To evaluate preliminarily the safety and efficacy of customized photorefractive keratectomy (PRK) to correct ametropia and irregular astigmatism after penetrating keratoplasty (PK).
MethodsThis pilot study included five eyes of five patients with a mean spherical equivalent of −5.1±1.46D (range from −2.75 to −6.50D). In all cases, ametropia and irregular astigmatism was corrected with topography-guided customized PRK. Ocular examinations with topographic analysis were performed preoperatively as well as at 1, 3 and 6 months after surgery.
ResultsAll eyes gained postoperatively at least three Snellen lines of uncorrected visual acuity. Mean refractive spherical equivalent was 0.62±0.63D (range from −0.25 to −1.75D) at 6 months postoperatively.
ConclusionOur pilot study suggests that customized PRK can be a safe and effective method for treating ametropia and irregular astigmatisms after PK. Future studies with larger samples and longer follow-ups should be performed to confirm these results.
Evaluar preliminarmente la seguridad y eficacia de la queratectomía fotorrefractiva personalizada (PRK) para corregir la ametropía y astigmatismo irregular tras queratoplastia penetrante (PK).
MétodosEste estudio piloto incluía un total de 5 ojos de 5 pacientes con un equivalente esférico medio de −5,1±1,46D (rango entre −2,75 y −6,50D). En todos los casos, la ametropía y astigmatismo irregular se corrigió mediante PRK personalizada guiada por topografía. Se realizaron exámenes oculares con análisis topográfico preoperatoriamente, así como a los 1, 3 y 6 meses tras la cirugía.
ResultadosTodos los ojos ganaron al menos 3 líneas de agudeza visual Snellen no corregida. El equivalente esférico medio fue de 0,62±0,63D (rango entre −0,25 y −1.75D) a los 6 meses tras la cirugía.
ConclusiónNuestro estudio piloto sugiere que la PRK personalizada puede ser un método seguro y eficaz para el tratamiento de la ametropía y el astigmatismo irregular tras PK. Deben realizarse futuros estudios con muestras de pacientes mayores y seguimientos más largos que confirmen estos resultados.
Keratoconus (KC) is characterized by progressive corneal protrusion and thinning, leading to irregular astigmatism and impairment of visual function. KC is among the best indications for doing a penetrating keratoplasty (PKP), with long-term graft survival rates surpassing those for any other indication.1,2 Generally accepted indications for PKP in KC are poor visual acuity with contact lenses, contact lens intolerance or inability to fit/wear contact lenses, and non-resolving corneal hydrops. The percentage of patients with KC eventually requiring PKP varies widely in different reports.3–7 Residual refractive error and corneal irregularity following PKP can be managed with spectacles or contact lenses. However, although advances in techniques and instrumentations for PKP, especially the introduction of femtosecond lasers, have greatly improved PKP results, high refractive errors, especially high astigmatisms, associated to high levels of corneal irregularity, may appear postoperatively in spite of an uneventful surgical procedure. These optical errors are hardly correctable and very disturbing for the patient.8–10 In order to reduce residual astigmatism after PKP, some options have been described: surgical approaches such as relaxing incisions,11,12 wedge resection as well as selective removal of sutures, which is a less predictable and stable method.13–16 Some of the most common related complications to this last method are the risk for wound dehiscence, transplant rejection, and unsolvable topographic and refractive fluctuations.17 Crystalline lens extraction with IOL implantation can correct ametropia but not corneal aberrations and some risks are associated to this procedures, such as endophthalmitis, secondary glaucoma, retinal detachment, or endothelial cell loss.18–20
The use of the excimer laser is a safe and effective technique to correct post-keratoplasty ametropia.21–24 However, conventional LASIK and PRK have limitations because they are unable to correct the irregularity of the post-transplantation corneal surface. Furthermore, some risks of the LASIK technique due to the creation of the flap should be considered, such as the creation of incomplete, irregular or even damaged flaps.25–27 PRK is a safe and reliable technique but the risks of corneal haze and refractive regression should be also considered.28–31
Customized topography-guided corneal ablation with excimer laser is a procedure that can be used to correct not only ametropia after penetrating keratoplasty (PKP), but also irregular astigmatisms.32 We have used this technique to treat ametropia and irregular astigmatism after PKP in five of our patients in the attempt of verifying its efficacy, predictability, and safety. Therefore, the purpose of the current study was to evaluate preliminarily the safety and efficacy of topography-guided customized PRK for the correction of irregular astigmatism after PKP.
