To evaluate the efficacy and safety of the low-power, high-frequency electrical current treatment administered by the Rexon-Eye device, in a cohort of patients affected by mixed-type dry eye disease (DED) of medium to severe level.
Patients and methodsIn this prospective, non-randomized, interventional clinical study, eighteen mixed type DED patients were treated. Treatment was a specific type of electrotherapy, Quantum Molecular Resonance (QMR®), administered by means of the Rexon-Eye® device (Resono Ophthalmic, Sandrigo, Italy) with a protocol of one 20-min session per week, for 4 weeks. Patients were examined at baseline and one month after the last treatment, utilizing the Ocular Surface Disease Index (OSDI) questionnaire and clinical signs: non-invasive tear break-up time (NIBUT), Oxford staining, meibum quality, meibography, meibomian gland expressibility, tear meniscus height (TMH), Schirmer's test, ocular inflammation expressed by MMP-9 concentration.
ResultsSubjective benefit in OSDI was reported (p = 0.013). Improvement was also observed in NIBUT (p < 0.001), Oxford staining (p = 0.002), expressible meibomian glands number (p = 0.001) and meibum quality (p < 0.001). A remarkable benefit was present in inflammation, as evidenced by the reduction of MMP-9 (p = 0.003). Changes, although not statistically significant, were also present in TMH (p = 0.076) and Schirmer's test (p = 0.675), whereas no change was observed in meibography score. No adverse event was reported.
ConclusionIn this mixed-type DED patients’ cohort, Rexon-Eye proved to be effective and safe in improving subjective and objective ocular parameters, as well as capable to normalize inflammatory markers.
Dry eye disease (DED), more broadly defined as tear dysfunction, is a common ocular condition that needs prompt diagnosis and careful treatment intervention.1,2 It is a multifactorial disease of the ocular surface characterized by loss of homeostasis of the tear film, with a potential discordance between dry eye signs and symptoms.1,2 The reported prevalence of DED varies widely, from 5% to 33%, depending on both population and diagnostic criteria; however, there is a consensus that is higher among women as compared to men and increases with age.2 The total estimated burden of DED has been placed at 9.3% of adult Americans with the prevalence being 11.3% among all adults greater than 50 years of age and as high as 22.8% among women greater than 75 years of age.2 Evaporative and aqueous‐deficient are recognized as the two main subtypes of DED with potential overlap in their presentation. Meibomian gland dysfunction (MGD), leading to an abnormality in the tear lipid layer, is the leading cause of evaporative dry eye disease.2
Tear film instability, hyperosmolarity, inflammation, and cellular damage, which are the main mechanisms contributing to the physio-pathological process, are regarded as triggers of the vicious cycle occurring in DED.3 Multiple inflammatory markers, including matrix metalloproteinase 9 (MMP-9), have been isolated from the tear film of patients with DED.4-6 MMPs secreted into tears in DED can destroy tight junctions in the ocular surface epithelium reflecting the loss of ocular surface barrier function.7,8 Tear film hyperosmolarity in DED triggers the stress-activated protein kinase, signaling a cascade which, in turn, leads to the release of MMP-9 from corneal epithelial cells. This cascade initiates a progressive inflammation cycle.7,8
Proper diagnosis of ocular surface diseases requires the careful observation of a patient's symptoms and the measurement of signs through various tests.1 There is, therefore, considerable interest in finding methods that can measure ocular surface, tear film and Meibomian glands parameters objectively, reproducibly, and noninvasively. The TFOS DEWS II recommended using automated noninvasive measurement techniques that allow for an objective assessment of the DED signs.9
The goal of DED treatment is to restore homeostasis of the ocular surface and tear film by breaking this vicious cycle.1,10 The TFOS DEWS II management and therapy report presents a stepwise approach to the treatment of DED, which ranges from education, environmental or dietary modifications, artificial tear substitutes, punctal plugs, topical and/or systemic anti-inflammatory medications up to surgery.1 However, alternative therapies, especially for MGD, are emerging on the market, namely the use of vibration, massage, thermotherapy or thermal pulsation.11
