non-inflammatory, progressive central or nearly central corneal ectasia (typically asymmetric) and decreased vision.1,2 Once diagnosed the progression of KC, if in the early stages, can be slowed down with corneal crosslinking, when available.3 KC is managed mainly with the use of either corneal rigid contact lenses4–6 or scleral rigid contact lenses.7 The back surface of these contact lenses will form a spherical front surface of the tear film thereby correcting the corneal irregularity that KC induces as the cornea thins and becomes more ectatic with time.8 The effect of corneal or scleral contact lenses on the morphological characteristics of the KC cornea or its progression is not well known.
The measurement of corneal thickness as an indicator of KC progression is common with the use of optical and ultrasound pachymeters.9,10 Central epithelial and total corneal thicknesses have been recently measured with optical coherence tomography and histology for higher resolution images that offer more precise measurements.11–15 These higher resolution images can more clearly detect morphological changes that can help categorize if KC is mainly an epithelial or a stromal condition or both.
The cornea consists of multiple layers that include the epithelium, anterior limiting membrane, the stroma, pre-Descemet's layer, Descemet's membrane and the endothelium.16,17 Histological studies have identified that each of these layers can be affected by KC.18 Normal corneal epithelium consists of 5–6 layers of squamous non-keratinizing epithelium arranged fairly uniformly thereby providing smooth anterior corneal surface. The stroma consists of a regularly arranged collagen matrix lamellar structure.19
Histopathological changes in KC include progressive thinning and irregularity of the cornea, Bowman membrane breaks, increased stromal density, spatial disorganization, and epithelial abnormalities.18,20 Thinning of the corneal stroma is an established fact in KC.21 However, significant differences and conflicting evidence has been presented in terms of the histopathological characteristics of the corneal epithelium and their impact on treatment approaches and rates of complications. Some studies involving histological analysis of corneas with KC demonstrate significant thinning of the central epithelium.22 On the contrary, other studies report thickened epithelia in KC23–25 or no difference in epithelial thickness between KC and normal controls.26
Analysis of corneal buttons obtained after penetrating keratoplasty in our laboratory revealed the existence of three patterns in the corneal epithelium of patients with keratoconus which have not been previously described. These patterns could have an important role in a more accurate interpretation of the new imaging techniques used for in vivo early diagnosis of the disease.27
The purpose of this study was to first characterize the central thickness of keratoconic corneae (ex vivo) and then to correlate these morphological patterns with their clinical history.
MethodsThis study is a retrospective examination of corneal penetrating keratoplasty specimens. The clinical decision to perform a penetrating keratoplasty was taken based on clinical criteria after all conservative measures had been exhausted. None of the patients having keratoplasty were immunocompromised, had chronic autoimmune conditions or were placed on prolonged immunosuppressive therapy. None of the patients were on topical eye or systemic medication that would affect the progression or long term health of the corneal epithelium. 56 penetrating keratoplasty specimens were selected from the archives of IOBA Ocular Pathology Laboratory of the University of Valladolid in Spain. All paraffin blocks were retrieved, 5μm-thick sections were made using a microtome (Leica Biosystems, Nussloch, Germany) and stained with hematoxylin and eosin (H&E) and periodic acid of Schiff (PAS). Histological slides of a section of the corneal buttons in their entirety were evaluated by two independent pathologists in a blind fashion using bright field microscopy (Leica DMLB4000, Leica Microsystems, Wetzlar Germany). Thickness measurements were taken from the microscopic slides and represent an average of 5 evenly spaced vertical measurements encompassing a high power view of the central area of corneal buttons. This study was performed in accordance with tenets of the Declaration of Helsinki and all procedures were approved by the University of Valladolid ethics committee.
Central corneal epithelial thickness was measured, and morphological features of the epithelium were studied in order to identify differences and possible patterns of alteration that were previously noted by the authors. Clinical history regarding duration of the condition prior to surgery and length of and tolerance to contact lens wear were compared and analyzed to find if a relationship was present with the pattern type.