MethodsThis study comprised of five eyes of five patients with significant residual ametropia (mean spherical equivalent −5.1±1.46D, range −2.75D to −6.50D) and irregular astigmatism after PKP that was treated by customized PRK. Patient age ranged from 49 years to 61 years. The sample included one male and four female patients.
All patients had undergone PKP at least 18 months before PRK, with removal of sutures at least 6 months before PRK. In all cases, PKP has been performed due to the presence of keratoconus of grade 3 or 4 according to the Amsler–Krumeich classification. After suture removal, no large changes in manifest refraction were observed. As a significant ametropia was present in the eye with previous PKP, a significant level of aniseikonia was present in all patients, not allowing them the use of spectacles. Furthermore, in all cases, the refraction was stable for at least 6 months after suture removal. All eyes from the sample were intolerant to contact lenses.
An informed consent about the risks, benefits, and alternative treatment methods was signed by all the patients. This study received approval of the Ethics Committee of the hospital where the study was conducted, following the tenets of the Helsinki Declaration.
Preoperatively and in each postoperative visit (3, 6, and 12 months after surgery), a complete ophthalmic examination was performed in all patients including: uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), corneal topography (Keratron, Optikon 2000 Inc.), corneal aberrometry (VISX WaveScan AMO Inc.), optical pachymetry (Oculus Pentacam HR, Oculus), air-puff tonometry (TonoRef II RKT-7700, Nidek) and endothelial cell analysis with a specular microscope (NonconRobo Sp-8000, Konan).
For the treatment, an acquisition of two very similar topography maps was performed, with a maximum difference of 3 micrometers between all the processed points in the 5mm central zone of the cornea. This process was aimed at ensuring the obtainance of reliable corneal surface data. The elevation data obtained with the topographer, the patient's manifest refraction as well as the aberrometric data were uploaded to the software of calculation of the ablation profile. The customized ablation profile was then transferred to the excimer laser VISX Star S4 IR (AMO Inc.) computer. Only corneal aberrometric data were used to design the laser ablation profile. The procedure was planned to achieve the correction of the spherocylindrical refractive defect and the minimization of corneal HOAs. The ablation profile obtained was transferred to the laser computer for optimal positioning of the laser beam.
In each eye, one drop of 4% lidocaine was instilled 4 times every 5min before treatment. The non-treated eye was occluded during surgery, whereas antibiotic drops of 0.3% netilmicin (Nettacin) were instilled in the affected eye. After epithelium removal with an Amoils rotating brush, the customized laser procedure was performed. To center the treatment, patient's pupil was automatically detected by the excimer laser and topographic and refractive data were utilized. We did not use the transepithelial technique because of the corneal anomalies and irregularities that are usually present in post-PKP corneas, and also considering that there are some papers stating the effectiveness of the surgical procedure used.7,15,16 One drop of netilmicin 0.3% (Nettacin), fluorometholone 0.1% (Fluaton) eye drops and a contact lens (Protek T&S, Contact Vision) were applied postoperatively. The postoperative treatment included netilmicin 0.3% eye drops 4 times daily for 1 month, clobetasone (Visucloben) eye drops 4 times a day for 1 week and then reducing during the next 3 weeks and hyaluronic acid 0.2% (Hyalistil eye drops 4 times daily for 3 months, then 3 times a day for the next 3 months).