Our study employed a specific type of electrotherapy device, Rexon-Eye® by Resono Ophthalmic, based on the QMR® stimulation. This technology produces and delivers, through electrical fields, an electrical stimulation with specific high-frequencies (from 4 to 64 MHz) and low intensity, which appears to be in resonance, i.e., has the same frequency, with the molecular bonds in biological tissue, and is thus able to maximize the transfer of power from the electrical stimulus signal to the biological tissue, with a minimum heat dissipation. This signal was also shown to obtain an important effect, i.e., the stimulation of the metabolism and natural regeneration of biological tissue and cells. It is possible to explain this effect by considering several phenomena generated by QMR and experimentally observed, such as a mechanical deformation of the cell membrane and an increase of calcium release and metabolism.12 Furthermore, using sophisticated micro-array techniques to evaluate gene expression, a more recent in-vitro study on mesenchymal stromal cells has shown that QMR is able to up-regulate genes involved in the extracellular matrix (ECM) remodeling, embryogenesis, wound healing and angiogenesis.13 It is of interest to note, for the possible application to the healing of corneal wounds, the positive results obtained by the QMR-based therapy in the healing of deep wounds in the limbs.14
Rexon-Eye was successfully employed to treat DED patients15 as well as MGD patients16 but was not clinically tested yet on mixed type DED patients. Our working hypothesis is that QMR can stimulate also in these patients the metabolism and natural regeneration of cells, resulting in the reactivation of the lacrimal and Meibomian gland tissue and benefit of the ocular annexes.15,16
The present study was therefore designed to assess efficacy and safety of QMR-based electrotherapy in a group of moderate to severe mixed type DED patients, utilizing objective diagnostic tools to evaluate symptoms, clinical signs as well as the inflammatory component.
Material and methodsThis prospective, non-randomized, interventional clinical study was conducted in the Ophthalmology Department of Konstantopouleio-Patission General Hospital, Athens, Greece. The study was in accordance with the tenets of the Declaration of Helsinki and the study protocol was approved by the hospital ethics committee (29,931/05.12.2019). All patients received oral and written information about the study and signed an informed consent form before receiving the examinations.
Patient selectionThe study population consisted of eighteen mixed type DED patients (17 female and 1 male; age range 42–81 years) that were randomly recruited. Patients were enrolled from the external diseases' outpatient clinic, presenting for their first appointment and were quasi-randomly allocated to treatment, by utilizing the last digit (odd or even) of their hospital number. The initial screening was performed with the OSDI questionnaire. Patients that reported an OSDI (Ocular Surface Disease Index) larger or equal to 13 were evaluated further with the DEWS II homeostasis markers of NIBUT (tear Non-Invasive Break Up Time) and corneal staining by Oxford scale. The ones with a NIBUT less than 10 s and/or corneal staining of more than 1 in Oxford scale were considered as DED cases according to DEWS II criteria and evaluated further in order to document the components of the mixed type of disease. A detailed ophthalmic history was evaluated, and a complete eye examination was carried out, including best corrected visual acuity (BCVA) assessment, intraocular pressure (IOP) measurement by Goldman applanation tonometry, slit lamp biomicroscopy and fundus examination. Participants with a history of ocular surgery, trauma, inflammation other than that attributed to DED, contact lens use, current or prior long-term topical ocular medication or other ocular pathology were excluded from the study. Patients with occasional usage of lubricant eye drops were included in the study, however a washout period of one week before commencement of treatment with Rexon-Eye was established by the study protocol, in order to avoid any confounding factors. Patients were advised against usage of any lubricant drops during the period of treatment.
TreatmentTreatment was delivered with the Rexon-Eye device (Resono Ophthalmic, Sandrigo, Italy), with a protocol of one 20-minute session per week, for four weeks. Treatment is administered by placing a custom designed mask over closed eyelids and closing the electrical circuit with a neutral plate the patient sits on. Special disposable facial tissues are worn between the mask and the eyelid surface, to evenly spread the electrical stimulation in the affected area and protect the eyes from potential transmission of bacteria and other pathogens. A custom unit scale is used by the device interface to display the applied power; the scale goes from 0 to 10, with 0 corresponding to no power applied. Intensity was set at 5 in our protocol, corresponding to an average power of 12 W, with 60 V voltage and 200 mA current between the mask electrodes and the neutral plate electrode. Patients were instructed to report any discomfort experienced during or after the sessions.