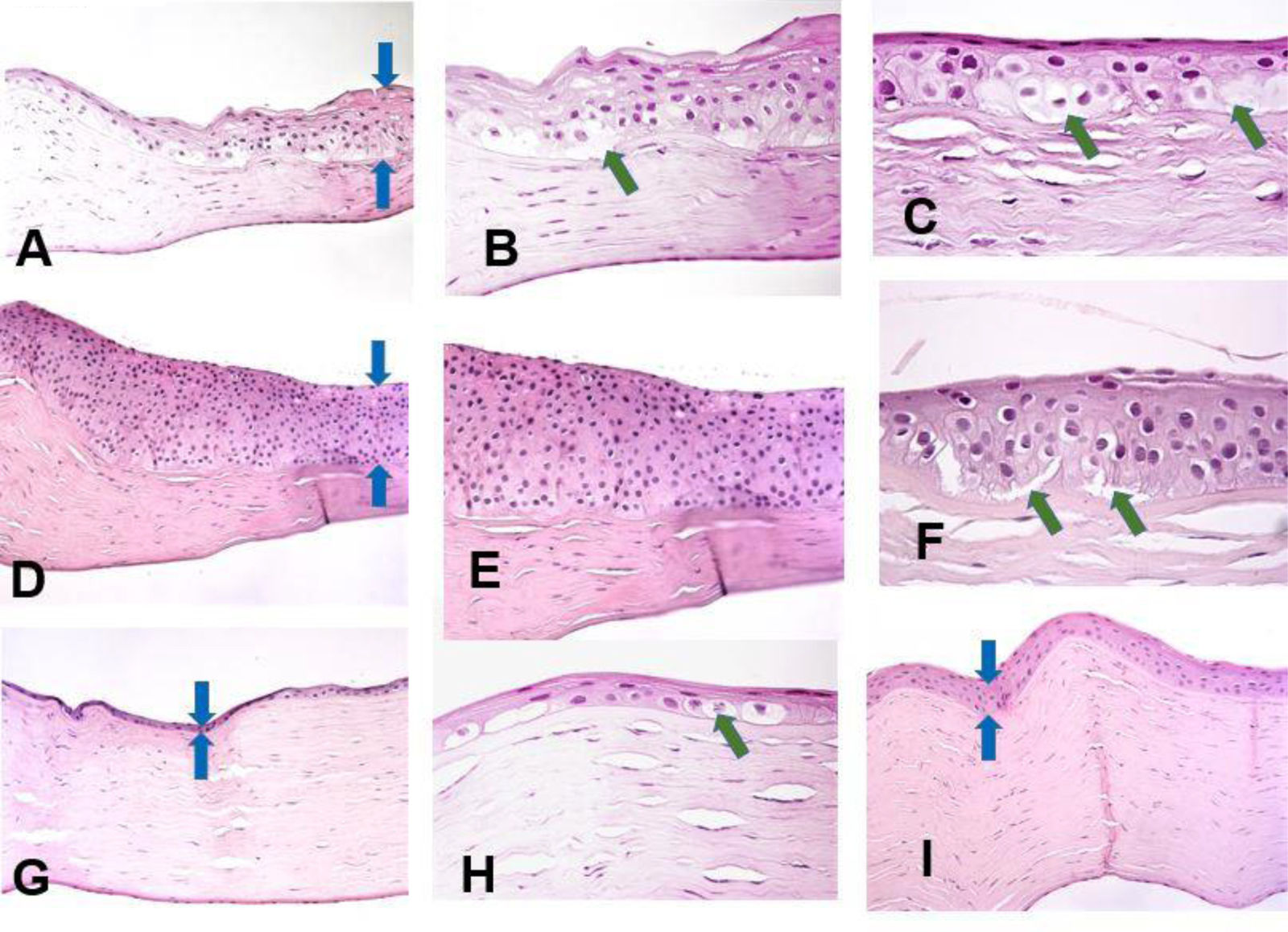
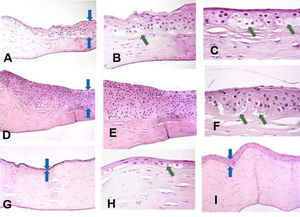
ResultsThree distinctive patterns of epithelial alteration of the central cornea were established. The most common pattern (Pattern 1) accounting for 19 of the cases was characterized by statistically significant increase in central cornea epithelial thickness with hypertrophy and pronounced hydropic changes (Fig. 1A–C) or hyperplasia with focal hydropic change (Fig. 1D–F, central epithelium indicated by blue arrows). The hydropic changes ranged from focal cellular swelling to patchy vacuolar degeneration to complete ballooning with cell loss and partial intraepithelial and sub-epithelial detachment (indicated by green arrows).