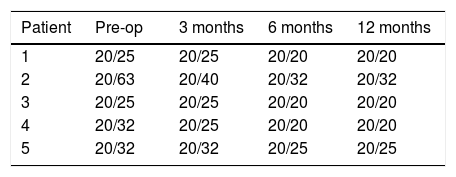
ResultsThe preoperative and postoperative patients’ data are shown in Table 1. All eyes had an UDVA equal to or better than 20/400 (1.1LogMAR) preoperatively. The treatment resulted in an improvement of UDVA that remained stable throughout the follow-up. After treatment, two eyes achieved 20/50 (0.4LogMAR) of UDVA and three eyes 20/100 (0.7LogMAR), with a gain of three Snellen lines.
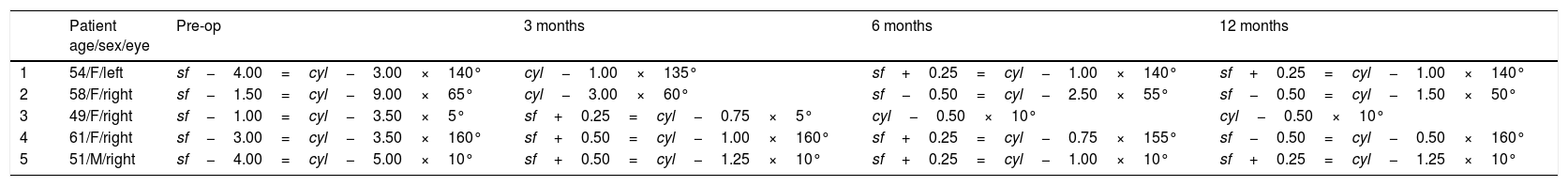
Refractive data of each patient preoperatively and postoperatively.
| Patient age/sex/eye | Pre-op | 3 months | 6 months | 12 months | |
|---|---|---|---|---|---|
| 1 | 54/F/left | sf−4.00=cyl−3.00×140° | cyl−1.00×135° | sf+0.25=cyl−1.00×140° | sf+0.25=cyl−1.00×140° |
| 2 | 58/F/right | sf−1.50=cyl−9.00×65° | cyl−3.00×60° | sf−0.50=cyl−2.50×55° | sf−0.50=cyl−1.50×50° |
| 3 | 49/F/right | sf−1.00=cyl−3.50×5° | sf+0.25=cyl−0.75×5° | cyl−0.50×10° | cyl−0.50×10° |
| 4 | 61/F/right | sf−3.00=cyl−3.50×160° | sf+0.50=cyl−1.00×160° | sf+0.25=cyl−0.75×155° | sf−0.50=cyl−0.50×160° |