Patient evaluationOur primary endpoint was change in OSDI, a 12-item patient-reported outcome questionnaire designed to provide rapid assessment of the range of ocular surface symptoms related to chronic dry eye disease, their severity, and their effect on the patient's ability to function.9
Our secondary endpoints included changes in NIBUT, TMH (Tear Meniscus Height), meibography score, number of expressible Meibomian glands on lower eyelid, quality of meibum, corneal staining by Oxford scale grading, Schirmer's test, and MMP-9.
NIBUT, TMH, and infrared meibography for the measurement of meibography score were assessed with the IDRA® ocular surface analyzer (SBM Sistemi, Turin, Italy). For the detection of expressible Meibomian glands, we used the Meibomian Gland Evaluator (MGE, TearScience Inc., Morrisville, USA), a tool that applies constant pressure analogous to that of a blink for 10–15 s and leads to the expression of approximately 8 meibomian glands, provided the secretion is liquid in consistency, in three positions: nasal, central and temporal.17 For the glands with a normal appearance that did not yield liquid secretion using the MGE, forceful expression was performed with digital pressure.
Corneal staining by Oxford scale was then assessed, since it requires fluorescein. Corneal staining was divided into six groups according to severity, from 0 (absent) to 5 (severe).18 We compared the overall appearance of the patient's corneal staining with a reference figure, simulating the pattern of staining encountered in dry eye disease. Schirmer's test with anesthetic was performed 10 min after the evaluation of corneal staining.
The inflammatory component of DED was evaluated with the MMP-9 test (InflammaDry, Quidel Co., San Diego, CA, USA), which assays tear MMP-9 levels producing a dichotomous, positive or negative, outcome.19 The eye with the worse symptoms was selected and documented for MMP-9 sampling at first visit, and at the last visit, the same eye was used for comparison.
Evaluation of Meibomian gland secretion quality is one of the most informative parameters, despite being a difficult one to evaluate. Based on previous publications,17 we followed a semiquantitative quality evaluation scale of 1 = clear, 2 = cloudy, 3 = granular and 4 = toothpaste. The evaluation of meibum quality was performed after all diagnostic procedures, in order to avoid any effect of the expressed secretions on the tear film.
Patients were evaluated at baseline and one month after the end of the treatment. All measurements in all visits were performed by the same investigator (AT).
Statistical analysisSince the applied treatment affected both eyes equally and intraclass correlation coefficient between the two eyes was high (close to unity), scores from both eyes were averaged for all the measured variables except for our primary outcome OSDI and MMP-9, where only 1 score per subject was obtained. All statistical analyses were performed using the SPSS software version 25 (SPSS Inc, Chicago, USA). Shapiro-Wilk test was used to test parameters for normality. Descriptive statistics were used to calculate mean, average and standard deviation of all data. Paired t-tests and Wilcoxon signed ranks test were used to compare the average values of measurements for each outcome before and after treatment. Values were considered statistically significant at p < 0.05. Graphs were generated using GraphPad Prism (GraphPad Software, La Jolla, CA).
ResultsPatient characteristics and parameter values before and after treatment are presented in Table 1. Of a total of 22 patients initially enrolled in the study, 18 patients completed the protocol, 17 women and one man, due to lost follow-up as they lived in rural areas and were not able to follow protocol intervals. Mean age was 59.66 ± 13.02 years.
Values of the measured parameters, (mean, SD, 95% confidence interval) at baseline (Before) and one month after the end of the treatment (After), with statistical significance of the difference.
Notes: All parameters follow normal distribution tested by Shapiro-Wilk test for normality. P values exported by paired t-test and Wilcoxon signed rank test, as appropriate.