Histopathology preparation of H&E stained penetrating keratoplasty specimens of patients with keratoconus. Distinctive patterns of epithelial alteration of the central cornea.
- a)
Increased central corneal epithelial thickness (blue arrows) with hypertrophy and pronounced hydropic changes. Original magnification 20×.
- b)
Increased central corneal epithelial thickness (green arrow) with hypertrophy and pronounced hydropic changes. Original magnification 40×.
- c)
Pronounced hydropic changes (green arrows) in the central corneal epithelium in keratoconus. Original magnification 63×.
- d)
Increased central corneal epithelial thickness with marked hyperplasia of the epithelium (blue arrows). Original magnification 20×.
- e)
Marked hyperplasia of the central corneal epithelium. Original magnification 40×.
- f)
Focal hydropic changes with partial epithelial detachment in the central corneal epithelium (green arrows). Original magnification 63×.
- g)
Patient with advanced keratoconus with unchanged central epithelial thickness (blue arrows). Original magnification 20×.
- h)
Markedly reduced central epithelial thickness with atrophy (blue arrows). Original magnification 20×.
- i)
Markedly reduced central epithelial thickness with atrophy and focal cell swelling (green arrow). Original magnification 63×.
The second pattern (n=14) was characterized by an unaltered central epithelial thickness (36.5±1.75μm), with morphological features of the central corneal epithelium comparable to those of healthy corneae (Fig. 1I). There were no hydropic, hypertrophic, hyperplastic or atrophic alterations present in this subset of cases. In these cases the overall central corneal thinning was due to stromal thinning, with little to no contribution from the epithelial component.
The third pattern (n=15) constituted of markedly reduced central epithelial thickness (19.9±1.2μm) with frank atrophy (Fig. 1G) or atrophy with focal swelling (Fig. 1H). The central corneal epithelium was composed of 1–2 layers of epithelial cells with focal degenerative changes. The epithelial thinning contributed significantly to the overall central corneal thinning.
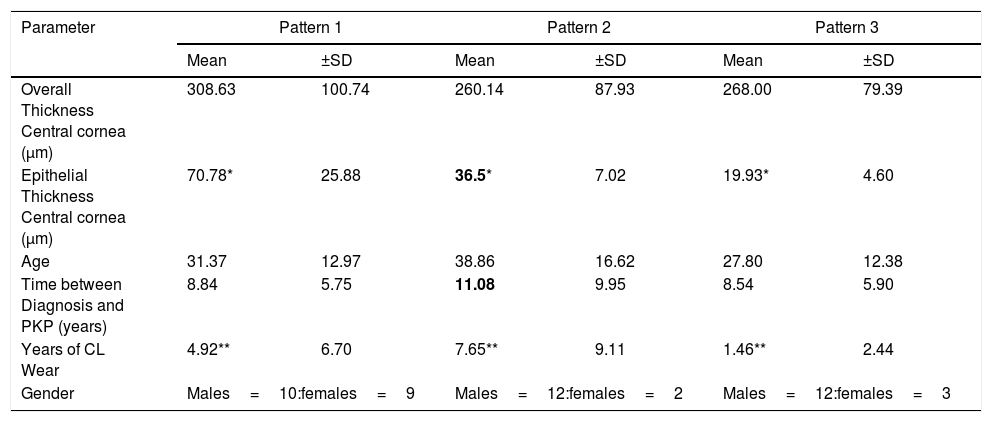
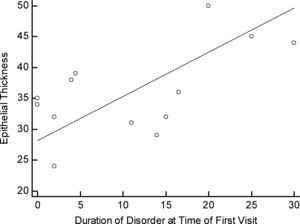
In the second part of the study that included all 48 patients for whom we had sufficient clinical history, 36 male and 14 female patients, and examined the relationship of clinical history and pattern type as shown in Table 1. Clinical factors that were examined included: age at the time of keratoplasty, contact lens tolerance and duration of lens wear. All specimens were derived from patients that either wore no contact lenses as they were intolerant but were previous corneal gas permeable lens wearers or were currently wearing corneal gas permeable lenses at the time of the keratoplasty. The results of central epithelial thickness (CET) were significantly different comparing each pattern (p<0.0001). Significant changes in CET having pattern 2 was correlated only with the duration of the condition taken from the first recorded visit (p=0.03) (Table 1).