| 5 | 51/M/right | sf−4.00=cyl−5.00×10° | sf+0.50=cyl−1.25×10° | sf+0.25=cyl−1.00×10° | sf+0.25=cyl−1.25×10° |
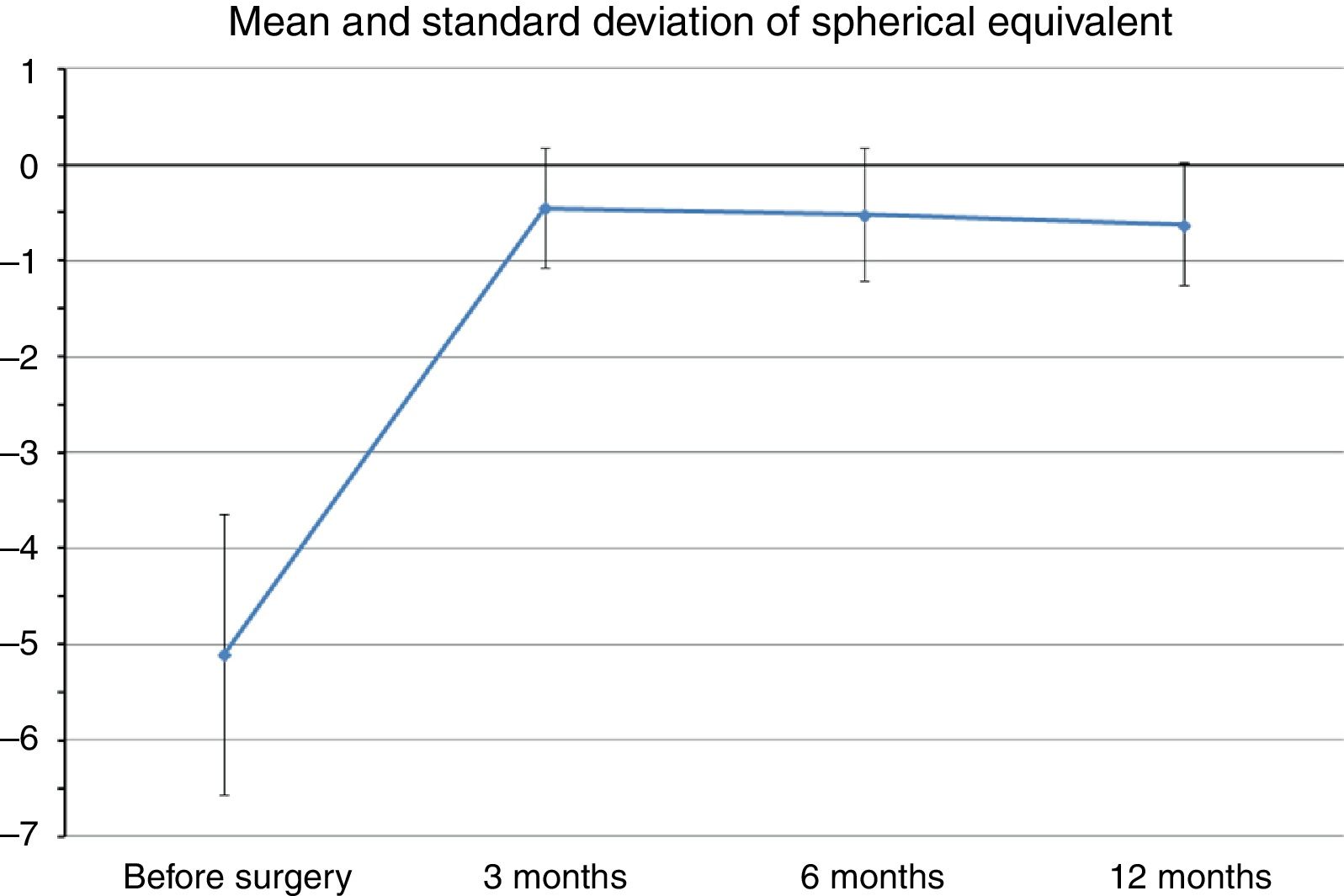
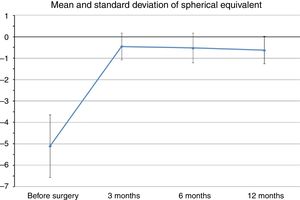
Mean preoperative SE was −5.1±1.46D (range −2.75 to −6.5D), whereas at the last postoperative visit mean SE was −0.62±0.63D (range −0.25 to −1.75D). Fig. 1 shows changes in mean SE during the entire follow-up. No patient lost lines of CDVA. A total of three patients gained two lines of CDVA, one patient gained three lines and another gained four lines. The visual outcomes remained stable at the last follow-up visit. Re-epithelization was observed in all cases in a period between 72 and 96h after PRK.
At the 12-month postoperative examination, three patients achieved 20/20 (0.0LogMAR) CDVA (Table 2). No patient needed secondary surgery. No patients had evident corneal haze, although two patients reported halos. Some subjective symptoms such as ocular discomfort, burning or slight pain was easily resolved after steroid therapy.