Abbreviations: OSDI, Ocular Surface Disease Index; MG, meibomian glands; NIBUT, non-invasive tear break-up time; TMH, tear meniscus height; MMP-9, Μatrix metalloproteinase 9; SD, standard deviation.
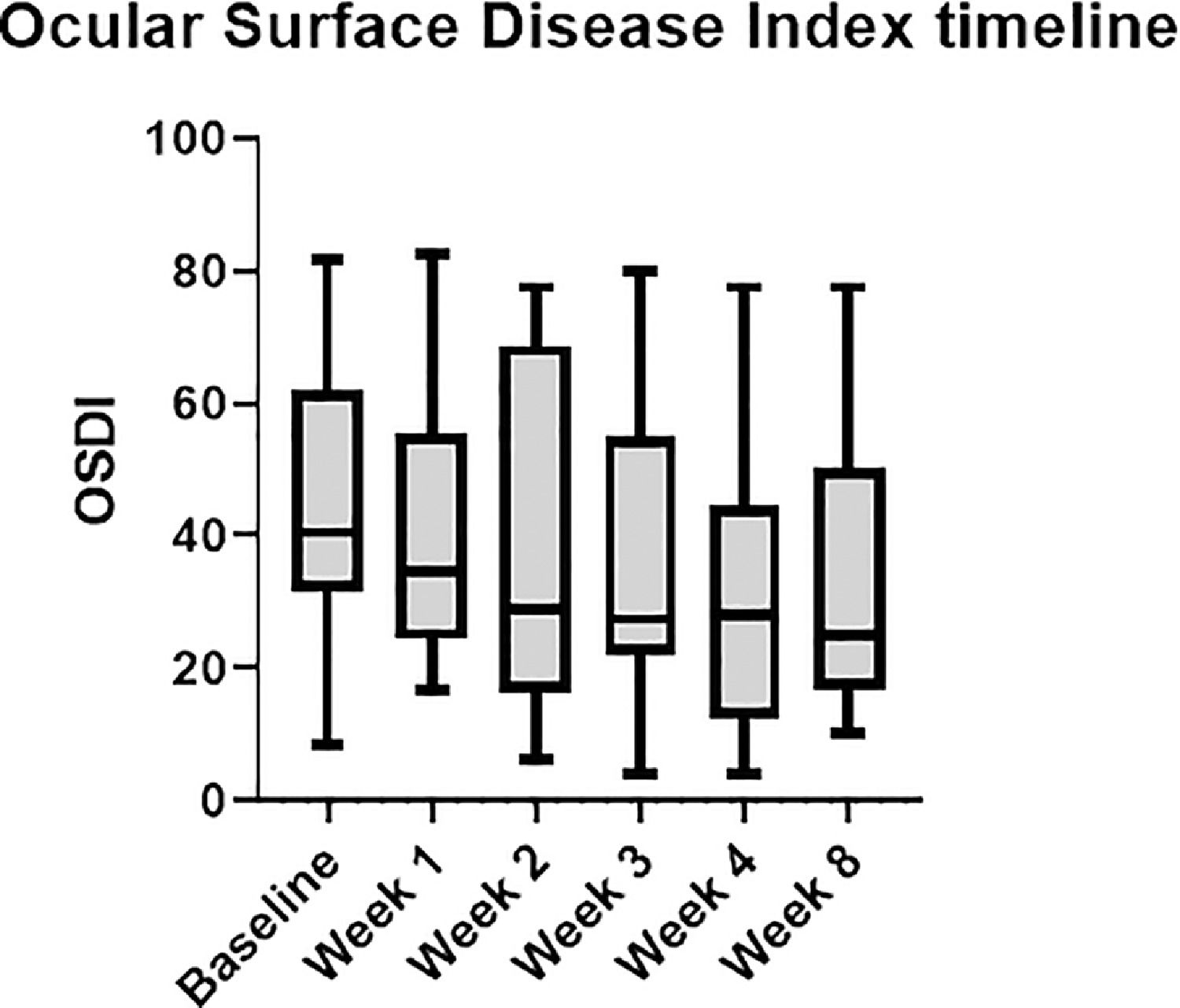
A statistically significant change in OSDI score was detected, from 45.46 ± 21.86 at the initial visit to 34.45 ± 23.79 (p = 0.013) at last visit, eight weeks after enrollment, which represents a 25% improvement. A monotonic decreasing trend was noticed during treatment (Figs. 1 and 2).