Table of central epithelium and total thicknesses comparing the 3 pattern types.
| Parameter | Pattern 1 | Pattern 2 | Pattern 3 | |||
|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | Mean | ±SD | |
| Overall Thickness Central cornea (μm) | 308.63 | 100.74 | 260.14 | 87.93 | 268.00 | 79.39 |
| Epithelial Thickness Central cornea (μm) | 70.78* | 25.88 | 36.5* | 7.02 | 19.93* | 4.60 |
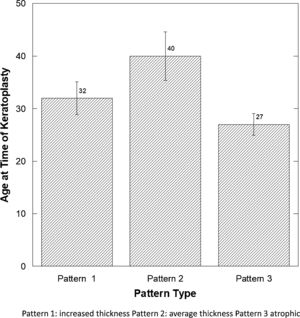
| Age | 31.37 | 12.97 | 38.86 | 16.62 | 27.80 | 12.38 |
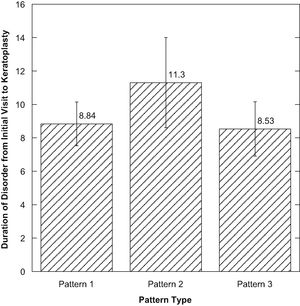
| Time between Diagnosis and PKP (years) | 8.84 | 5.75 | 11.08 | 9.95 | 8.54 | 5.90 |
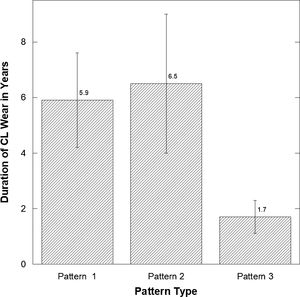
| Years of CL Wear | 4.92** | 6.70 | 7.65** | 9.11 | 1.46** | 2.44 |
| Gender | Males=10:females=9 | Males=12:females=2 | Males=12:females=3 | |||
For the 48 corneae examined, within the central area of the cornea, the thickened (hypertrophic and hydropic) epithelium thickness (pattern 1, n=19) was 70.89±25.88μm and the total thickness was 308.63±100.74μm. The epithelial thickness and total thickness centrally was 36.5±7.02μm and 260.14±87.93μm with pattern 2, having moderate hyperplastic and focal hydropic changes (n=14). The epithelial and total corneal thicknesses were19.93±4.60μm and 268.00±79.39μm, respectively with pattern 3, having atrophy and atrophy with focal swelling (n=15).