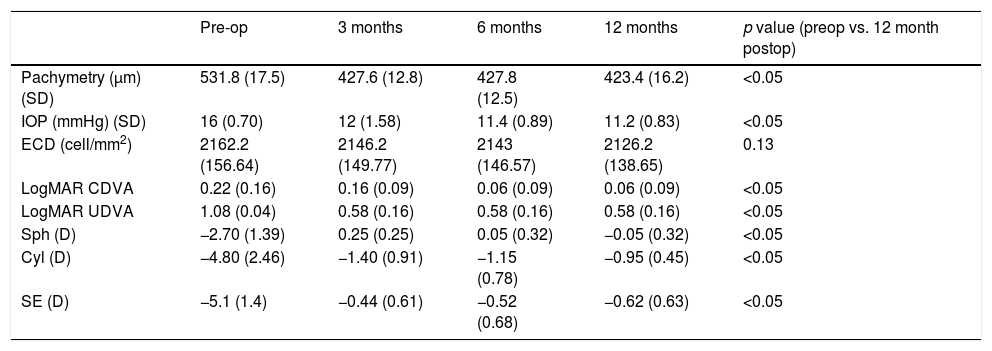
Table 3 shows the results obtained in the measurement of central corneal thickness, intraocular pressure and endothelial cell count. Corneal thickness was reduced on average 108μm with the laser ablation, remaining in all cases more than 400μm of residual cornea. Mean intraocular pressure values were similar during the postoperative follow-up without statistically significant differences (p=0.09, paired Student's t-test). Mean endothelial cell density did not change significantly between the preoperative and the 12-month postoperative visits (p=0.13, paired Student's t-test), with a cell loss of less than 2% at one year.
Mean value±standard deviation of pachymetry, intraocular pressure (IOP), endothelial cell density (ECD), corrected distance visual acuity (CDVA), sphere in diopters (Sph), cylindrical value in diopters (Cyl), and spherical equivalent in diopters (SE) during the follow-up.
| Pre-op | 3 months | 6 months | 12 months | p value (preop vs. 12 month postop) | |
|---|---|---|---|---|---|
| Pachymetry (μm) (SD) | 531.8 (17.5) | 427.6 (12.8) | 427.8 (12.5) | 423.4 (16.2) | <0.05 |