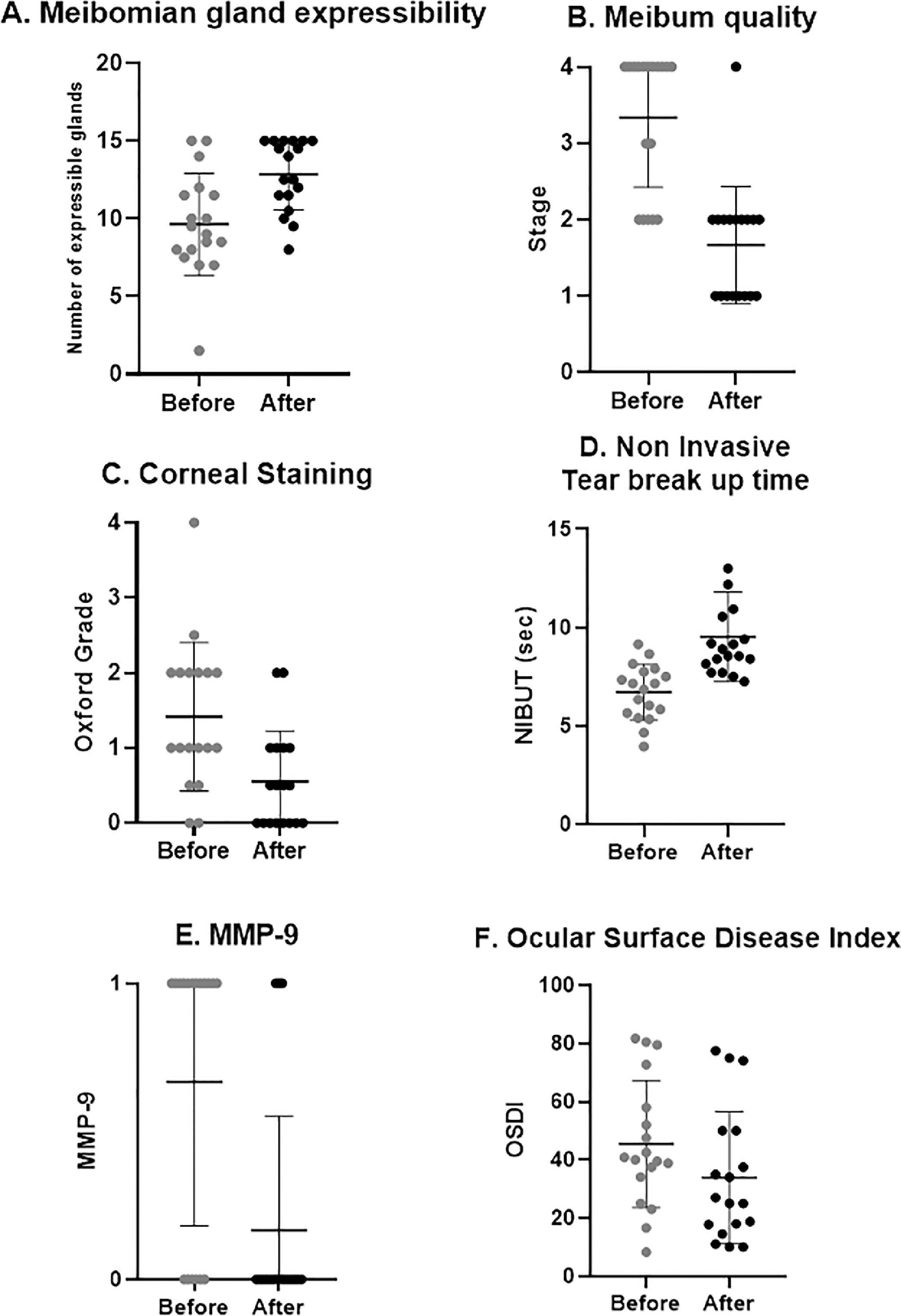
Clinical outcomes after treatment. A, number of expressible meibomian glands significantly increased (p = 0.001). B, meibum quality significantly improved (p < 0.001). C, corneal staining significantly decreased (p = 0.002). D, NIBUT significantly increased (p < 0.001). E, number of patients with positive MMP-9 test significantly decreased (p = 0.003). F, OSDI significantly decreased (p = 0.013).