There were no significant differences among the three pattern types when compared (p>0.05) considering the duration of the disorder from initial diagnosis to keratoplasty (Fig. 2). There was a positive correlation between the time of the first visit to keratoplasty for those with pattern 2 compared to corneal epithelial thickness (R=0.600, p=0.03) (Fig. 3).
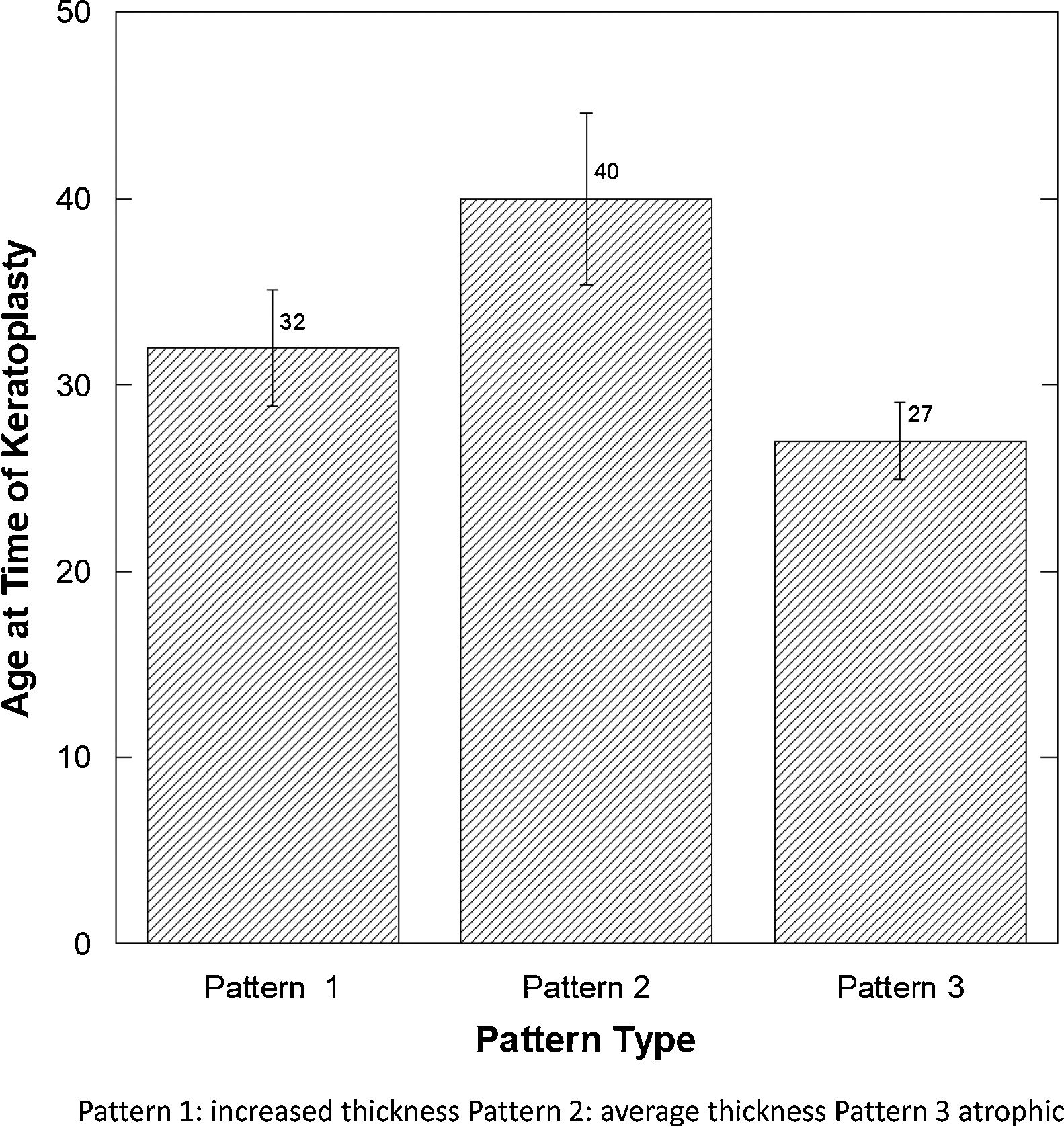
There was a significant difference in the age at the time of keratoplasty (Fig. 4), comparing those with pattern 3 (atrophic epithelium) to patterns 1 and 2 (p<0.02) (Fisher p<0.03).
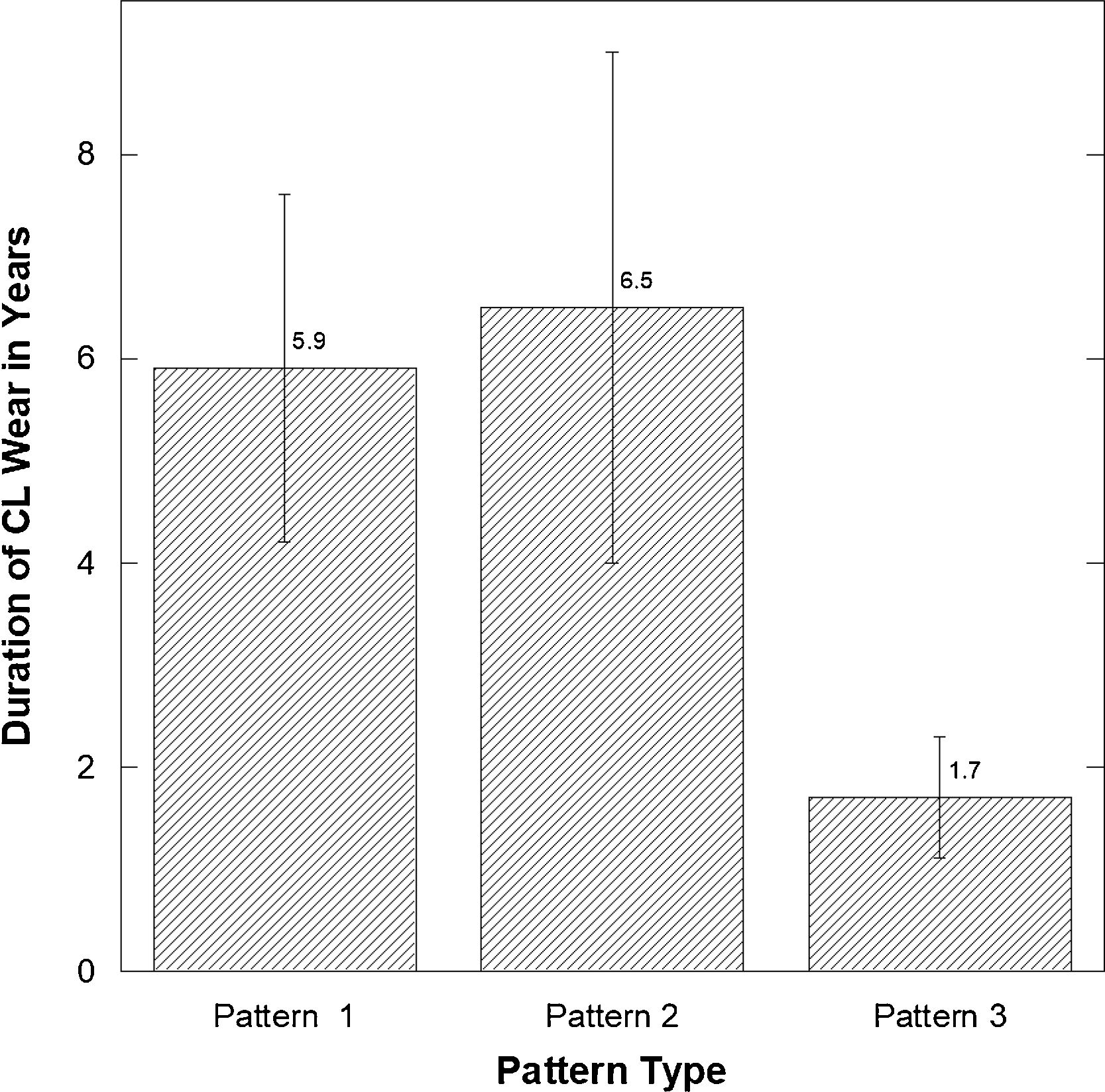
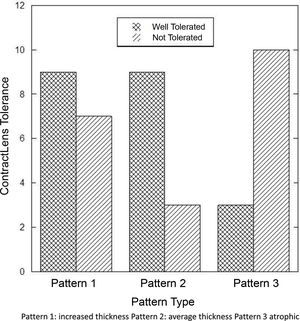
There was a significant difference in the length of CL wear (Fig. 5) comparing those with patterns 1 and 2 versus 3 (least number of CL years) (Pattern 1 versus 3: p=0.05 and p=0.033 Pattern 2 versus 3).
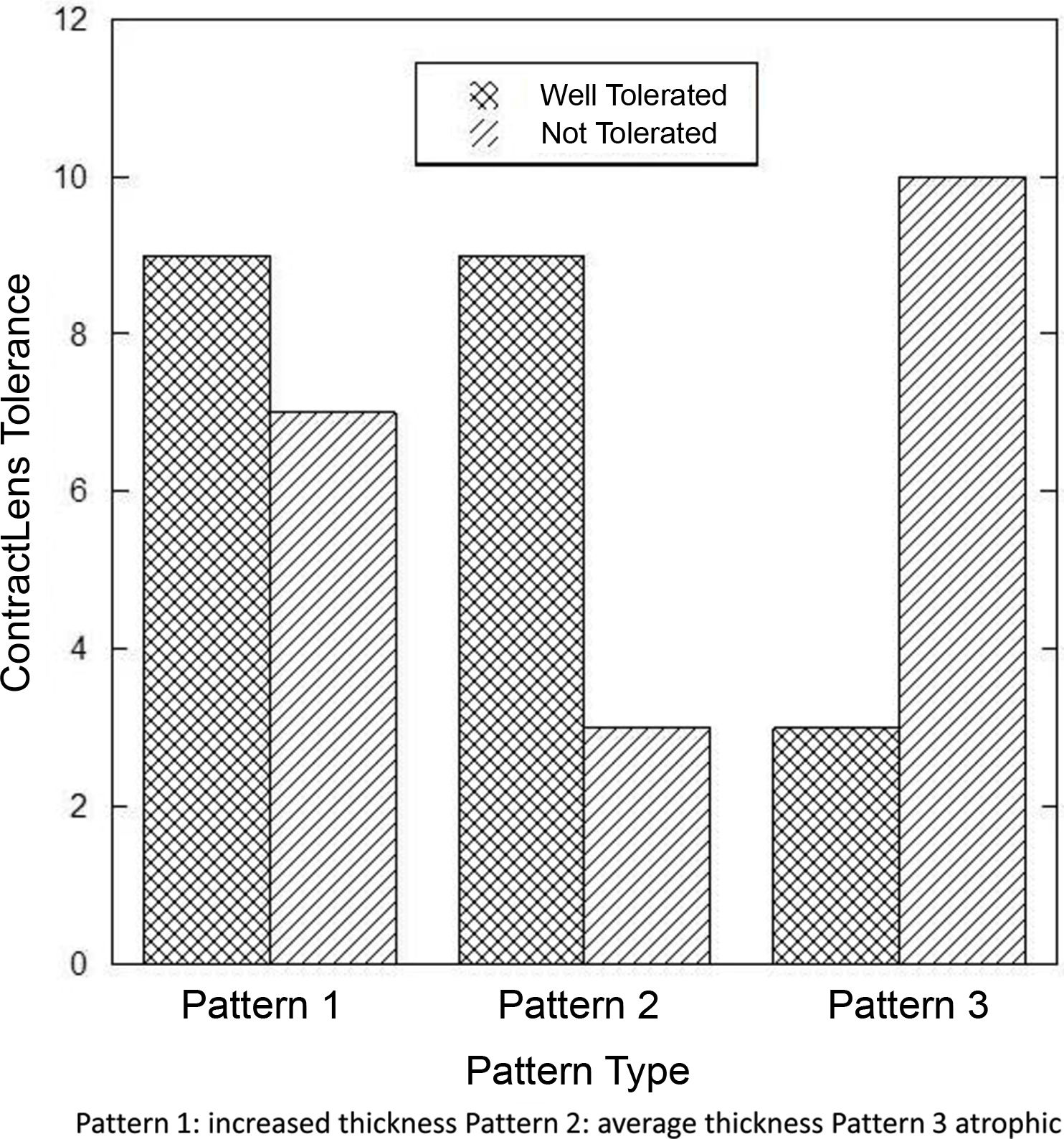
Those with pattern 3 were found to be significantly less tolerant to contact lenses (p<0.03) as shown in Fig. 6.