| IOP (mmHg) (SD) | 16 (0.70) | 12 (1.58) | 11.4 (0.89) | 11.2 (0.83) | <0.05 |
| ECD (cell/mm2) | 2162.2 (156.64) | 2146.2 (149.77) | 2143 (146.57) | 2126.2 (138.65) | 0.13 |
| LogMAR CDVA | 0.22 (0.16) | 0.16 (0.09) | 0.06 (0.09) | 0.06 (0.09) | <0.05 |
| LogMAR UDVA | 1.08 (0.04) | 0.58 (0.16) | 0.58 (0.16) | 0.58 (0.16) | <0.05 |
| Sph (D) | −2.70 (1.39) | 0.25 (0.25) | 0.05 (0.32) | −0.05 (0.32) | <0.05 |
| Cyl (D) | −4.80 (2.46) | −1.40 (0.91) | −1.15 (0.78) | −0.95 (0.45) | <0.05 |
| SE (D) | −5.1 (1.4) | −0.44 (0.61) | −0.52 (0.68) | −0.62 (0.63) | <0.05 |
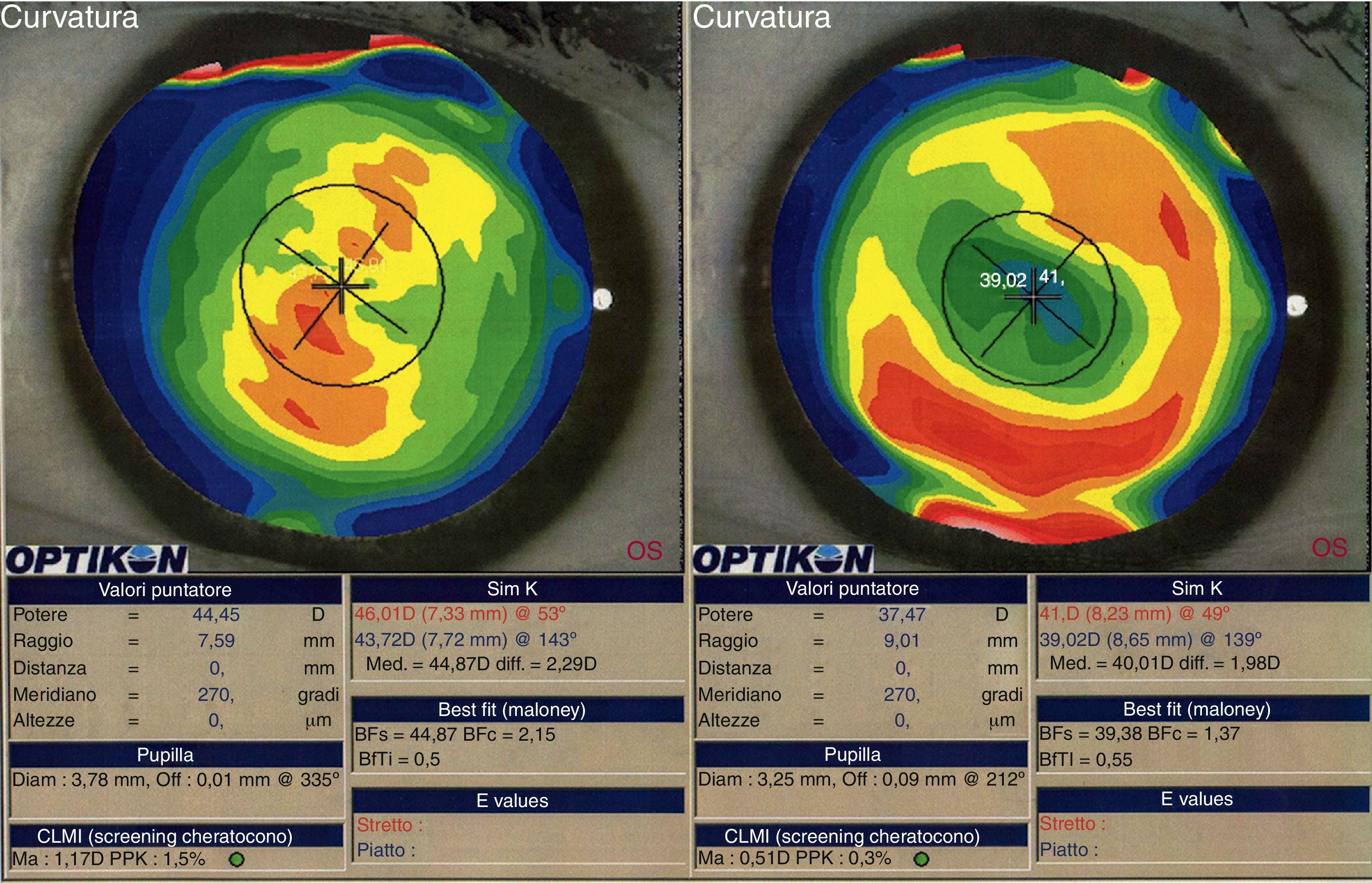
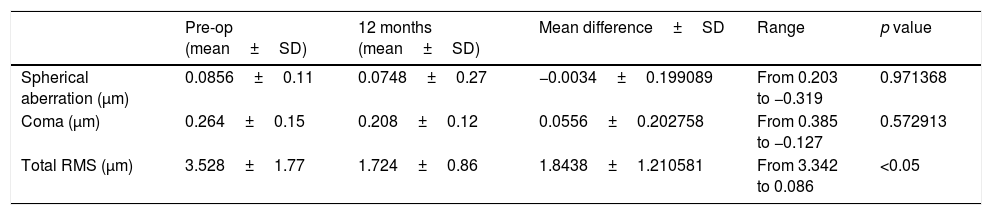
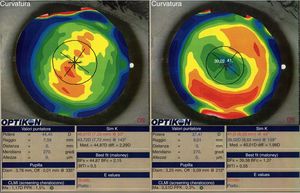
We did not observe a statistical significant change in spherical aberration (SphA) and coma at 12 months after surgery (Table 4). In addition, aberrometry showed a reduction of total aberrations root mean square (RMS). Indeed, a statistically significant difference in total RMS was found one year after treatment (p=0.027, paired Student's t-test). Fig. 2 shows an example of preoperative and postoperative corneal topographies in one case from the sample.