After treatment, NIBUT increased by 42%, from 6.71 ± 1.40 to 9.53 ± 2.28 s (p < 0.001, Fig. 2).
TMHTear meniscus height values were normalized, decreasing by 31% from 0.52 ± 0.25 to 0.36 ± 0.20 mm (p = 0.076).
MeibographyMeibomian gland score as recovered by IR meibography did not show any significant change, from 15.05 ± 7.95 to 14.88 ± 7.21 percent (p = 0.926).
Meibomian gland expressibilityThe number of expressible Meibomian glands on lower lid improved after treatment by 33%, from 9.63 ± 3.27 to 12.83 ± 2.26 (p = 0.001, Fig. 2).
Meibum qualityA significant improvement, by 50%, was noticed in meibum quality at the end of treatment, from grade 3.33 ± 0.90 to grade 1.66 ± 0.76 (p < 0.001, Fig. 2).
Staining by oxford scaleAt the end of treatment, a significant decrease, by 61%, in corneal staining was detected, with Oxford grade decreasing from 1.41 ± 0.98 to 0.55 ± 0.66 (p = 0.002, Fig. 2).
MMP-9At baseline, twelve out of eighteen patients had positive MMP-9. One month after treatment, only three patients had positive MMP-9, a 75% improvement (p = 0.003, Fig. 2).
Schirmer'sOne month after treatment, there was a slight but not statistically significant improvement in Schirmer's values, from 8.75 ± 4.98 to 9.19 ± 5.63 mm (p = 0.675).
Safety evaluationCompliance was observed in all study patients regarding sessions of treatment scheduled by the study protocol. All patients reported a pleasant feeling during treatment and none of them asked for interruption during stimulation. At the end of each session, a routine eye check was performed, and no adverse events were reported.
Discussion and conclusionOur study evaluated the safety and efficacy of the treatment with the QMR-based electrotherapy administered by Rexon-Eye in a mixed-type cohort of medium to severe DED patients.
Electromagnetic fields play an essential role in cellular functions, interacting with cellular pathways and tissue physiology. Cells interact with the surrounding environment through receptors and ion channels that transmit chemical, mechanical, and electrical signals.20
In this context, the Quantum Molecular Resonance (QMR) produces and delivers, through electrical fields, an electrical stimulation with specific high-frequencies (4–64 MHz) and low intensity. This unique stimulation can obtain an important effect, i.e., the stimulation of the metabolism and natural regeneration of biological tissue and cells.
As regards the specific application of QMR to ophthalmology, previous results showed that this technology is able to effectively and safely treat symptoms and signs of DED.15,16 Our results confirm those of these two previous studies and extend them to the specific case of mixed DED.
In case of DED, the available severity criteria are confounded by complex disease subtypes and a lack of standardization. Therefore, the selection of single criteria for assessment of disease severity is fraught with difficulties.12 In the DEWS II diagnosis report, several diagnostic tests are listed, including questionnaires, tear film tests, epithelial abnormalities assessment strategies, and other approaches.9 The report indicated that the most appropriate and efficacious protocol to diagnose and monitor DED is based on a combination of symptoms, signs, and clinical tests, since any one of these alone would miss some patients.9
Considering these guidelines, several subjective and objective clinical parameters have been considered in our study. Patient-reported symptoms reflected by OSDI, our primary outcome, were significantly alleviated. What is more interesting, a gradual improvement in OSDI scores in each one of the four session visits of our therapeutic protocol reflects the effect of each session as well as the additive effect of the whole four-sessions treatment. From our secondary outcomes, both NIBUT and corneal staining, which according to DEWS II are two of the critical diagnostic signs of DED, were statistically significantly improved. There was also a significant improvement in the quality of MG expression, which is clinically translated into a better quality of tear film. In addition, a significant improvement was also observed in inflammation, as documented by the marked reduction in MMP-9.