DiscussionNormally the thickness of the corneal epithelium of approximately 48±5 microns does not vary across the cornea form limbus to limbus (whereas the stromal layer does as it thickens toward the periphery).28 This study attempted to categorize central epithelial thickness in keratoconus affected pathological corneae where the epithelial thickness did not appear to be consistent across its entirety. The variation in central epithelial thickness lead to the characterization of 3 distinct central corneal epithelial thickness patterns where there was thickening of the epithelium (pattern 1), to unaltered of the epithelium (pattern 2), to significantly thinner epithelium (pattern 3), all compared theoretically29 to average eye. Central corneal epithelium in keratoconus responds with characteristic compensatory changes ranging from hypertrophy and hyperplasia to atrophy.22 This study has found 3 distinctive histologic patterns that could potentially have a diagnostic and prognostic value. To the best of our knowledge these patterns have not been previously described in the literature. The stroma in keratoconus undergoes changes in a progressive stepwise fashion therefore the particular measurements that were found may represent varying degrees of alterations at different stages. Stromal changes in keratoconus involve spacial disorganization of collagen fibers and abnormal extracellular matrix remodeling resulting in overall thinner, more compact, but weaker stroma. Multiple factors could be responsible for the similar overall central thickness of the cornea found with Pattern 2, one being varying degrees of swelling of the stroma likely due to impaired barrier function and Bowman membrane breaks even if normal appearing in thickness and morphology. Morphologically healthy but functionally impaired epithelium could cause stromal changes through facilitating the exposure to mechanical, chemical and biological environmental agents including the normal flora. The thicker stoma in the atrophic epithelium group (Pattern 3) could be attributed to a possible compensatory mechanism to the increased sheer stress from the mechanical friction of the protruded area with less epithelial cushioning, which could have caused a compensatory stromal re-enforcement through alternative matrix and fiber remodeling. Contact lenses may impact the changes found in the epithelium but there are other potential factors that remain unaccounted for in this small retrospective study of histological patterns including sex (women) where the majority had Pattern 1, characterized by thickened epithelium.