Difference between preoperative and after 12 months after surgery of Corneal Wavefront Aberrometry.
| Pre-op (mean±SD) | 12 months (mean±SD) | Mean difference±SD | Range | p value | |
|---|---|---|---|---|---|
| Spherical aberration (μm) | 0.0856±0.11 | 0.0748±0.27 | −0.0034±0.199089 | From 0.203 to −0.319 | 0.971368 |
| Coma (μm) | 0.264±0.15 | 0.208±0.12 | 0.0556±0.202758 | From 0.385 to −0.127 | 0.572913 |
| Total RMS (μm) | 3.528±1.77 | 1.724±0.86 | 1.8438±1.210581 | From 3.342 to 0.086 | <0.05 |
SfAb, spherical aberration; Coma, coma aberration; RMS, root mean square; SD, standard deviation.
Customized transepithelial photorefractive keratectomy (PRK) has been shown to be effective in reducing post-keratoplasty ametropia.33 In our series, a mean correction of SE of 87.9% (range 79–94.3%) was found, with a strong improvement in UDVA. No patient lost any Snellen line of UDVA or CDVA. Therefore, the visual acuity improved in all patients with no regression during a period of 12 months postoperatively. As a consequence of the laser treatment, all patients could use spectacle correction after surgery, as a high reduction of ametropia was achieved.
Also CDVA was benefited from the laser treatment, improving on average three Snellen lines, even reaching 20/20 in three patients. The absence of haze and the negligible loss of endothelial cells demonstrated the safety and stability of the treatment. Also, although a reduction in total RMS was observed, coma and spherical aberration were not statistically reduced. This reduction in total corneal aberrations was the consequence of a change in some other HOAs induced by the excimer laser. It should be also noted that the small number of our series is a clear limitation for achieving statistical significance. The customized ablation allowed us to obtain a corneal surface much more uniform than preoperatively. Using the laser spot technology, with variable diameter and modulated frequency, local irregularities can be corrected with saving of corneal tissue.34 This saving of corneal tissue allows treatment of high levels of irregular astigmatism, inducing simultaneously a regular and smooth surface. The topo-aberrometric information transmitted to the excimer laser allows a precise treatment planning that combined with a highly precise alignment and an accurate centering lead to excellent levels of visual rehabilitation. Visual acuity improvement is related to the regularization of the corneal surface and the enhancement of topographic parameters. The reduction of the total aberrations lead to an improvement in the quality of the corneal front surface and so to a better image optical quality, as observed in other studies.35 In our study, we have achieved the goal of reducing as much as possible the high astigmatism that was present in our post-PKP eyes to obtain a better visual acuity and a smoother corneal surface. The UDVA and CDVA improved without regression during the 12-month postoperative follow-up, which shows acceptable levels of predictability, efficiency and stability of treatment. The correction with spectacles after the treatment led to a binocular vision greatly appreciated by the patients and inducing a benefit in their daily life activities. Our results should be confirmed in further studies with longer follow-up periods and larger samples in order to define the customized PRK as a primary treatment strategy to correct stable ametropia after PKP.
Conflicts of interestThe authors have no conflicts of interest to declare.