Control of inflammation seems to play a pivotal role in improving symptomatology, since it is a major component in the pathophysiology underlying the long-standing and more severe types of DED.4,5 Our purpose was to evaluate the efficacy of treatment protocol not only in symptoms and signs but also regarding the response to the existent inflammation. In this test, levels above 40 ng/ml produce a positive result, however it is a nonspecific marker as regards the source of ocular surface inflammation.19 Our results document an improvement by 75% (p = 0.003), with only three patients producing a positive MMP-9 test after treatment, compared to twelve out of eighteen patients before treatment. To the authors’ knowledge, this is the first study to evaluate the effect of Rexon-Eye treatment in inflammation.
The improvement in the quality of MG production and the reduction of inflammation allowed an overall improvement in tear film homeostasis, which is eventually what one requests from a DED treatment. Reduction of MMP-9 accompanied by improvement of patient-reported symptoms and objective clinical signs confirms the anti-inflammatory effect of Rexon-Eye, as well as the correlation between inflammation and clinical outcomes. The probable mechanism behind the relationship between improvement of meibum quality and tear inflammatory component could be explained by the pathogenesis of MGD. Increased meibum viscosity may arise from the changes in meibum composition.13 Ocular surface inflammation and quality of Meibomian gland secretions are interactively involved in the cascade of the pathophysiologic mechanism of MGD. Increase in MG viscosity promotes to meibum stasis that can in turn promote bacterial growth, which potentially could lead to the increased release of esterases and lipid-degrading lipases.10 It has been shown that increased enzyme activity increases meibum melting temperature, generating also free fatty acids that can lead to hyper-keratinization and inflammation.10 These changes in lipid composition lead to further Meibomian gland obstruction, ocular surface instability, and increased tear evaporation, contributing to the development of DED and patient discomfort.10 In our patients, the number of expressible glands on lower lid improved after treatment by 33%, alongside meibum quality, which improved from a mean granular composition to a mean clear to cloudy composition, supporting this hypothesis.
From our results there is a suggestion that the improvement in inflammation in conjunction with improvement in MG quality might be important therapeutic targets in patients with MGD. The QMR treatment significantly reduces the expression of proinflammatory molecules, such as matrix metalloproteinases, which is increased in MGD.14,21 This improvement is related to the stabilization of the ocular surface, also supported by the normalization in the tear meniscus height, whose average decreased from 0.52 to 0.36 mm, possibly attributed to control of inflammation.
The limitations of this study include a relatively small sample size and lack of a control group. Potential risks of placebo effect and investigator bias were greatly reduced by the objective tools used to assess clinical outcomes. By implementing IDRA, InflammaDry, MGE, and the strict guidelines of DEWS II criteria, our effort aimed at standardizing treatment outcomes to ensure that the measurement quality of the acquired data is less likely to be compromised.
The follow-up period after treatment termination was indeed short. Although a previous study showed that clinical benefits from Rexon-Eye therapy are basically maintained one year after the treatment,11 further investigations are needed to confirm these long-term results also in the specific population of mixed-type DED patients. In addition, since Rexon-Eye is a fairly new technology in dry eye disease treatment, different protocols should be examined in the future, depending potentially on the severity of disease. It would also be of interest to examine the changes in dry eye parameters in the course of treatment in order to investigate future amendments in treatment protocols.
Future studies with larger sample sizes and longer follow-up times might be helpful in replicating and extending our findings, which however established Rexon-Eye as a significant therapeutic option that effectively widens our armamentarium in the treatment of DED.
We would like to thank Resono Ophthalmic, Sandrigo, Italy for providing us the Rexon-Eye device used in this study.