Nowadays, the use of in vivo techniques such as ultra-high resolution OCT have increased interest in the study of the corneal epithelial layer and new therapeutic procedures have also been developed. However, the application of these innovations in the prognosis and treatment of KC has not been explored in detail.30–33 On the other hand, the advantage of this histological study is that there is no eye movement and no disturbances, unlike OCT where these would degrade the image resolution. Histology also provides a precise visualization of the morphology of the epithelial cells, not visible with OCT, which could subsequently reveal potential mechanisms of disease progression.
Contact lens fitting as well as other treatment methods like corneal cross-linking are highly dependent on the epithelial properties and involve epithelium based variations in the techniques with different degrees of treatment success and failure.34–36
In a study performed by Mathew et al. the authors describe similar outcomes in 12 corneae examined histologically.20 They found a range for the central epithelial thickness from 13.5 to 91.6μm with an average of 42.2μm; the average in this study was 42.4μm across our three patterns. Our results also provide more evidence that keratoconus affects a wide area of the central cornea. This has been shown in a study by Brautaset et al. using commercially available instrumentation examining overall corneal thickness and comparing central thicknesses compared to controls.37 Using a higher resolution OCT and topographical measurements, Li et al., found only in the inferior zone that the epithelial thinning was significantly different than the normals he measured and related their results to the difficulty in OCT detecting the back surface of the epithelium die to the lack of Bowman's membrane in most cases.38 They could not relate epithelial thinning due to the lack of information regarding age and contact lens wear. There was also a large amount of variability in epithelial thickness apically using OCT in a study by Rocha et al. who concluded that regional irregularity of the epithelium should be directly measured when considering corneal cross-linking where the underlying stroma which consequently will also be variable in thickness may be clinically relevant.39
Our results are unique as we have collected clinical data to find whether these differences in epithelial thickness are related to the duration of contact lens wear and of the condition, lens tolerance and age at the time of the PKP. The atrophic epithelial pattern (pattern 3) tends to be present in significantly younger patients who are significantly less likely to tolerate lenses. This implies that central epithelial atrophy is not the natural progression of the disorder in all cases because patients with atrophic epithelial pattern are not older than the rest of the study population. The implication of this study may be that younger patients with the atrophic pattern are as mentioned less likely to tolerate lenses and therefore may benefit from different treatments and strategies for conservative management, of their keratoconus since they have significantly lower contact lens wear time and or tolerance.
The limitations of the study is its relatively small sample size and the limited data included in the clinical history at the time of the keratoplasty. One of the possible benefits of our work could be a greater use of cost-effective conservative techniques such as cross-linking.40 A significant decrease in the number of keratoplasties performed in patients with keratoconus was found in those patients treated with cross-linking which is normally performed on younger patients. Another technique that could be influenced would be the use of intrastromal corneal ring segments, which were approved by the FDA for the treatment of keratoconus in 2004.41
Further studies are necessary to determine the accuracy of the current imaging techniques in determination of progressive epithelial changes in keratoconus and establish their value in terms of patient management. Future work is also necessary to establish the significance of these morphological patterns by using ultra-high resolution optical coherence tomography (UHR-OCT). Also, its potential use of some of our findings as predictive factors in the progression of keratoconus needs to be elucidated.
ConclusionPatients with advanced keratoconus have various central corneal epithelial changes that can be detected with histology. Although each central epithelial pattern type was distinctive and different comparing the three patterns, there was no correlation with years of CL wear but only with the duration of the condition. Patients with atrophic epithelium showed significantly reduced tolerance to contact lens wear compared to patients with intact or hypertrophic central epithelium.
Funding sourcesFunding provided through the International European Union Partnership Grant.
Conflict of interestNo author has any conflicts of interest associated with any of the information or products discussed in this manuscript.
We are grateful to the Office of Research of the University of Waterloo (Waterloo, Ontario, Canada) for the funding provided to us through the International European Union Partnership Grant. We also would like to thank Centro de Oftalmología Barraquer (Barcelona, Spain) and IOBA, University of Valladolid (Valladolid, Spain) for providing us with the corneal samples for diagnosis.